Surveying Maize Seedlings as The First Step for Re-Evaluating Cold Tolerance in Maize Inbred Lines
Manja Božić1*, Ana Nikolić1, Ksenija Marković1, Nenad Delić1, Marija Milivojević1, Jelena Vančetović1, Mihai Miritescu2 and Dragana Ignjatović-Micić1
1R&D, Maize Research Institute Zemun Polje, S. Bajića 1, 11000 Belgrade Serbia
2Saaten Union Romania SRL &Rapool Ring Romania SRL, Str. General Praporgescu Nr. 1-5, Bucharest Romania
Submission: January 28, 2021; Published: March 08, 2021
*Corresponding author: Manja Božić, R&D, Maize Research Institute Zemun Polje, S. Bajića 1, 11000 Belgrade Serbia
How to cite this article: Manja Božić, Ana Nikolić, Ksenija Marković, Nenad Delić, Marija Milivojević, et al .Surveying Maize Seedlings as The First Step for Re-Evaluating Cold Tolerance in Maize Inbred Lines. Agri Res & Tech: Open Access J. 2021; 25 (4): 556311. DOI:10.19080/ARTOAJ.2021.25.556311
Abstract
The consequences of the current climate changes sustain the strategy of early sowing (early spring) to avoid drought during the flowering period and thus secure maize yields. Earlier sowing dates increase the risk of exposure of the plants to cold during the early development stages and thus re-evaluation of elite material is required to select more tolerant genotypes. The first step of re-evaluation is to define characteristics that could be used for fast survey of the elite material, before performing more profound analyses. The aim of this paper was to analyse morphological characteristics (radicle length, coleoptile length, seedling weight) of 5 day old seedlings of two inbred lines LK and Mo17 (according to breeders experience tolerant and susceptible, respectively) subjected to chilling stress (10°C day/8°C night) for 24h and 48h, as well as to perform morphological (root and shoot characteristics) and physiological (chlorophyl, flavonoid and anthocyanin contents) analyses of the plants after seven day recovery. Control plants were grown under optimal conditions (30°C day /20°C night, ≈60% humidity), at an 8h photoperiod. The results were not straightforward, as the susceptible Mo17 showed faster recovery (statistically insignificant differences between control and treatment plants for most of the analysed traits), while the tolerant LK was a more robust genotype throughout the experiment. Chlorophyl, flavonoid and anthocyanin contents were not informative. The results identified survival rate and root characteristics as informative for fast screening of seedlings for determination of cold tolerance.
Introduction
Global warming can have severe negative effects on maize production due to high temperatures and water shortage, especially during summer seasons when flowering and grain filling occur. The consequences of the current climate changes sustain the strategy of early sowing (early spring) to avoid drought during the flowering period and thus secure maize yields [1]. However, maize is sensitive to low temperatures and it has been well documented that suboptimal temperatures (10–15°C) can cause reduction in growth and biomass production capacity while under low temperatures (2-8°C) irreparable damage and loss of plants can occur [2,3].
Lower temperatures of early spring are unfavourable for seed germination, emergence, transition phase from heterotrophic to autotrophic growth, as well as the early seedling development [4]. Seed germination is a complex process which starts with water absorption by the dry seed and ends when radicle elongates [5]. Rapid and successful germination is a key stage for the establishment of vigorous seedlings and is also considered as a major component of final crop yield [6]. In the context of climate change, understanding the effects of low temperature on maize seeds during germination and early seedling growth and development (which strongly depends on temperature) is becoming of extreme importance.
Earlier sowing dates increase the risk of exposure of the plants to cold during the early development stages and hence require re-evaluating cultivated materials and to select inbred lines that are more tolerant to low temperatures [7]. The molecular mechanisms of chilling tolerance or sensitivity during germination and early seedling development, in contrast to the effect of cold on seedlings or at the whole plant level, are not well studied [8]. Maize Research Institute Zemun Polje has developed a project for studying mechanisms underlying maize chilling/cold tolerance and developing more tolerant genotypes. The first step is to test elite inbred lines known to be tolerant or susceptible to low temperatures based on breeders’ experience for morphological and physiological traits at germination and early development stages. After surveying the elite material, selected inbred lines will undergo more profound analyses at genomic, transcriptomic, physiological and biochemical levels. In this paper, we present the results of: 1) morphological analyses of 5 day old seedlings of two inbred lines, designated as cold tolerant and cold susceptible, subjected to chilling stress, and 2) morphological and physiological analyses of the plants after recovering from the stress for seven days. The aim of the experiment was to define characteristics that could be used for fast survey of the elite material.
Materials and methods
Plant material and growth conditions
Maize seeds of two genotypes (Mo17 and LK) were sterilized with 1% NaOCl (sodium hypochlorite, commercial bleach) and then placed on moist filter paper. The filter paper was rolled up and placed in a growth chamber, where the seedlings were grown for 5 days in optimal conditions (30/20° C, ≈60% humidity), at a 8h photoperiod.
Stress treatment
Chilling stress in 5-day old seedlings of both genotypes was induced by exposure to low temperatures (10°/8° C) for both 24h and 48h. Control plants were left in optimal conditions for the same time periods. After each chilling treatment has finished, half of the seedlings were sampled immediately and the other half were sown in pots containing a mixture of soil and sand (3:1) and moved to the greenhouse and grown there under optimal conditions for another 7 days to recover before further sampling.
Post-treatment measurements
Seedlings of both genotypes sampled immediately after chilling stress treatment (both 24h and 48h) were used for fresh weight (5 day S-W – seedling weight), coleoptile (5 day S-CLseedling coleoptile length), and radicle length measurement (5 day S-RL - seedling radicle length). Chlorophyll (CH), flavonoid (FL) and anthocyanin (AN) content, were measured on seedlings that were left to recover after 7 days, using DUALEX® optical sensor (Force A, France). Recovered plants were harvested and their fresh root (RFW) and shoot weight (SFW), as well as root (RL) and shoot length (SL) were measured. The plants were then dried for 24h at 110° C, after which they were measured again and their dry root (DRW) and shoot weight (SDW) was determined. Root and shoot moisture contents (RMC and SMC, respectively) were also determined.
Statistical analysis
The data analysis for this paper was generated using the Real Statistics Resource Pack software (Release 7.2) (Zaiontz, 2020). Statistical significance of the data and results was verified through t-test with and marked at significance level p < 0.05 (*), p < 0.01 (**) and p < 0.001 (***). Graphs were designed in Microsoft Excel.
Results and Discussion
It is well known that germination of maize seed is altered at suboptimal temperatures in a genetic dependent manner [8]. The results of our experiment confirm this, as significant differences between tolerant and susceptible inbred lines were detected in the survival rate, as well as plant morphological and physiological responses - LK inbred line has a better genetic potential for overcoming the growth inhibition caused by the low temperature stress. The survival rate was determined by the number of plants grown during the recovery period. The percentage of survived plants in both Mo17 and LK controls was 100%. Additionaly, 27% of Mo17 24h-control-plants were in V1 stage and 73% in V2; while among the Mo17 48h-control-plants 47% were in V1 and 53% in V2 stage. LK 24h-control-plants were mostly in the V2 stage (87%), while the rest reached the V3 stage (13%), and it was the same for the LK 48h-control-plants (87% V2, 13% V3). On the other hand, the percentage of survived plants after both treatments was 80% for Mo17 and 100% for LK. After 24h stress, 33% of the Mo17 survived plants reached V1 and 67% reached V2, while after 48h stress 69% reached V1 and only 31% V2. In LK, all survived plants after both stresses were in V2 stage. This is in accordance with Enders et al. [9], who showed that different genotypes have different growth rates after exposure to cold stress, as well as that plants exposed to shorter periods of cold treatment had lower amounts of growth inhibition.
Low temperature stress in the early maize growth stages retards seed germination, seedling emergence, and vegetative growth, negatively affecting morphogenesis, photosynthetic characteristics, and yield [10]. Results of 5 day old seedlings analyses (5 day S-RL, 5 day S-CL and 5 day S-W) are given in Table 1. It can be seen that absolute average values for all three traits were higher in LK genotype, in both controls (C) and treatments (T), indicating a better seedling vigour. Similar results were obtained for root (Table 2) and shoot (Table 3) morphological traits (except for SMC) after seven days of recovery. It was previously shown that genotypic differences in cold tolerance exist for the development of the root [11] and the shoot [12] and that strong primary root holds promise for the improvement of early vigour under chilling conditions [13]. More favourable root and shoot characteristics found for LK plants indicate higher tolerance to cold stress compared to Mo-17. Additionally, SL and SW (Table 4) confirm the superiority of LK genotype. According to the t-test (given in the same Tables), significant differences between control and treatment Mo-17 plants after 24h stress were found for two traits and for 48 stress for four traits. On the other hand, a higher number of significant differences between control and treatment plants were found for LK – six for 24h stress and eight for 48 stress. Common significant differences in both Mo-17 and LK were found in four out of 11 analysed traits (5 day S-RL, 5day S-CL, RL and RDW), only under 48h stress. As expected, greater impact on recovery was found for the 48h stress because duration of the exposure to low temperatures is one of the pivotal factors of the plant response to the stress [3].
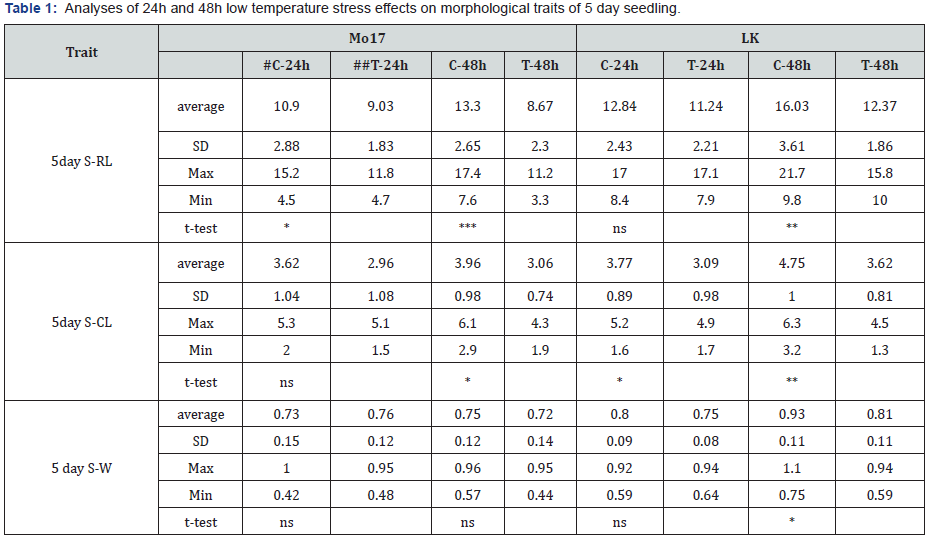
5 day S-RL- seedling radicle length (cm), 5 day S-CL- seedling coleoptile length (cm), 5 day S-W – seedling weight (g); #C-control, ##T-treatment; *,**,*** –significant at p˂0.05, 0.01 and 0.001, respectively, ns –non-significant
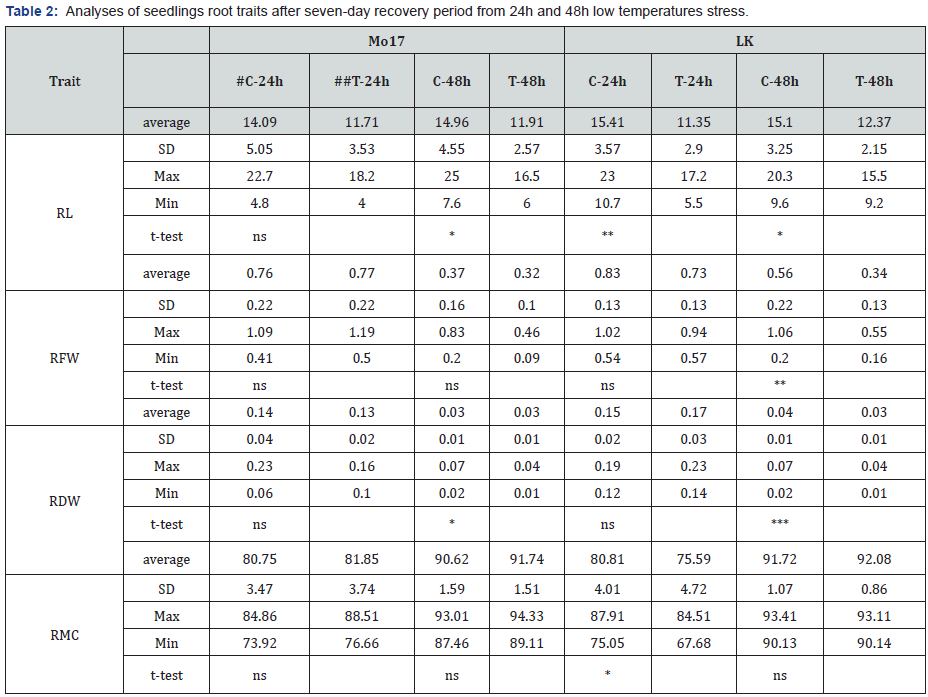
RL – root length (cm), RFW – root fresh weight (g), RDW – root dry weight (g), RMC (root moisture content) #C-control, ##T-treatment *,**,*** –significant at p˂0.05, 0.01 and 0.001, respectively, ns –non-significant
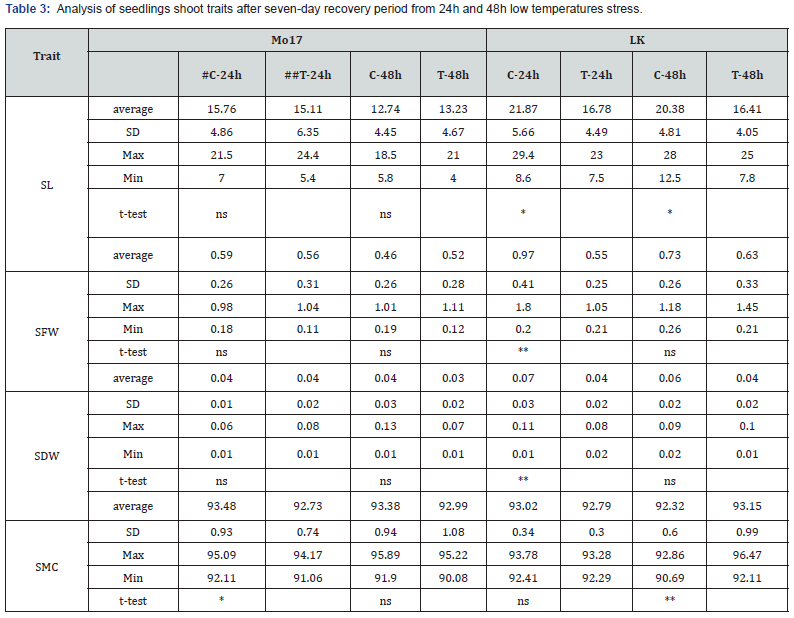
SL – shoot length, SFW-shoot fresh weight, SDW – shoot dry weight, SMC – shoot moisture content #C-control, ##T-treatment *,**,*** –significant at p˂0.05, 0.01 and 0.001, respectively, ns –non-significant



The t-test results were confirmed by the percentage of changes of morphological traits under 24h and 48h chilling stresses (Figure 1 - 5day old seedlings; Figure 2 - root traits after recovery; Figure 3 - shoot traits after recovery). The impact of both 24h and 48h stress on root (Figure 2) and shoot (Figure 3) traits during recovery was more pronounced in LK plants. A reduction in temperature slows down most physiological processes and growth arrest might be the strategy for fast recovery after a mild stress [7]. It seems that the susceptible Mo-17 displayed faster recovery seven days after the stress ceased, but better recovery efficiency was not sufficient to display better chilling tolerance. LK was a more robust genotype with higher survival rate. The results indicate higher seed and seedling vigour of LK genotype which was shown to be one of the most important characteristics for chilling tolerance during early development stages [13].

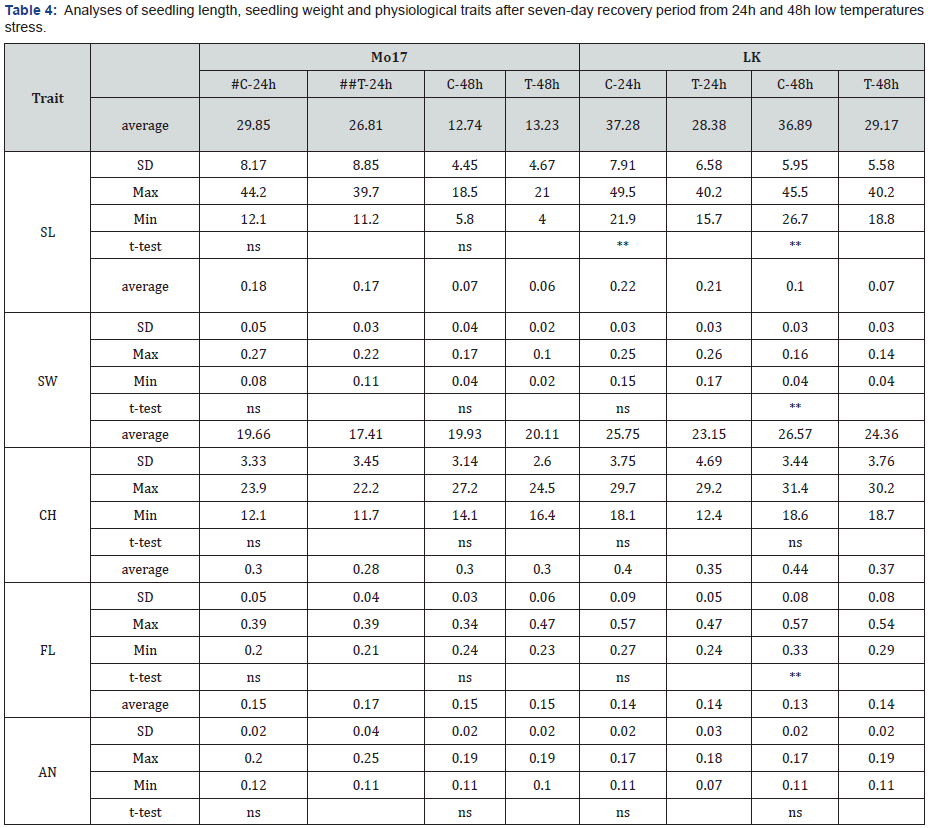
SL-seedling length, SW-seedling weight, CH –chlorophyll, FL –flavonoids, AN –anthocyanins #C-control, ##T-treatment *,**,*** –significant at p˂0.05, 0.01 and 0.001, respectively, ns –non-significant
Besides morphological traits, CH, AN and FL contents were also analysed on the recovered plants. Since seedling leaf tissues are developmentally unable to synthesize structurally protective compounds in their cell walls, they often increase vacuolar solutes, like anthocyanin, to remain turgid under low water potential conditions [14]. Flavonoids were also found to be over accumulated in response to a wide range of abiotic stresses [15], as they outperform antioxidants, owing to their strong capacity to donate electrons or hydrogen atoms [16]. Chlorophyll content reflects plant photosynthesis efficiency and under suboptimal temperatures the photosynthetic capacity is low [17]. However, no significant changes of these compounds were found either in Mo-17 or LK plants after seven day of recovery, except for FL in LK plants after 48h stress (Table 4). The same as for morphological traits, percentage of changes was higher for LK plants (Figure 4). As FL, AN and CH changes occur during the stress, it is possible that their measurement in recovered plants is not an indicator of cold tolerance.
Conclusions
The tolerant LK inbred line was a more robust genotype and had higher average values for almost all analysed traits under both 24h and 48h cold stresses, although the seven-day recovery was faster in the susceptible Mo-17 genotype. CH, AN and FL content measurements on the recovered plants did not reveal any relevant data on cold tolerance and thus should not be used for fast screening. Among the analysed traits for fast screening of seedlings for determination of cold tolerance, survival rate and root characteristics showed to be informative.
References
- Kucharik CJ (2006) A multidecadal trend of earlier corn planting in the central USA. Agron J 98(6): 1544–1550.
- Leipner J, Stamp P (2009) Chilling stress in maize seedlings. In: Bennetzen JL, Hake SC (eds.), Handbook of maize: Its biology. Springer, New York, pp.291–310.
- Farooq M, Aziz T, Wahid A, Lee D-J, Siddique KHM (2009) Chilling tolerance in maize: agronomic and physiological approaches. Crop and Pasture Science 60(6): 501-516.
- Huang J, Zhang J, Li W, Hu W, Duan L, et al. (2013) Genome-wide association analysis of 10 chilling tolerance indices at the germination and seedling stages in maize. J Integrat Plant Biol 55(8): 735– 744.
- Bewley JD (1997) Seed germination and dormancy. Plant Cell 9(7): 1055–1066.
- Ellis RH (1992) Seed and seedling vigour in relation to crop growth and yield. Plant Growth Regul 11: 249-255
- Riva-Roveda L, Escale B, Giauffret C, Périlleux C (2016) Maize plants can enter a standby mode to cope with chilling stress. BMC Plant Biology 16: 212.
- Noblet A, Leymarie J and Bailly C (2017) Chilling temperature remodels phospholipidome of Zea mays seeds during imbibition. Scientific REports 7: 8886
- Enders TA, St Dennis S, Oakland J, et al. (2018) Classifying cold‐stress responses of inbred maize seedlings using RGB imaging. Plant Direct. 3: 1-11.
- Hund A, Fracheboud Y, Soldati A, Stamp P (2008) Cold tolerance of maize seedlings as determined by root morphology and photosynthetic traits. European Journal of Agronomy, 28(3): 178-185.
- Richner W, Kiel C, Stamp P (1997) Is seedling root morphology predictive of seasonal accumulation of shoot dry matter in maize? Crop Sci 37(4): 1237–1241.
- Lee EA, Staebler MA, Tollenaar M (2002) Genetic variation in physiological discriminators for cold tolerance -early autotrophic phase of maize development. Crop Science 42: 1919-1929.
- Hund A, Fracheboud Y, Soldati A, Frascaroli E, Salvi S, Stamp P (2004) QTL controlling root and shoot traits of maize seedlings under cold stress. Theor Appl Genet 109(3): 618–629.
- Gould KS (2004) Nature’s Swiss Army Knife: The Diverse Protective Roles of Anthocyanins in Leaves. J Biomed Biotechnol 5: 314–320.
- Nakabayashi R, Saito K (2015) Integrated metabolomics for abiotic stress responses in plants. Current Opinion in Plant Biology 24: 10-16.
- Hernandez I, Alegre L, Van Breusegem F, Sergi Munné-Bosch, et al. (2009) How relevant are flavonoids as antioxidants in plants? Trends in Plant Science 14(3): 125–132.
- Zaidi PH, Yadav M, Maniselvan P, Khan R, Shadakshari TV, et al. (2010) Morphophysiological traits associated with cold stress tolerance in tropical maize (Zea mays L.). Maydica 55(3): 201-208.






























