Trends on New Cooperage Toasting/Charring Technology TCT. Radio Frequency Volumetric Heating and Infrared Surface Toasting Principles and Application: Influence on the Heartwood Composition and Quality
Nicolas Vivas*, Magali Picard, Nathalie Vivas de Gaulejac and Marie Françoise Nonier
Demptos Research Center, Bordeaux University, France
Submission: October 21, 2021; Published: October 27, 2021
*Corresponding author: Nicolas Vivas, Demptos Research Center, Bordeaux University, Institute of Molecular Sciences, 351 cours de la Libération, 33405 Talence, France
How to cite this article: Nicolas V, Magali P, Nathalie V d G, Marie F N. Trends on New Cooperage Toasting/Charring Technology TCT. Radio Frequency Volumetric Heating and Infrared Surface Toasting Principles and Application: Influence on the Heartwood Composition and Quality . Agri Res & Tech: Open Access J. 2021; 26 (3): 556336. DOI: 10.19080/ARTOAJ.2021.26.556336
Abstract
This paper addresses the problem of predicting soil properties using a combination of optical spectroscopic and electrical impedance methods. A comparative analysis between the most common machine learning methods such as Random Forest, Naive Bayes, Support Vector Machine, Decision Tree and Artificial Neural Network was performed using our research dataset consisting of 50 soil samples. The results indicate that none of the methods showed the best performance for nutrients prediction when only optical or electrical impedance spectroscopy measurements were used. Then, the influence of the principal components was validated to improve the machine learning performance. Their negative influence on the overall accuracy was found. Finally, the influence of the nutrient category on the prediction was validated, where similar results were found for 3-level grade and 5-level grade systems indicating a possibility for more precise and accurate soil characterization. In addition, the work shows the importance of repeated measurements for each soil sample, which can improve the overall accuracy.
Keywords: Oak heartwood, Binding, Toasting, Radio frequency, Infrared, Aroma compounds
Abbreviations: RF: Radio Frequency; IR: InfraRed
Introduction
In the cooperage field, heating treatment represents one of the key step of casks production. First, heating is necessary to permit the binding of the staves and promote the general shape of the casks. In addition, it ensures the degradation of some polymers, such as polysaccharides and lignins, leading to the formation of various aroma compounds which directly contribute to the quality of the final product [1]. Consequently, the wood heating treatment directly impacts the diversity of composition and quality of both spirits and wines aged in these casks. In traditional systems used in barrel making process, heat energy is transferred from a wood fire (heat source) to the product (wood). In this case, it is necessary to heat materials for much longer times to ensure the interior of products be heated to an appropriate temperature, which in turn may bring about overheating on the surface of products. Indeed, conventional heating methods relies mainly on two heat transfer mechanisms, convection at the surface and conduction within the wood, which alternately have dominated effects at different stages during heating process. At the beginning of 1990 decade, some other systems appeared with gas heat or with electric heat system as a bread toaster. In the same time, several modern techniques were also developed using radio frequency probe for broad applications. In this context, two devices presented a high interest in cooperage: the Radio Frequency (RF) heating and InfraRed (IR) surface toasting. This system presented the main advantage to control with high fine tunings the toasting intensity, with maximum of consistency in the heat application to the wood. Thanks to a drastic decrease of the heat variability, a better homogeneity in the cask product was possible, leading to a reproducible cask’s composition, and thus a better control of the influence of wood on wine and spirit aging.
Brief of new Cooperage Toasting/Charring Technologies
Radio frequency heating
In contrast to conventional methods, RF heating, also known as high frequency dielectric heating, is a volumetric heating technique where electromagnetic waves (10 to 300 MHz) directly couple with the product, involving a direct transfer of electromagnetic energy. Thus, RF heating is different, since it heats at molecular level. RF treatment involves heating with electromagnetic waves. The heated product forms a “dielectric” between two metal capacitor plates, which are alternatively charged positively and negatively by a high frequency alternating electric field. three in other words, when the dielectric material (such as wood) with polarized molecules and charged ions is subjected to an alternating electrical field, one phenomenon that occurs is that positive ions within the material move towards negative regions of the electromagnetic field and inversely for negative ions, which is often referred to as “ionic migration.” In addition to the migration of charged ions, polar molecules, such as water, are forced to constantly align themselves appropriately with the electric field which is known as “dipole rotation” [2,3]. These phenomena lead to frictional interactions between molecules, resulting in heat generation. The interaction between wood and RF energy is also governed by the relative complex wood permittivity (i.e the ability of wood to store energy) and can affected by various factors, such as the frequency used, the wood density and moisture content [4].
Infrared (IR) Radiation Heating
IR radiation is based on the transfer of energy by radiation between the emitting source, made up of electric heating elements, and the heated product which absorbs all or part of this energy. IR radiation falls between the region of visible light and microwaves of the electromagnetic spectrum. It is propagated as a wave and gets converted into heat when interrupted by the food surface. According to wavelength, it can be distributed into three regions: near-IR (0.78–1.4 mm), mid-IR (1.4–3.0 mm) and far-IR (3.0– 1000 mm). Practically, products absorb IR energy most efficiently by changing the molecular vibrational state resulting in radiative heating. The exposure of IR radiation to the surface material results in its penetration into product, the radiation was transformed into thermal energy and the product is then heated intensely [5]. It was reported that IR heating results in high rate of heat transfer compared to conventional heating, and the product is more uniformly heated, resulting in better quality characteristics [6]. The macromolecular composition and structure of the product as well as the wavelength of IR radiation determines the depth of penetration IR radiation into the product. Although appealing because it is fast and produces heating inside the material, total energy absorption in IR heating is limited due to the inability of IR radiation to penetrate deeper inside the product [7]. Optimum use of energy through synergic effect may be obtained by combining IR heating with other forms of energy such as microwave or radio frequency modes of heating. Generally, solid materials absorb infrared radiation in a thin surface layer, but it was important to note that moist porous materials are penetrated by radiation to some depth, and their transmissivity depends on the moisture content [8].
Materials and methods
Generals
For this specific study, some experiments were performed in laboratories with small apparatus permitted the heat treatment of oak heartwood pieces. The 2x5 cm French oak pieces (Q. petraea from center of France or Tronçais forest) obtained from Demptos cooperage were used. Different Radio Frequency or Infrared programs (time, targeted temperature, or wavelength) were applied. The control sample corresponded to barrel toasted with traditional method by a wood fire. Two pieces of stave (2x5 cm) were chosen in the middle of the barrel after light, medium or heavy toast. The same procedure was carried out by infrared toasted of a 2x5 cm oak piece from the same wood pool used for the barrel production. Experiments were performed in triplicate. The TCT treatment was decomposed in two parts: first, the wood sample was heated until 130°C or 140°C by radio frequency in order to obtain the same effect as pre-heating before binding. Then and before temperature decrease, the second step of the real toasting by infrared radiation was applied. Infrared with short or medium wavelengths required 1 or 2 minutes to obtain light, medium, or heavy toasting, depending on the energy intensity. Radio frequency system reached the target temperature (130°C) in only few seconds. The toasting started when the temperature was maintained during few minutes. For the charring process, wood samples were toasted until the formation of a complete black layer with dense white smoke was observed, but before burning for laboratory security reasons. Liquid extraction was then performed after a 24-h period necessary to rebalance the temperature and humidity of wood sample. Each sample was extracted by 250 mL of acetone/water mixture (7/3, v/v) during 2 weeks in dark, at room temperature and under nitrogen atmosphere to prevent oxidation. Due to the large polarity of the solvent mixture used, a broad range of compounds were extracted. Solutions were then filtered, and acetone was removed by evaporation under vacuum at 35°C. Furanic, phenolic aldehydes derivatives and ellagic acid were then analyzed by liquid chromatography. For better clarity and concision of the data interpretation, only the main by-products issued from the thermolysis of the oak heartwood polymers were studied: hydroxyl-methyl-furfural from cellulose; furfural from hemicellulose; vanillin and syringaldehyde from lignins; and ellagic acid from C-glucosidic-ellagitannins. Only hydroxyl-methyl-furfural and vanillin were selected for global comparison (Figures 1-5).
Radio Frequency and infrared radiation heating systems
Experimental equipement, adaptated for laboratories investigation on small peace of oak, are provided by E.A Technology Ltd. (Capenhurst, Chester, CH1 6ES, GB).
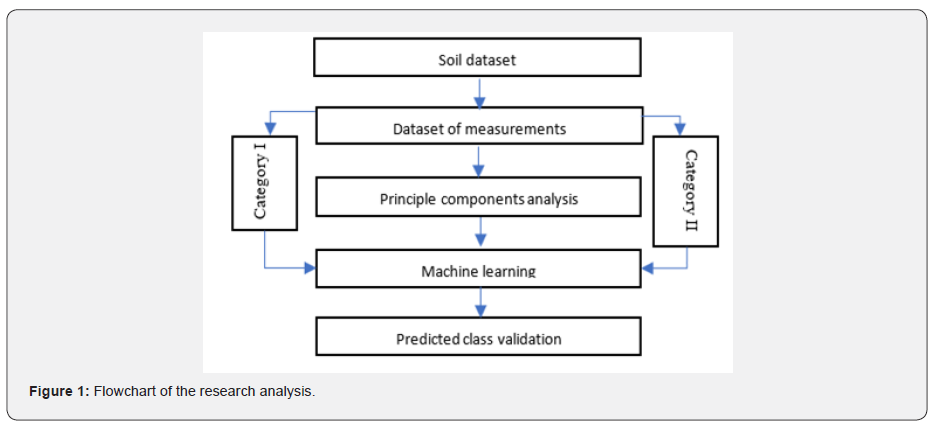
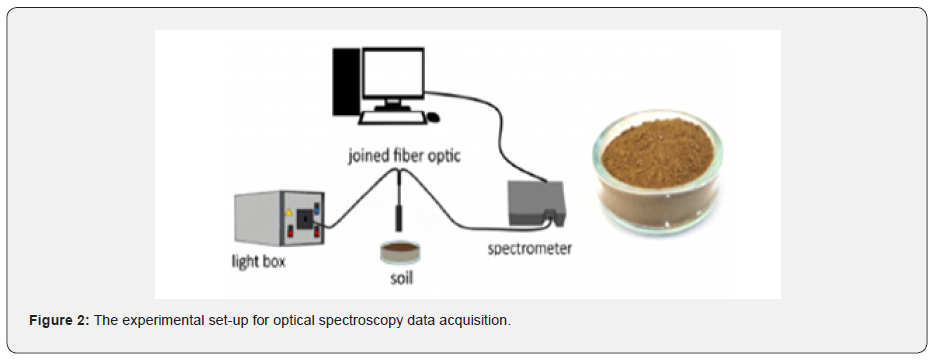
LC-ES/MS analysis of wood aldehydes, furanics and pyranics compounds
HPLC/UV-Visible analyses were performed with a Waters separation module system, a Waters UV-Visible detector, and Millenium32 chromatography manager software. UV-visible spectra were recorded at 280 nm and 320 nm (for specific detection of phenolic aldehydes). The column was a reverse-phase Interchim C18 (10 m packing, 250 x 4.7 mm i.d.) protected with a guard column of the same material; solvent A, water/formic acid (98:2, v/v); solvent B, acetonitrile/water/formic acid (80:18:2, v/v). The column was placed at ambient temperature. The elution program was performed at a constant flow of 1 ml/min, passing from 5 to 30% of B in 40 min., and then rising to 40% of B in 10 min., and finally to 100% of B in 5 min., followed by washing and re-equilibrating the column during 15 min. The injection volume was 20l. MS measurements were performed on a LCQ ThermoTM instrument with an electrospray ionization source, in negative-ion mode with a spray voltage of 4.5 KV, capillary temperature: 275°C.
Pyrolysis-GC-MS experiment
A “PYROJECTOR SGE II” was used as pyrolyzer, in conjunction with a Varian 3400 CX model gas chromatography apparatus equipped with a Varian Saturn 4D ion trap detector. Samples (approximately 0.1-0.2 mg) were placed in the pyrolyzer and triplicate pyrolysis experiments were carried out at different temperatures: 300°C, 400°C, 450°C, 500°C, 600°C. General profiles for pyrolyzates were obtained using EI-MS. Separation of the pyrolysis products was achieved using a fused-silica capillary column: RTX-20 WCOT(30 m x 0.25 mm i.d x 1m phase thickness, 80% dimethyl and 20% diphenylpolysiloxane). Helium was used as the carrier gas at a nominal flowrate of 1 ml/min. The inlet mode was spitless. The gas chromatography oven was operated using the following programme: isothermal for 10 min at 50 °C, then raised from 50 °C to 280°C at 6°C/min. The mass spectrometer was set at 70 eV. Spectra were produced using a ChemStation software package. Identification was achieved by mass fragmentometry, by comparison of their mass spectra and relative retention times with those of compounds reported in the literature and the National Institute of Standards library (NIST). When possible, the identifications were accomplished by comparison with authentic standards. Quantification was based on peak areas (total integral of identified compounds equals 100). The syringyl/guaiacyl ratios (S/G) were calculated by dividing the sum of the peak areas of syringyl units by the sum of the peak areas of guaiacyl units.

Result
Preliminary results on radio frequency and infrared heated treatment of oak wood, comparison with traditional casks fire binding/toasting process. Preliminary results are presented on and. The effect of RF/IR heat treatment on wood pieces and the practical effect of traditional binding/toasting in cooperage were investigated. Only French oak was analyzed in this study and further research are required on different oak origins, especially on American oak. illustrates the impact of the various heating procedures on the content of HMF and vanillin, chosen as indicators of heat treatment. Regarding the untoasted oak (control sample), heat treatment promoted the formation of HMF and vanillin at different levels but always higher than in control. Interestingly, a global evolution of theirs contents according to the toasting intensity (light, medium, heavy toast) was emerged. Using either traditional binding/toasting in cooperage or with RF/IR heat treatment, same evolution trend was observed. For the first stage, both methods were similar, and combining RF and IR heat treatments well-reproduced the main impact of a traditional binding/toasting. More precisely, RF/IR heat treatment promoted a higher concentration of HMF and vanillin for each toasting level (LT, MT, HT), since the reaction was more intense than in the traditional binding/toasting procedure. The differences observed were significant and represented up to 25 to 50% higher concentration of the two assayed compounds for RF/IR heat treatment. Concerning the charring trial, visual aspect of the wood surface was very close to traditional charring with gas fire and corresponded to what expected for this kind of treatment. These results were in accordance with examination made by J.S Swan on this experimental sample (IR charring versus oven charring) and other samples issued from a real charring treatment in a Scotland cooperage. Consequently, a typical charring was also possible using RF/IR heat treatment. In our study context, charring promoted the degradation of the majority of the wood polymers such as polysaccharides and lignins, leading to higher concentrations in HMF and vanillin than heavy toasting. Surprising different results were obtained with an oven charring or gas charring. Indeed, the content of aroma compounds in these two last procedures was lower, especially for compounds issued from polysaccharides. RF/IR heat treatment allowed to reach, in the same time, the standard level of a visual charring, with conservation of a large amount of aroma compounds. In a second series of experiment, the specific effect of a pre-heated operation by RF system before the post-toasted treatment by IR was investigated. Interestingly, since the pre-heated treatment by RF was usually necessary for binding the stave, this operation also improved the aromatic compounds produced during the second heated treatment by IR. presents the effect of (i) RF treatment at 130°C (just before binding) following by the IR toasting, and (ii) RF pre-heating at 140°C, maintained at this temperature for 3 min and followed by the normal process of IR post-heating to obtain low, high, and medium toasting levels respectively.
This highlighted that the 3-min delay at 140°C significantly improved the thermolysis reaction with an increase of HMF and vanillin content in the treated oak samples. For low, medium, and high toasting’s, the variation of the concentration was impacted by a factor 2 and similarly concerned polysaccharides pyrolysis (HMF) and lignins pyrolysis (vanillin) products. It is reasonable to imagine similar observations about the melanoïdins formation and their main pyrolysis products, like maltol or cycloten (not confirmed in this work by specific experiments). After targeting an optimisation of pre-heated treatment by RF system, the IR heating treatment was then improved. A regular RF pre-heated (to reach 130°C) and a series of toasting with medium or short wavelength for 1 or 2 min were thus compared. Data revealed that (i) the exposition time to IR for 1 or 2 min increased the concentration of HMF and vanillin in oak samples and (ii) medium wavelength promoted all the pyrolysis reactions, with higher concentration of aroma products at the end of the heat treatment application. Higher content in HMF and vanillin was obtained for the procedure with medium wavelength during 2 min. An IR heating with medium wavelength option for 1 min represented thus a good compromise between time of treatment and balance of aroma compounds.
Finally, the reproducibility of the heating system was studied by comparing traditional fire binding/toasting and RF/IR methods. For both medium and heavy toastings, RF binding and IR toasting gave the best consistency in the production of HMF and vanillin (i.e lower variability in the content of these two compounds), suggesting best robustness of the RF/IR method than traditional one.
A first approach of the specificity of pyrolysis chemistry reaction for RF/IR heat treatment
An overview of pyrolysis reaction. According to the polymer composition of oak wood, various molecules formed by pyrolysis derived from polysaccharides, or carbohydrates and lignins. During pyrolysis, polysaccharides produced different furanic and pyranic compounds, such as 2-, 3- and 2,5-furfuraldehyde, 4-hydroxy-5,6-dihydro-(2H)-pyrane-2-one, 5-hydroxymethyl-2-furfuraldehyde (also called HMF, using as a key compound in our investigation) or hydroxymethyl-pyranone. The 2-, 3-, and 2,5-furfuraldehydes came from pyrolysis of the pentose units of hemicellulose, whereas the 5-hydroxymethyl-2-furfuraldehyde from the glucose units of cellulose. The ratio between the heights of m/z 96 (in pyrolysis/MS analysis) and 126 peaks was, on average, 1.9 ± 0.2 [9]. Although cellulose is more abundant in wood, hemicellulose is more easily thermally degraded. The main products originating from pyrolysis of lignins are classified as guaiacyl “G“units (monomethoxylated) and syringyl “S“ units (dimethoxylated). Among the G units, 2-methoxyphenol and 4-formyl-2-methoxyphenol (also called vanillin, using as a key compound in our investigation) were identified. Similarly, 4-vinyl-2,6-dimethoxyphenol and 4-formyl-2,6-dimethoxyphenol (syringaldehyde) were identified within the S-units. The S/G ration varied during the course of pyrolysis: 1.25 at around 150°C, 18 at 195°C, 2.2 at 220°C and 0.77 at around 250°C [9]. This result suggested that, for temperature below 220°C, pyrolysis degrades the S units of the lignins selectively whereas, at higher temperatures, the phenomenon is reversed and lignins, in G units, were more affected.
Cellulose, although much more abundant in oak than hemicellulose, is degraded less, whatever the temperature level. The crystalline structure of a part of the cellulose explains its resistance [10]. Using CPMAS 13C NMR, the proportion of crystalline cellulose in Q. robur and Q. petraea was present at 45% [11]. During pyrolysis, the furanic derivatives released by thermal degradation of arabinose and xylose were predominant. Depolymerization of lignins was high at temperature of around 150°C. Beyond this temperature, a sharp decrease was observed in the thermo-degradation products. In accordance with [12], the increase in temperature led to depolymerization at first, followed by cross-linkage of the lignin at higher temperature. The new polymers produced have higher molecular masses and fusion points. Careful examination of S/G ratio showed that the different kinds of lignins were transformed during pyrolysis. From 150°C to 220°C, the S/G ratio is greater than 1 and above 250°C, it becomes less than 1. During the first phase of thermolysis, the lignins located in the secondary walls, rich in S units, were degraded. At higher temperature, thermal degradation concerned more particularly the primary walls containing lignins rich in G units [13]. Some specificity of the RF/IR treatment in comparison with traditional fire toasting. Based on specific experiment, the practical aspects of pyrolysis chemistry for FR/IR heat treatment were studied in comparison with traditional fire binding/toasting. The heat treatment on main oak heartwood polymers, as well as cellulose, hemicellulose, lignins and ellagitannin composition, was evaluated. In both experiments and for all targeted compounds, RF/IR heat treatment gave more thermal degradation by-products: HMF, furfuraldehyde for polysaccharides; vanillin, syringaldehyde for lignins; and ellagic acid for ellagitannins. Concerning polysaccharides, effects were discriminated according to the nature of polymers. For cellulose, the more resistant form of polysaccharides to pyrolysis because partially crystallized, RF/IR promotes a large increase in the content of HMF (+72, +74%) the main key aroma compound derived from this polymer. In contrast, and although the quantity of specific by-products were higher by RF/IR treatment, hemicellulose (easily pyrolysis polysaccharide) presented a less answer to RF/IR treatment. Indeed, a limited increase (+11, +20%) of the furfuraldehyde concentration (key compound of hemicellulose) was noted comparing to traditional fire binding/toasting. This difference was clearly highlighted by the ratio HMF/furfuraldehyde comparison: 0.04-0.02 for traditional fire toasting and 0.11-0.06 for RF/IR treatment. Concerning lignins, the chemistry pyrolysis was not affected by the heating system, since a more or less similar ratio S/G was found using either traditional fire binding/toasting and FR/IR treatment: 2.8-3.0 for the first experiment and 2.2 for the second. However, and similarly to polysaccharides, the final concentration of by-products enlarged in a significant proportion (+61% to +71%). Finally, interesting findings were obtained for ellagitannins. In comparison to traditional fire binding/toasting, RF/IR treatment degraded a larger amount of ellagitannins, observable by the increase of ellagic acid (+65% to +77%), the mains stable by-product of C-glucosidic ellagitannins thermal degradation.
Discussion and conclusions
The aim of this study was to investigate a new generation of cooperage heat treatment by electric devices in order to substitute the traditional fire system. This study was conducted mainly to push-on the rate of casks binding and toasting in the industrial process. Radio frequency and infrared system appeared as a great compromise between the faster capacity to operate and their ability of mimic the global chemical process occurred during the traditional fire method. However, some differences were also observed, in favor to the RF/IR equipment. However, before anything else, several potential difficulties due to fast heating procedure needed to be clarified: The binding can promote the stave breakage, since we don’t take enough time to reach in the same time the plasticity of the wood (by temperature application), the repartition of the tension (surface tension of the lignins and the mains polymers) and the plasticity capacity homogenization on the whole volume of the staves material. To solve this specific problem, it was probably highly recommended to increase the duration of binding, from few seconds to a minimum of 20 seconds. Pre-moisturizing casks may help to reach this point. Another issue was the constant temperature repartition within the wood. Theatrically, in the FR and IR systems, the temperature was constant in every part of the wood in accordance with our program selection. However, wood structure, especially wood porosity, was not uniform and led to some change in the physical properties of the stave. Thus, this phenomenon has to be taken into account when a program of toasting was designed. This issue may also be an advantage when the variety of the toasting profiles was considered, that sometimes represented a part of the complexity of casks and the ageing quality. Concerning the specific point of toasting and aroma production, the RF/IR method clearly initiated a higher production of aroma compounds, even coming from some particular pyrolytic resistant polymers like cellulose. Better degradation of ellagitannins was also observed, resulting in a final low polyphenol impact of the cask and an improve of mouthfeel properties of aged wines and spirits. In comparison with traditional fire toasting method, the consistency and the reproducibility of the toasting, underlining by a lower variability in the content of HMF and vanilla, was also obtained using RF/IR treatment. Indeed, application of a specific program of treatment, such as pre-heating for few minutes (2-3 min) by radiofrequency at 140°C-150°C, enhanced the aroma compounds production. This was particularly useful to increase aroma potential of wood casks, preserve energy by a faster IR toasting or retreat old casks (5- 10- fill casks). This also facilitated the production of a minimum of aromatic compounds coming from pyrolysis of the residual polymers which were still active in aroma production. Overall, the RF/IR combination system as a binding/toasting system presents a high interest in the barrel-making process, due to the fastness of the cooperage facility, as well as the final product quality. Considering our first investigations, the application of this technique for industrial casks production was thus highly recommended.
References
- Vivas N, Bourgeaois G (1998) La maîtrise de la chauffe traditionnelle en tonnellerie. Proceedings of Cooperage Sciences and Techniques Conference 4: 29-35.
- Piyasena P, Dussault C, Koutchma T, Ramaswamy HS, Awuah GB (2003) Radio frequency heating of foods: principles, applications and related properties: A review. Critical Reviews in Food Science and Nutrition 43(6): 587-606.
- Zhou X, Wang S (2019) Recent developments in radio frequency drying of food and agricultural products: A review. Drying Technology 37(3): 271-286.
- Nelson SO, Trabelsi S (2012) Factors influencing the dielectric properties of agricultural and food products. Journal of Microwave Power and Electromagnetic Energy 46(2): 93-107.
- Rastogi NK (2021) Infrared Heating in Drying Operations. Reference Module in Food Sciences.
- Ginzburg AS (1969) Application of IR Radiation in Food Processing. Leonard Hill Books, London, UK.
- Krishnamurthy K, Khurana HK, Soojin J, Irudayaraj J, Demirci A (2008) Infrared heating in food processing: An overview. Comprehensive Reviews in Food Science and Food Safety 7(1): 2-13.
- Lampinen MJ, Ojala KT,Koski E (1991) Modeling and measurements of infrared dryers for coated paper. Drying Technology 9(4): 973-1017.
- Vivas N, Absalon C, Vivas de Gaulejac N, Benoist F (2000) In Situ analysis by pyrolysis-Mass Spectrometry of the macromolecules in heartwood of Q. robur L. Journal des Sciences et Techniques en Tonnellerie 6(1): 85-89.
- Barnoud F (1980) La cellulose, (In:) “Les polymers végétaux: Polymères pariétaux et alimentaire non azotés“, Monties B (Edn.) Bordas, Paris 1: 66-86.
- Vivas N (1997) Recherches sur la qualité du chêne Français de tonnellerie (Q petraea Liebl, Q robur L) et sur les mécanismes d’oxydoréduction des vins rouges au cours de leur elevage en barriques. Ph D University of Bordeaux, Tome I 205p, Tome II P. 52.
- Lapierre C, Monties B, Vassal Gonthier A, Dworkin A (1986) Differential calorimetric study of pine and poplar lignins between 300 and 525°K. Journal of Applied Polymers Science 32(8): 4561-4572.
- Monties B (1992) Composition chimique des bois de chê (In:) “Le bois et la qualité des vins et des eaux-de-vie“, Guimbertaud G (Edn.), Vigne et Vin Publications Internationales, Bordeaux 59-72.






























