Contributions of Effective Microorganisms and Organic Materials on Microbial Activities
Ene DU*, Orji OA and Nwonuala AI
Crop/Soil Science Department, Rivers State University,Nigeria
Submission: October 13, 2020; Published: November 23, 2020
*Corresponding author: Ene DU,Crop/Soil Science Department, Rivers State University,Nigeria
How to cite this article: Ene DU*, Orji OA and Nwonuala AI. Contributions of Effective Microorganisms and Organic Materials on Microbial Activities. Agri Res & Tech: Open Access J. 2020; 25 (2): 556302. DOI: 10.19080/ARTOAJ.2020.25.556302
Abstract
A pot experiment was conducted in the screen house of the Teaching and Research Farm of Rivers State University (RSU)from March to July, 2018 to determine the effects of Effective Microorganisms Activated Solution (EMAS) and organic materials (poultry manure (PM) and dry grasses (DG)) on microbial population and activities. The six treatments made up of 2 levels of EMAS and 3 levels of organic materials were replicated three times and arranged in a Completely Randomized Design (CRD). The treated soils were incubated for 4 weeks and samples collected for microbial count and carbon dioxide evolution. Microbial activities were monitored weekly for 4 weeks. The results show a significant difference (p<0.05) between soil+ effective microorganisms activated solution (SO+EMAS), soil + poultry manure + effective microorganism activated solution(S+PM+EMAS), soil + dry grasses + effective microorganism activated solution (S+DG+EMAS), soil + poultry manure (S+PM), soil + dry grasses (S+DG) and the control soil only (SO). The population of heterotrophic bacterial were significantly different, S+EMAS had the highest (9.82x109CFU/g) and the control (SO) had the least population (1.38x109CFU/g) before planting, SO had the highest population count (4.63x108 CFU/g) and S+DG had the least population count of bacteria (4.10x107CFU/g) after harvest, also a significant different exist among the treatments on CO2 evolution, S+PM had the highest mean value (0.06mg), S+EMAS had the least value (0.002mg). The results show that soils amended with EMAS improved bacterial count, lowers CO2 evolution, soils amended with PM improved CO2 evolution. Hence recommending that EMAS and organic matter (especially PM) should be used for the improvement of soil microbial population to enhance nutrient availabilities for plants and to create a sustainable agriculture.
Keywords:Effective microorganisms (EM); Effective microorganisms activated solution (EMAS); Microbial activities
Introduction
Microbial amendments have received considerable attention in sustainable agriculture for enhancing crop production, improving soil quality, and reducing inputs of chemical fertilizers and pesticides. Hussain et al. [1]. Microbial amendments are also known as biofertilizers, biodynamic fertilizers or microbial inoculants which are comprised of living microorganisms that can function as chemical fertilizer adjuvant biocontrol agents, and plant growth factors according to Li & Zhang [2], one popular microbial amendment is effective microorganisms (EM) developed by Teruo Higa, at the University of Ryukyus, Okinawa Yamada &Xu [3], which has been used as inoculants to change soil microbial diversity and the microbial interaction between soils and plants, thus effective microorganisms has been widely reported to improve soil quality and productivity of crops over a wide range of agro ecological conditions Yamada & Xu [3], also Effective microorganisms increased biological soil activities and improved physical and chemical soil properties through rapid humification of fresh organic matter when these effective microorganisms were mixed with animal manure or green manure Valarini et al.,[4]. Thus, the major microbes of EM are the photosynthetic bacteria (Rhodospeudomonas spp.), Lactic acid bacteria, (Lactobacillus spp.) and yeasts (Saccharomyces spp.) Zuraini et al., [5]. The photosynthetic bacteria are self-sufficient self-sustaining microorganisms, they produce energy from the sun and soil heat and utilize it to change exudates from root systems, gases such as ammonia and soil organic fraction into building materials of cells such as amino acids, nucleic acids and sugars, these nutrients can all be assimilated directly into the plants to advance plant growth and as well in the soil system to promote and sustain the growth and establishment of new beneficial microorganisms. For instance, vesicular-arbuscular mycorrhiza (VAM fungi), which enhance the plants assimilation capability of soil phosphates, increases in the root zone in the existence of amino acids secreted by the beneficial microorganisms, Ouwehand [6]. Lactic acid bacteria in effective microorganisms are identified to produce lactic acid from sugar and carbohydrates through photosynthetic bacteria and yeasts. Lactic acid has sterilizing property and its occurrence in the soil regulates the proliferation of nematode population and brings about protection against nematode related plant diseases, which also participate in the synthesis of lignified organic materials and cellulolytic in the soil, Ouwehand [6]. The yeast in EM produces hormones and enzymes that are recognized to promote plant cell and root division. They make use of the amino acids and sugars synthesized by the photosynthetic bacteria and plant roots and in turn enhance growth factors for the lactic acid bacteria. Hence can be concluded that, the various species of organisms in EM balance each other and are in a mutually beneficial relationship with the roots of plants in the soil ecosystem. Plants would hence grow better in soils inhabited and dominated by this effective microorganism, Pei-Feng et al. [7]. Thus, the objectives of this research was to assess the effect of EM and organic materials on microbial population and activity in soil.
Materials and Methods
The Experimental Site
This experiment was carried out in a screen house located at the Teaching and Research Farm and the Soil Science Laboratory of Rivers State University (RSU) located at Port Harcourt (40 46’ N, 70 10’ E). Port Harcourt is in the humid forest zone which has an average elevation of ten meters above sea level. The mean annual rainfall is 2400mm, usually in a monomodal distribution lasting from March to November. There is usually a dry spell between December and March with little or no rain, the wettest months are between July and October. Temperature varies from 27OC from February to April (warmest months) to 25OC in July and August (the coolest months). Relative humidity remains high throughout the year and varies from 78% in February (the driest month) to 89% in July and September (rainy months). Ikpe et al. [8].
Experimental Materials
Top soil excavated from a building site at the new Marine engineering Department were collected in RSU, the soil were air dried and sieved with 2mm sieve, 5kg of the soil was placed into plastic container with known volume (3815cm3). The Organic materials such as Poultry manure (PM) were obtained from the Rivers State University Teaching and Research Farm of the poultry unit, air dried and broken into smaller pieces, 111g of the PM were weighed and mixed with the soil at 50t/ha for PM treated soil, Dry grasses such as elephant grass (Pennisetum purpureum), carpet grass (Axonopus compressus), buffalo grass (Stenotaphrum secundatum) were collected from the Teaching and Research Farm of Rivers State University, chopped into smaller pieces and air dried, 70g of the dry grasses were weighed (30t/ha) and homogenize with the soil for dry grass treated
sample
Effective Microorganisms (EM1) was prepared as initiated by Ene, D.U in this research, 2 cups of top soil were added into 500ml of rain water and allowed to stand for 2hrs and thereafter filtered, molasses, egg white, yoghurt, and yeast were added and the mixture made up to 1000ml with rain water then mixed together, allowed to incubate for 31days under dark environment. 5 liters of EMAS (Effective Microorganism Activated Solution) was prepared as follow
Water (94%), 4700ml, EM1 (3%) = 150ml and Molasses (3%)= 150ml as described thus, 150ml of molasses were mixed with 1000ml of tap water, 150ml of EM1 were added and mixed with 1000ml of water, both solutions were mixed together and 2700ml of water added and stirred very well, the solution incubated on a plastic pot closed tight to prevent air for the period of 10 days, to obtain a pH level of pH 3.8 for EMAS.
Experimental Design
The 2 factor experiment consisting of 3 levels of organic materials Dry grasses (DG), poultry manure (PM) and No organic material (SO)), and 2 levels of EMAS (+EMAS and -EMAS) were replicated thrice and fitted into a completely randomized design (CRD) at 50t/ha poultry manure and 30t/ha dry grass. The various treatments were laid out on a table in the screen house in a completely randomized pattern. The EMAS for EMAS treated pots and water in water treated pots were added to field capacity and then incubated for 30 days after covering with black polythene material
Cultivation and enumeration of bacterial
1gram of the incubated soil sample were collected and put into a sterile specimen bottle, the samples were immediately taken to the laboratory for microbial analysis Adoki & Orugbani [9]. The total number of bacteria in untreated and treated soil samples were determined, using dilution technique method nutrient agar medium. The rhizosphere soil samples were processed using soil sample suspended in a conical flask containing 10ml of sterilized distilled water and thoroughly shaken for 15 minutes. About 1ml of sample were serially diluted with sterilized distilled water up to 10-6, 0.1ml portion of each sample was pipetted and plated out on the solid medium. A glass spreader sterilized with alcohol and flame was used to spread the inoculums evenly on the plates. The plates were incubated at 370C for 24hrs. colony forming units was counted and calculated.

CFU = colony forming Unit
Carbon Mineralization Study (CO2 evolution) 200g samples of each treatments were put into kilner jars of 1413.9cm3 volume, using the apparatus as stated by Valarini et al. [4] to stimulate the aerobic activity of the microorganisms and incubated with 15ml of 1N NaOH in 30ml size beakers placed on the soil samples. Back titration with 1N HCL (using phenolphthalein indicator) of each was done weekly, after the addition of 2ml of 3N BaCl2. The use of BaCl2 precipitate the trapped CO2in the beakers. The covers of the jars were greased with the petroleum jelly, to prevent carbon dioxide leakage for both the blank. A triplicate blank titration was also run for each weekly determination. The amount of CO2 evolved, expressed in milligram were calculated using the formula: Milligram

Where:
B= Volume of acid needed to titrate blank (cm).
T= Volume of the acid needed to titrate sample (ml).
N= Normality of the acid for titration
E= Equivalent weight to convert to mg (c=6).
200= weight of the soil sample used (g)
Data collection
Bacterial count was collected before planting, after incubation and after harvest
The CO2 evolved from the different treatments was collected weekly for 4 weeks
Statistical Analysis
Data collected from the various parameters was subjected to analysis of variance (ANOVA) at P< 0.05. Means were separated using Tukey’s Pair Wise Comparison at 95% confidence intervals.
Results
Initial properties of the soil and organic materials
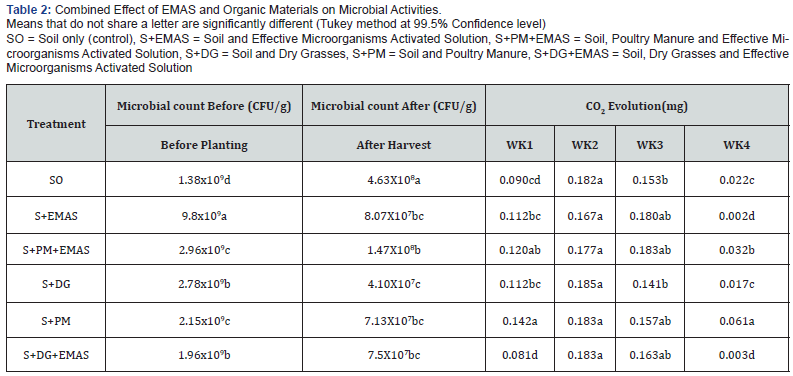
The results of the initial microbial activities of the soil and the experimental materials is as shown in Tables 1 & 2. the soil had a bacterial population count of 1.0x107 CFU/g, the rate of carbon dioxide evolved follows that, the 3rd week had the highest of 0.218mg and 4th week had the lowest 0.005mg, the soil is loamy sand with a pH of 5.3, it contain an organic carbon of 12.0 g/kg and 20.6 g/kg of organic matter, the nitrogen content is 2.7g/kg and a C/N ratio of 4.46. The poultry manure had an organic carbon of 365.3g/kg, organic matter of 619.8g/kg, total nitrogen of 70.9g/ kg and C/N ratio of 5.15 while the dry grasses had an organic carbon of 419.9g/kg, 732.99g/kg of organic matter, total nitrogen of 50.97g/kg and a C/N ratio of 8.32.
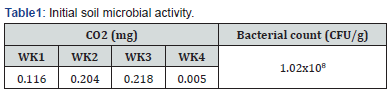
Effect of effective microorganisms on heterotrophic count
The results of the total heterotrophic count of bacteria present in the various treatments is shown on Figure 1. indicated a significant different at (p˂0.05) among the treatments, the combination of soil and EMAS (SO+EMAS) shows the highest colony forming unit (9.8x109CFU/g), which shows that, the addition of EMAS to the soil significantly increased the bacterial count both before planting and after harvest.
Effect of effective microorganisms on total carbon dioxide evolved
The results of the effect of effective microorganisms on total carbon dioxide evolved weekly for 4 weeks is shown in Figure 2. As shown in the results, there is no significant different at (p˂0.05) observed in week 2 among the treatments, the combination of soil and activated EM (SO + EMAS) and NO EMAS. The result indicates that, amendment of soil with EMAS negatively affects CO2 evolution compare with the result on Table 2. However, the rate of microbial activities is of its maximum in week 2 & 3 and drops drastically in week 4.
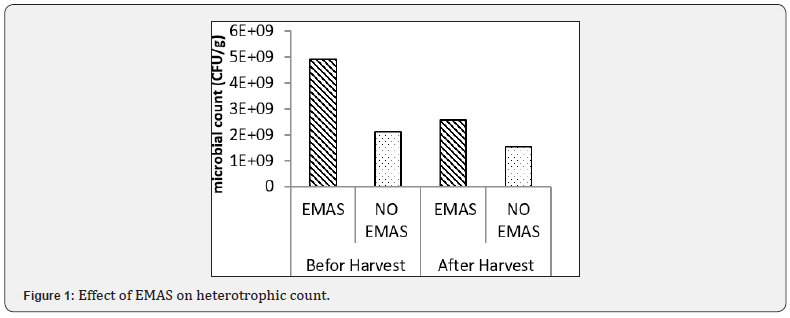
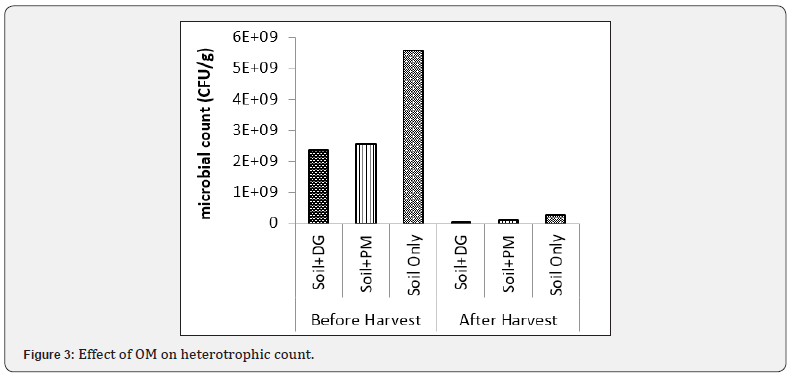
Effect of organic materials on heterotrophic count
The result of the effect of organic materials on total heterotrophic count is shown in Figure 3. among the treatments, the control (soil only) had the highest heterotrophic count (5.50x109CFU/g), whereas, soil combined with dry grasses (SO+DG) had the least heterotrophic count (2.37x109CFU/g) after incubation, the heterotrophic count drastically dropped of which SO had (6.35x108CFU/g) and SO+DG also had the least (5.8x107 CFU/g) after harvest this shows that, the amendment of soil with organic materials (DG & PM) negatively affected heterotrophic count both before planting and after harvest, with the SO having the higher heterotrophic count than both the DG & PM amended soils.
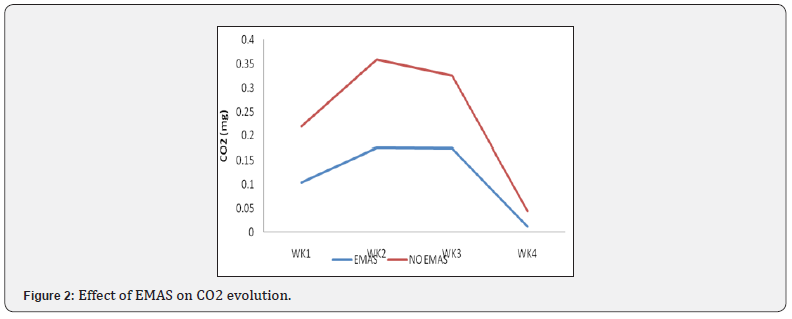
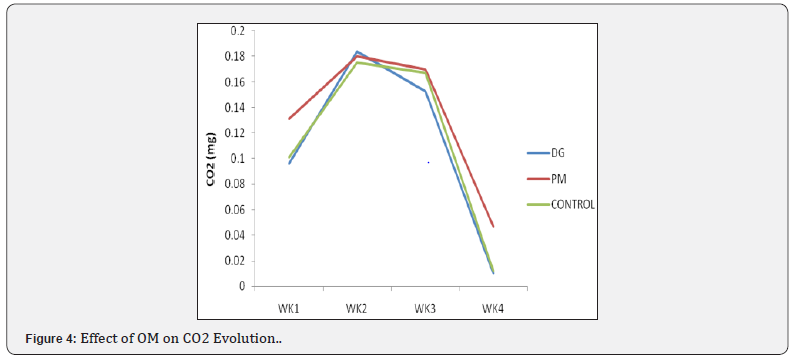
Effect of organic materials on carbon dioxide evolved
The results of the effect of organic materials on total carbon dioxide evolved after 4 weeks is shown in Figure 4. there is a significant (p˂0.05) different between the treatments however, the combination of soil and poultry manure without activated EM (SO+PM) recorded a higher CO2 evolved at the 4th week (0.061mg) while the treatment soil and dry grasses with no activated EM (SO+DG) recorded the least (0.017mg).
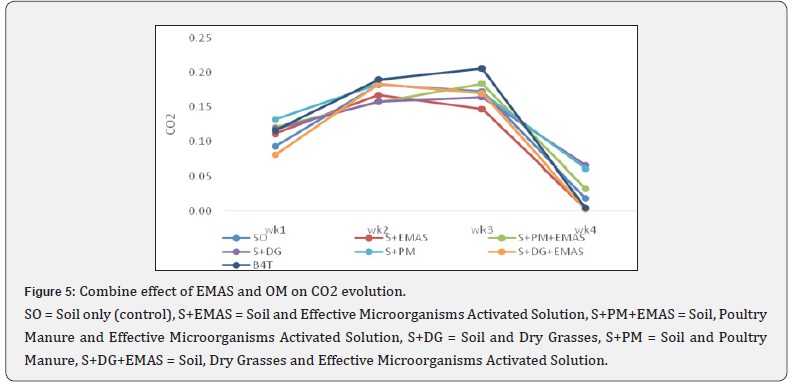
Combined effect of EMAS and organic materials as affected heterotrophic count and carbon dioxide evolved
As show in Table 2 the effect of EM `and organic materials on heterotrophic bacteria population differs significantly (p<0.05). the combination of soil and activated EM (SO+EMAS) had the highest (9.82x109CFU/g) bacteria population before planting but after harvest, the control treatment soil with no EMAS (SOEMAS) recorded the highest (4.63x108CFU/g) and soil + dry grasses with no activated EM (SO+DG-EMAS) recorded the least (4.1x107CFU/g), in Figure 5 there is a significant (p<0.05) different among the treatments and the control, soil + poultry manure with no activated EM (SO+PM-EMAS) recorded the highest (0.06mg) evolved whereas, soil with activated EM (SO+EMAS) recorded the lowest (0.002mg) CO2 evolving among the treatments.
Discussions
Effect of activated effective microorganisms (EMAS) as affected by microbial activities
The results of the effect of effective microorganisms EMAS affected by microbial activities as shown in (Figures 1 & 2). The combination of soil and activated EM (SO+EMAS) had the highest heterotrophic count compared with the control treatment without the addition of activated EM (NO EMAS), from the results, the addition of activated EM on the various treatments increases the heterotrophic count of bacteria, this result agreed with the findings of [10] which reported that inoculation with bacterial EM and fungi EM had a stimulating effect to increase the microbial density in soil significantly then water only, the principal activity of EM appear to increase the bio-diversity of soil microflora, thereby increasing plant yield Mohan [11]. The variations in microbial population indicate the impact from the root exudates which differed in chemical and in quantity among plants, which produced by plant can cause immediate and profound response in the microbial population. Among the treatments, the total carbondioxide evolved at the 3 week was recorded the highest in soils treated with EMAS while at the 4 week, EMAS treated soil had the least carbon dioxide evolved. As reported by Stoklasa (1905), the amount of CO2 evolved was dependent on the mechanical condition of the soil, its fertility and crop grown and that intensity of CO2 produced shows the presence not only of active bacterial but also of easily available organic matter, the reduction of CO2 evolved as shown in Figure 2 indicates the ability of EM to reduce the rate of CO2 evolved into the environment hence, reducing environmental pollution which is in accordance with the research findings of Reganold et al; (1990) which state that, environmental pollution problems cannot be solved without using microbial methods and technologies in coordination with agricultural production.
Effect of organic materials as affected by microbial activities
The results of organic materials as affected by microbial activities are shown in Figure 2. the soil heterotrophic count of bacterial indicates a variation among the treatments when compare with the control, however, SO+DG had the highest bacterial count (2.78x109CFU/g) whereas, the control treatment SO had the least count (1.38x109CFU/g) before planting while after harvest the control treatment had the highest heterotrophic count, this findings agrees with research findings of Kee-Choon et al. [12], which reported that soil organic matter was not affected by EM, which suggest that EM did not influence in situ decomposition of SOM or that the microbial density in liquid EM was too low to supplement microbial activities in soil. The carbon dioxide evolved as affected by organic materials is shown in Fig.4 also shows the rate of CO2 evolved reduced with respect to time of which, SO had the least CO2 evolved and this agreed with the findings of Stoklasa (1905) which stated that, the determination of CO2evolved by a soil under given degree of moisture and temperature in a length of time was also believed to furnish a reliable and accurate method of determination of bacterial activity, from Figure 4. is shown that the flush of CO2 observed after the addition of the treatments is not thoroughly understood but may result from (1) nonliving soil organic matter becoming more susceptible to microbial attack, which induces the rapid mineralization of C from exposed aggregates Adu and Oades [13] and (2) the contribution of cellular lysing from water-induced osmotic shock to an easily mineralization C pool that is consumed by the surviving soil microbes Halverson et al. [14].
Combined Effect of EMAS and organic matter on microbial activities
In Table 2 SO, S+DG, S+PM and S+DG+EMAS follows the same pertain of CO2 evolution, in which, the rate of mineralization intensively increases during the first week to the second week and decrease in intensity from the third week and drastically drops on the fourth week, but S+EMAS and S+PM+EMAS also indicates an intensive mineralization during the first week to second week and tend to maintain an average mineralization until the third week then an accentuated decrease in the fourth week. This result can be summed up in three phases as, first phase is the intensive mineralization phase (first week), second phase is the maintenance of average mineralization (2rd & 3rd week) and the third phase is the accentuated decrease in activity. This result is in line with Valarini [4] which reported that, the third phase is represented by an accentuated decrease in activity caused by limiting nutritional factor, because respiration depends on the number of microorganisms present in the soil. There is a significant difference in the population of heterotrophic bacteria enumerated. The number of heterotrophic bacteria increased after the addition of the treatment S+EMAS recorded the highest population of heterotrophic bacteria (9.82x109CFU/g) but decrease after the harvest (8.07x107CFU/g). This result showed that microbiological parameters are sensitive indicators of changes and improvements in structure and soil fertility in a very short time.
Conclusion
The effect of microbial count and activity shows a significant increase in the population of heterotrophic bacteria, and reduces the rate of CO2 evolution according to Higa [15], the presence of EM in the soil reduced the rate of carbon dioxide released into the environment. However the combined effect of EMAS and PM had a greater effect on biological properties of the soil, EMAS should be combined with organic materials because the rate of mineralization was observed to be slow when only organic materials are used but the addition of effective microorganisms the rate of mineralization is faster which in turn enhances nutrients availability and crop performance for sustainable agriculture.
References
- Hussain T, JavaidT, Parr JF, Jilani G, Hagi MA (1999)Rice and Wheat Production in Pakistan with effective microorganisms. Am. J Alt. Agric 14(1): 30-36.
- Li Z, Zhang H (2000) Application of microbial fertilizers sustainable agriculture. J. Crop Prod 3(1): 337-347.
- Yamadu K, Xu H (2001) Properties and application of an organic fertilizers inoculated with effective microorganisms. J. Corp Prod. 3(1): 255-268.
- Valarini PJ, Dyaz Alvarez MC, Gasco JM, Gureerro F, Tokeshi H (2003) Assessment of soil properties by organic matter and EM-microorganism incorporation R. Bras. Ci. solo 27(1): 519-525.
- ZurainiZ, Sanjay G,NoresahM(2010)Effective microorganism (EM) technology for water quality restoration and potential for sustainable water resources and management. Proceedings of the international congress on Environmental Sake, Fifth Biennial Meeting held between 5th-8thJuly, Ontario Canada.
- Ouwehand A (1998)Antimicrobial components from lactic acid bacteria.Inlactic acid bacteria microbiology and functional aspects.Salminer S, Von Wright A, Marcel Dekker Inc,New York, 139-159.
- Pei-Feng S, Wei-Ta F, Li-Ying S, Jyuan-Tu W, Shih-Feng F, et al. (2014) Indole- 3 – Acetic acid- producing Yeasts in the Phyllosphere of the Carnivorus Plant DroseraIndica L. Plos one 9(12): l4196.
- Ikpe FN, Ndegwe NA, Gbaraneh LD, Torunana JMA, William TO, Larba A (2003) Effect of sheep browse diet on fecal matter decomposition and N and P cycling in the humid lowlands of West Africa. Soil Science 168(9): 646-659.
- Adoki A, Orugbani T (2007) Influence of nitrogenous fertilizer plants effluents on growth of selected farm crops in soil polluted with crude petroleum hydrocarbons. Afri. J. Agric. Res. 2 (11): 569-573.
- KiehlJC (2001) Producao de compost organico e vermicomposto. InfAgropec22 (1): 40-42 e 47-52.
- Mohan B (2008) Evaluation of organic growth promoters on yield of dry land vegetable crops in India. Journal of Organic Systems 3 (1): 23–36.
- Kee-Choon Kim, Yeong-Suk Kwon, Oh-Hoon Kwon, Tae-Ryong (2001)Efects of organic amendments on soil microbial community in Red Pepper Field.Korean Journal of Soil Science and Fertility.
- Adu JK, Oades JM (1978) Physical factors influencing decomposition of organic materials in soil aggregates. Soil Biol. Biochem 10 (2): 109-115.
- Halverson LJ, Thomas MJ, Firestone MK (2000) Release of intracellular solutes by four soil bacteria exposed to dilution stress. Soil Sci. Soc. Am. J. 64(5): 1630–1637.
- HigaT (2012) Kyusei Nature Farming and Environmental Management through Effective Microorganisms – The Past, Present and Future.






























