Phytochemical Screening and Antimicrobial Activity of Selected Spices and Herbs against Staphylococcus Aureus Bacteria
Alemu Mekonnen Tura*
Department of Chemistry, Arbaminch University, Arbaminch, Ethiopia
Submission: October 22, 2019; Published: December 12, 2019
*Corresponding author:Alemu Mekonnen Tura, Department of Chemistry, Arbaminch University, Arbaminch, Ethiopia
How to cite this article: Alemu Mekonnen Tura. Phytochemical Screening and Antimicrobial Activity of Selected Spices and Herbs against Staphylococcus Aureus Bacteria. Agri Res& Tech: Open Access J. 2019; 23(3): 556236. DOI: 10.19080/ARTOAJ.2019.22.556236
Abstract
Spices have been defined as plant substances from indigenous or exotic origin, aromatic or with strong taste, used to enhance the taste of foods. Herbs and spices have been used during the middle ages for flavoring, food preservation and medicinal purposes. The aim of this study was to evaluate and compare the phytochemicals and antimicrobial properties of extracts of nine types of commercial herbs and spices commonly used in the production of ready meals. Phytochemical screening tests was conducted for nine spice species and found that extract contains a variety of Phytochemicals like saponins, tannins, flavonoids, terpenoids, glycosides and reducing sugars and among which there is higher level of precipitation for phenol and flavonoids. As they are essential source of antimicrobial agents against pathogens, their extracts were tested for its antimicrobial activity by well diffusion method using Nutrient agar against human pathogenic bacteria like Staphylococcus aureus. The findings showed potential antibacterial properties of the extracts against the organisms tested. Among the nine-spice extract tested, ethanol extract of garlic shows higher inhibition zone. Agar well diffusion assay for antimicrobial activity yielded the inhibitory zone of 7±1.15mm diameter for black cumin, 12.50±1.12mm diameter for ginger, 10.30±0.73mm for cloves and 13.45±0.58mm diameter for garlic extract indicating that garlic was the most effective spice in inhibiting the microbial growth. Finding of the study concluded that Spices possesses the anti-microbial capabilities against infections (diseases) caused by Staphylococcus aureus and seems to be useful in the discovery of antibiotic drugs.
Keywords: Herbs, spices, Phytochemical tests, Antimicrobial activity, Disc diffusion method
Introduction
Nature has been a source of medicinal agents for thousands of years and an impressive number of modern drugs have been isolated from natural sources; many of these isolations were based on the uses of the agents in traditional medicine. This plant based traditional medicine system continues to play an essential role in health care, with about 80% of the worlds inhabitants relying mainly on traditional medicines for their primary health care [1,2]. The medicinal power of these plants lies in phytochemical constituents that cause definite pharmacological actions on the human body. The search for new pharmacologically active agents obtained by screening natural resources such as plant extracts has led to the discovery of many clinically useful drugs for the treatment of human diseases. The most important of these bioactive constituents of plants are alkaloids, tannin, flavonoid and phenolic compounds [3,4]. Among these plants, Spices which include leaves (coriander, mint), buds (clove), bulbs (garlic, onion), fruits (red chili, black pepper), stem (cinnamon), rhizomes (ginger) and other plant parts, have been defined as plant substances from in digenous or exotic origin, aromatic or with strong taste, used to enhance the taste of foods. The term ‘herb’ is used as a subset of spice and refers to plants with aromatic leaves [5]. Spices and herbs have played a dramatic role in civilization and in the history of nations. The delightful flavour and pungency of spices make them indispensable in the preparation of palatable dishes [6]. In addition, they are reputed to possess several medicinal and pharmacological properties in particular, antimicrobial activity against different types of microbes [7-12].
Apart from enhancing the taste and flavour of food, spices have been widely believed to exert digestive stimulant action [13]. Spices have been generally believed to intensify salivary flow and gastric juice secretion, thereby aiding digestion [14]. Several pharmacological preparations available to correct digestive disorders employ certain spices besides other plant substances [15]. A few medicinal properties of spices such as tonic, carminative, stomachic, diuretic, and antispasmodic have long been recognized [16-18]. These attributes, largely empirical, nevertheless efficacious, have earned them pharmacological applications in the indigenous systems of medicine as digestive stimulants and to relieve digestive disorders [13]. Plant extracts and natural compounds purified from plants have been used by humans for many centuries for the treatment and alleviation of a variety of inflammation related diseases, including cancer [19]. Spice derived phytochemicals have an enormous potential in the prevention and treatment of cancer [20-22]. They can induce apoptosis, suppress proliferation of tumor cells, inhibit invasion and angiogenesis, and prevent even bone loss. These phytochemicals mediate their effects through multiple targets and yet pharmacologically they are highly safe [20].
Phytochemicals are natural bioactive compounds found in plants. Phytochemicals are divided into two groups; primary and secondary compounds. These classes are according to their functions in plant metabolism. Amino acids, sugars, proteins and chlorophyll are known as primary compounds while secondary compounds consist of alkaloids, terpenoids, phenolic compounds and many more [23]. Plant produces these chemicals to protect itself, but recent research demonstrates that many phytochemicals can protect humans against diseases. There are many phytochemicals in fruits and herbs and each works differently. Many plant extracts have been shown to inhibit the growth of microorganisms. These extracts consist of chemicals and are usually considered to play a role in defense reactions of plants against infections by pathogenic microorganisms [24]. Along with their protective abilities, Spices and herbs are believed to be the natural chemical component responsible for the antimicrobial effects against some pathogens. Various studies have shown that Spices and herbs are known to be effective against gram-negative as well as gram-positive bacteria, such as Escherichia coli, Salmonella, Staphylococcus, and Streptococcus species. Therefore, screening and testing the efficacy of plants are undertaken to explore their antibacterial activity [25,26].
There has been a need to increase alternative antimicrobial agents leading interests of evaluating extracts from plants known to have medicinal value for the manufacturing of herbal antimicrobial agents by Pharmaceuticals Company. The increase in resistance to many commercially produced synthetic antimicrobial agents by microorganisms has been increasing with time hence the need of searching for new antimicrobial agents. Most of the bacteria land fungal pathogens have shown the capability of developing resistance to some of the available commercially available antimicrobial agents. The aim of this study is to ascertain the phytochemical properties and antimicrobial activity of some spices and herbs from Arba Minch zuria woredas and to determine if the plant extracts from antimicrobial tendencies against the bacteria pathogens.
Materials and Methods
Description of Study Area
The study was conducted in Arba Minch University (Abaya campus) which is found in Gamo-Gofa zone. It located at Arba Minch town, 505km south of Addis Ababa in the vicinity of unique natural and anthropogenic diversity. Arba Minch town is located (situated, in great raft valley with average temperature and rain fall 30 Celsius and 575mm respectively. Moreover, general elevation of the zone ranges from 600 to 3300 meters above sea level. Therefore, the spices sold in this town are expected to be cultivated in this zone’s woredas.
Chemicals and Apparatus
Chemicals
Ethanol, Sulphuric acid (H2SO4), Ammonium Hydroxide (NH4OH), sodium Hydroxide (NaOH), Fehling Solution, Chloroform (CHCl3), Hydrochloric acid (HCl), Mayer’s reagent, Benedict’s reagent, tannic acid (C76H52O46), Lead acetate (Pb(CH3COO)2), Ammonia (NH3), Ferric Chloride (FeCl3), Acetic anhydride (C4H6O3), McFarland Standard, Muller Hinton agar (MHA) and Distilled water.
Apparatus
The apparatus used for this project were: Sieve, Refrigerator, Polyethylene plastics, Stirrer, Beaker, Electronic balance, Reagent bottle, Test tube, Filter paper, Dropper, Hot plate, water bath, Petri dish, Cotton Swab, Hood and Incubator
Sampling and Sample Preparation
Sample collection
The samples of fenugreek, black cumin, ginger, garlic, Ethiopian cumin, turmeric, cinnamon, cloves, coriander and aframomum was purchased from vendors in a market located in Sikela and Secha, Arba Minch town and voucher specimens was deposited at the Arba Minch University Chemistry Laboratory.
Sample Preparation
Each sample was separately cleaned of dirt and dust particles and then sun dried to constant weight before rubbing and grinding to a powder using a desk top mill fitted with a 500 μm sieve. The ground spices was then put in polyethylene plastic, sealed and stored at 4°C in a refrigerator until required for analysis.
Preparation of Extracts
Previous studies indicate that methanol is a good solvent to extract the bioactive chemicals in spices [20]. Extracts was prepared by mixing ground spice with ethanol, in a 1:10 ratio followed by stirring at room temperature for 2 hours and filtering through Whatman No 1 filter paper. Residues was re-extracted with additional methanol for a further 2 hours and filtered as described. The volume of the combined extract was removed by evaporation and the concentrated extracts was subsequently stored at 4°C until use.
Procedure of Phytochemical Analysis
Test for Carbohydrate
Molisch’s test:
To the extract the few drops of alcoholic alpha naphtol solution was added. Then few drops of concentrated Sulphuric acid was added along the sides of test tube. The appearance of purple and violet color shows the presence of carbohydrate.
Fehlings test:
To the extract equal amount of Fehlings A and B solution added. The solution was heated in a boiling water bath. Brick red precipitation of cuprous oxide was formed for all extracts, if reducing sugar was present.
Benedicts test:
To the extract benedict reagent was added. The tubes were heated in a boiling water bath. Red precipitation indicates positive result for each extract.
Tests for Proteins:
Xanthoproteic test: 1mL of extract and 1mL of concentrated H2SO4 was mixed in test tube. Formation of white precipitate which on boiling turned yellow. On addition of NH4OH, yellow ppt. turned orange.
Test for glycosides:
Borntrager’s Test: 25mL of dilute sulphuric acid was added to 5mL extract in a test tube and boiled for 15 minutes, cooled and neutralized with 10% NaOH, then 5mL of Fehling solution added. Glycosides are indicated by a brick red precipitate.
Test for Steroids:
Salkowski Test: To 2mL of aqueous extract, 2mL of chloroform and 2mL of concentrated H2SO4 were mixed. The solution was shaken well. As a result, chloroform layer turned red and acid layer showed greenish yellow fluorescence.
Tests for alkaloids:
One gram of the extract was dissolved in 10mL of 10% HCl on a water bath and filtered, and the pH was adjusted to 6.5. Then 0.5mL of the filtrate was put in test tubes, and the following reagents were added drop by drop in each test tube: Dragen druff’s reagent (Bismuth potassium iodide solution); Mayer’s reagent (potassium mercuric iodide solution); Wagner’s reagent (iodine in potassium iodide solution); 1% picric solution (observed for some minutes); and 10% tannic solution. The observation of turbidity or precipitation with the reagents in each test tube indicates the presence of alkaloids or the aqueous extract was evaporated in a test tube. To the residue dilute HCl was added, shaken well and filtered. With the filtrate following tests was performed.
Hager’s Test:
To the 2-3mL of filtrate Hager’s reagent was added. If Yellow precipitate was formed showing the presence of alkaloids.
Mayer’s Test:
To the 2-3mL of filtrate Mayer’s reagent was added. Formation of yellow precipitate showed the presence of alkaloids.
With tannic acid:
To 1mL of extract 2-3 drops of the tannic acid solution reagent was added. Appearance of amorphous or crystalline precipitate represents the presence of alkaloid.
Tests for flavonoids
With Lead acetate:
To small quantity of aqueous extract 10% of lead acetate solution was added. Formation of yellow precipitate showed the presence of alkaloids.
With Sodium Hydroxide:
On addition of an increasing amount of sodium hydroxide, the aqueous extract showed yellow coloration, this decolorized after addition of acid.
Test for saponins:
About 0.5 g of the extract was boiled with 10mL of distilled water in a water bath for 10 min and filtered. The filtrate (about 5mL) was made up to 10mL with distilled water and shaken vigorously for 5 min for a visible, stable persistent froth. The frothing was mixed with two drops of olive oil and shaken vigorously, then observed for formation of a fairly stable emulsion.
Test for anthraquinones:
0.5gm of the extract was boiled with 10mL of sulphuric acid and filtered while hot. The filtrate was shaken with 5mL of chloroform. The chloroform layer was pipetted into another test tube and 1mL of dilute ammonia was added. The resulting solution was observed for color changes.
Test for Tannins:
For 2mL of extract few drops of 1% lead acetate was added. A yellowish precipitate showed the presence of tannins. OR To a portion of the extract diluted with water, 3-4 drops of 10% ferric chloride solution was added. A blue color was observed for Gallic tannins and green color indicates for catecholic tannins.
Test for Terpenoids:
2ml of extract was dissolved in chloroform. Few drops of acetic anhydride were added followed by 1ml of concentrated sulphuric acid. The appearance of pink colour in chloroform layer shows the presence of terpenoids.
Test for volatile oils:
2mL of extract was shaken with 0.1mL dilute NaOH and a small quantity of dilute HCl. Formation of white precipitate shows the presence of volatile oils.
Test for reducing Sugars:
To 0.5mL of plant extracts, 1mL of water and 5-8 drops of Fehling’s solution was added and heated over water bath. Brick red precipitate indicates the presence of reducing sugars.
Antimicrobial activity of Spices
Pathogenic microorganism Staphylococcus aureus was selected for the present investigation. The microorganism was originally obtained from Biology department, Microbiology laboratory. The antibacterial activities of the extracts were tested against the selected bacterial strains. Agar well diffusion method was used for screening for antimicrobial activity. Ten wells were made on each Mueller Hinton agar plate seeded with the test organism using a 6-mm cork borer. Nine of the wells contained the extracts while the tenth well (made at the center of the plate) contained ethanol as a control. 20mL of sterilized agar medium was poured into each sterile Petri plates and allowed to solidify. The test bacterial cultures were evenly spread over the appropriate media by using a sterile cotton swab. Then a well of 0.5cm was made in the medium by using a sterile cork borer, 150 μL of each ethanol and sample extracts were transferred into separate wells. After these, plates were incubated at 37oC for 24-48 hours. After incubation period, the results were observed and measure the diameter of inhibition zone around the each well [27].
Result and Discussions
Phytochemical screening
Phytochemical research is closely related to the needs of finding new and effective pharmaceuticals. As an initial step the phytochemical screening research allows to determine qualitatively the main groups of chemical constituents present in the plant. Phytochemical screening was done using color forming and precipitating chemical reagents on the dried extracts to generate preliminary data on the constituents of the plant extract. Phytochemical screening of the ethanolic extract of fenugreek, black cumin, ginger, garlic, turmeric, cinnamon, cloves, coriander and aframomum were used to study the presence of contained Carbohydrate, Proteins, glycosides, Steroids, alkaloids, flavonoids, Saponins anthraquinones, Tannins, Terpenoids, Volatile oils and Reducing Sugars and have various medicinal values. There are nine samples selected for phytochemical analysis on the bases of their test accordingly in which the result is shown below.
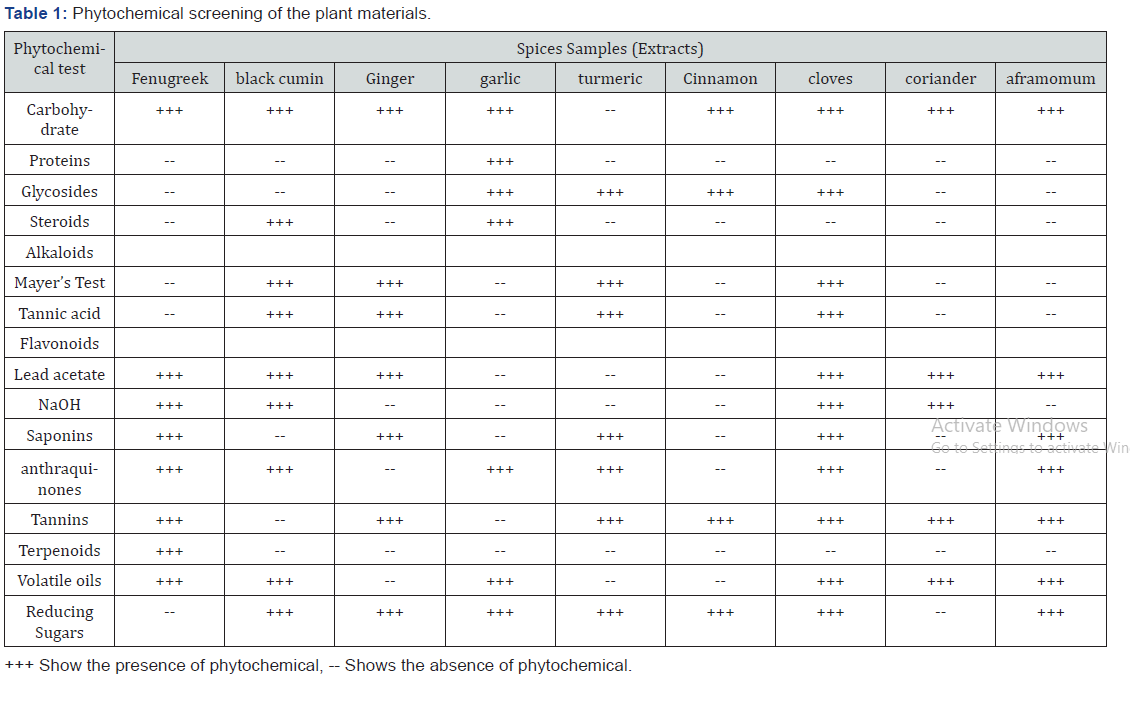
The present study carried out on nine extracts of spices i.e., fenugreek, black cumin, ginger, garlic, turmeric, cinnamon, cloves, coriander and aframomum and revealed the presence of medicinal active constituents. The phytochemical active compounds of these spices were qualitatively analyzed separately, and the results were summarized in (Table 1) Analysis of spices extracts revealed the presence of Alkaloids, flavonoids, glycosides, phenols, saponins, steroids and tannins in most of the selected Spice extracts which could be responsible for the observed antimicrobial property. Alkaloids were absent in some selected spice extracts studied. The presences of phytoconstituents like flavonoids, saponins, tannin and phenols in the crude extract are likely to be responsible for the antimicrobial activities. In this screening process Carbohydrate, Proteins, glycosides, Steroids, alkaloids, flavonoids, Saponins anthraquinones, Tannins, Terpenoids, Volatile oils and Reducing Sugars show different types of result in ethanolic extract. This means as showed form the result carbohydrate is present in all extracts except in turmeric, but proteins give positive result only in garlic extracts. The medicinal value of plant lies in some chemical substances that have a definite physiological action on the human body. Different phytochemical has been found to possess a wide range of activity, which may help in protection against chronic diseases. For example, alkaloids protect against chronic diseases. Saponins protect against hypercholesterolemia and antibiotic properties. Steroids and Terpenoids show the analgestic for central nervous system activity. Phytochemical constituents such as alkaloids, flavonoids, tannins, phenols, saponins, and several other aromatic compounds are secondary metabolites of plants that serve a defense mechanism against prediction by many microorganisms, insects and other herbivores. Alkaloids which are one of the largest groups of phytochemicals in plant have amazing effects on humans based on their toxicity against cells of foreign organisms. Therefore, the compounds detected may be responsible for the antibacterial activity of the herb extracts. Flavonoids forms complex with soluble proteins and with bacterial cell walls and exhibited a wide range of biological activities such as antioxidant, anti-inflammatory, antimicrobial, anti-angionic, anticancer and anti-alergic. Saponins which are one of the active constituents involved in plant disease resistance because of their antimicrobial activity. Traditionally, saponins are subdivided into triterpenoid and steroid glycoside. Tannins are phenolic compound which act as primary antioxidants or free radical scavengers. Steroids have been reported to have antibacterial properties, the correlation between membrane lipids and sensitivity for steroidal compound indicates the mechanism in which steroids specifically associate with membrane lipid and exerts its action by causing leakages from liposomes. Tannins bind to proline rich proteins and interfere with the protein synthesis.
According to Nuran et al. [23], several phenolic compounds like tannins present in cells of plant are potent inhibitors of many hydrolytic enzymes such as proteolytic macerating enzymes used by plant pathogens. In addition, herbs that has tannins as their main components are astringent in nature [28,29]. According to Sulaiman et al. the phytochemical screening of different spices extracts demonstrated the presence of flavonoids and saponins which supported these findings. The presence of these metabolites probably explains the various uses of this plant in traditional medicine [23].
Evaluation of antibacterial activity
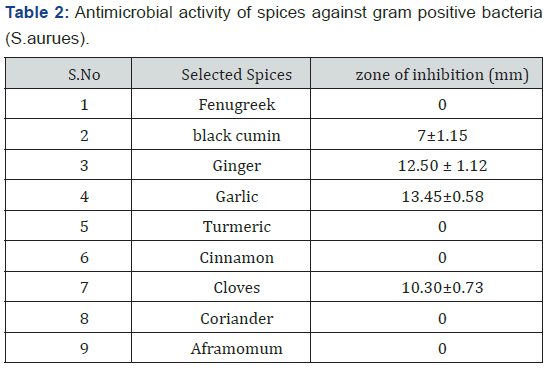
Crude extracts have moderate inhibition effect in comparison with the standard drug against the tested bacteria and fungi due to naturally occurring combinations of phytochemical constituents. Presences of constituents like flavonoids and tannins in the extract are likely to be responsible for the antimicrobial activity. The results of the present study suggested that several Phytochemicals as well as antioxidant properties are present in all the nine spices extracts. The presence of those Phytochemicals can be correlated with the fact that solvent extracts showed antimicrobial activity against the bacterial and fungal strains. Phytochemicals give plants their colors, flavour, smell and are part of a plant ‘s natural defense system and protect them against herbivorous insects and vertebrates, fungi, pathogens, and parasites. In this study, one Gram positive bacterial pathogen Staphylococcus aureus was selected, and antibacterial activity of spices extracts of Fenugreek, black cumin, Ginger, Garlic, Turmeric, Cinnamon, Cloves, Coriander and Aframomum against Staphylococcus aureus bacteria was conducted. Spice extracts were prepared, filtered and evaporated as per standard procedures. The antimicrobial activity of individual extracts against the selected microorganisms was assessed by presence or absence of inhibition zone. The zone of inhibition was estimated by agar well diffusion assay and the diameters of zones are as shown in (Table 2).
The Ethanol extracts of nine medicinal plants were tested against the pathogenic microbes viz., Staphylococcus aureus, a most common bacteria of which virulent strains and a wound infecting pathogen which can cause septicemia, endocarditis and toxic shock syndrome. Out of nine spice extracts tested for antimicrobial activity, 4 spice species showed antibacterial activity by inhibiting microorganisms. The results of the antimicrobial activity of plant extracts tested against microorganisms by disk diffusion method are shown in (Table 2) above. Activity zone of inhibition against staphylococcus aureus bacteria is Fenugreek (0mm), black cumin (7 ±1.15mm), Ginger (12.50±1.12), Garlic (13.45±0.58mm), Turmeric (0mm), Cinnamon (0mm), Cloves (10.30±0.73), Corainder (0mm) and Aframomum (0mm). Ethanol extracts of garlic showed highest inhibitory activity against the test organisms. Maximum zone of inhibition (13.45±0.58mm) was obtained with ethanol extract of garlic (Allium sativum) against Staphylococcus aureus. The plant extracts showed antibacterial activity but not antifungal activity.
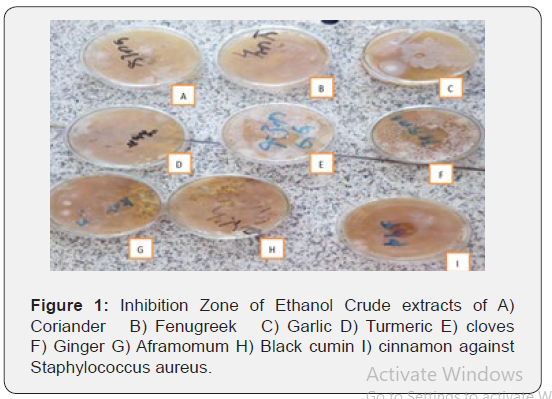
The antimicrobial properties of many medicinal plants have been previously studied. In the present study, antibiotic potential of spices extracts of nine important medicinal plants has been determined against one pathogen namely Staphylococcus aureus. For the comparison positive controls was used. Positive controls did not show inhibitory action on any of the test organisms. The findings match with that of other workers like Baljeet (2015) who
conducted antimicrobial activity of garlic on bacteria [25]. From the present studies among all the liquid extract tested, the ethanolic extract of black cumin, ginger, turmeric, garlic, and cloves exhibited maximum antimicrobial activity against gram positive (Staphylococcus aureus) bacteria. Though the results of this study agree with results of other workers, diameter of zone of inhibition formed varies from other study results. Probably the sources of microorganisms used may be the reason for the difference. Moreover, the effectiveness of plant extract against a particular pathogen is affected by various intrinsic and extrinsic factors (Figure 1).
Conclusion
The present study demonstrates that the selected spices contain natural antioxidants and phytochemicals which may be accountable for their use in treating infections, curing certain diseases and as ingredients in traditional medicine. The inclusion of these spices or their extracts in the production and development of functional foods should be encouraged. The results of this study also provide ample evidence that most of the spices were still under- utilized either as medicinal plants or flavouring agents. Based on these findings, this study suggests the need to lay emphasis on the consumption of spices as well as create more public awareness of their health benefits. From the above study, it is concluded that the presence of phytochemical constituents revealed in the ethanol crude extract of Spices could contribute for their antimicrobial activities. The crude extract was active against the tested bacteria and Staphylococcus aureus. These findings suggest that the selected phytoextracts possesses antimicrobial properties and could be used for biocontrol of bacterial cultures. Antimicrobial activity was confirmed by the selected spice species and the results revealed that plant extracts varied in their efficacy for inhibiting the bacterial growth of the tested pathogens.
Acknowledgment
The authors are grateful to Arba Minch University College of natural science department of chemistry for providing equipment and laboratory facilities.
References
- Abhijit Balasaheb Shinde, Yogini Ramkrishna Mulay (2015) Phytochemical Analysis and Antibacterial Properties of Some Selected Indian Medicinal Plants. International journal of current microbiology and Applied Sciences 4(3): 228-235.
- Murugan M, Mohan VR (2011) Evaluation of phytochemical analysis and antibacterial activity of Bauhinia purpurea and Hiptage benghalensis L. Kurz. Journal of Applied Pharmaceutical Science 1(9): 157- 160.
- Mohamed Sham Shihabudeen, Hansi Priscilla, Kavitha Thirumurugan (2010) Antimicrobial activity and phytochemical analysis of selected Indian folk medicinal plants. International Journal of Pharma Sciences and Research 1(10): 430-434.
- Solomon Girmay Berehe, Aman Dekebo Boru (2014) Phytochemical Screening and Antimicrobial Activities of Crude Extract of Lepidium Sativium Seeds Grown in Ethiopia. International journal of pharmaceutical Sciences and research 5(10): 4182-4187.
- M Douglas JH, Smallfield B (2005) Herbs, spices and essential oils Post-harvest operations in developing countries, in Herbs, spices and essential oils. Food and Agriculture Organization of the United Nations: Rome.
- Parthasarathy VAC, Bhageerathy Zachariah, John T (2008) Chemistry of spices: CABI.
- Bin Shan YZC, John D Brooks, Harold Corke (2007) The in vitro antibacterial activity of dietary spice and medicinal herb extracts. International Journal of Food Microbiology 117: 112-119.
- Aljit S, Arora JK (1999) Antimicrobial activity of spices. Int J Antimicrob Agent 12(3): 257-262.
- Singh GM, Sumitra De Lampasona, MP Catalan (2006) Chemical constituents, antifungal and antioxidative potential of Foeniculum vulgare volatile oil and its acetone extract. Food control 17(9): 745-752.
- Shahedur Rahman, A.K.P, Rezuanul Islam, Mahboob Hossain Khan (2011) Antibacterial activity of natural spices on multiple drug resistant Escherichia coli isolated from drinking water, Bangladesh. Annals of clinical microbiology and antimicrobials 10(1): 10.
- Anna M Witkowska DKH, Mercedes Alonso-Gomez, Martin Wilkinson (2013) Evaluation of Antimicrobial Activities of Commercial Herb and Spice Extracts Against Selected Food-Borne Bacteria. Journal of Food Research 2(4): 37-54.
- R Hema SKN, Elanchezhiyan (2009) Antimicrobial activity of some of the South-Indian spices and herbals against food pathogens. Global Journal of Pharmacology 3(1): 38-40.
- Kalpana Platel KS (2004) Digestive stimulant action of spices. Indian J Med Res 119: 167-179.
- Platel K, SK (2000) Influence of dietary spices and their active principles on pancreatic digestive enzymes in albino rats. Nahrung 44(1): 42-46.
- Platel, K Rao, Alkananda, G Saraswathi, K Srinivasan (2002) Digestive stimulant action of three Indian spice mixes in experimental rats. Nahrung 46(6): 394-398.
- Filomena Conforti GS, Dimitar Uzunov, Francesco Menichini (2006) Comparative chemical composition and antioxidant activities of wild and cultivated Laurus nobilis leaves and Foeniculum vulgare subsp. piperitum (Ucria) coutinho seeds. Biol Pharm Bull 29(10): 2056-2064.
- Ndukwu BC, Ben-Nwadibia NB (2005) Ethnomedicinal aspects of plants used as spices and condiments in the Niger delta area of Nigeria. Ethnobotanical Leaflets 205(1): 10.
- Syed Zahoor Husain, R N M, Mubashera Javaid, Sadia Bibi (2008) Ethonobotanical properties and uses of medicinal plants of Morgah biodiversity park, Rawalpindi. Pakistan Journal of Botany 40(5): 1897-1911.
- Navindra P, Seeram HI, Shishir Shishodia, Bharat B (2006) Aggarwal, preventive and Therapeutic Effects of Plant Polyphenols through Suppression of Nuclear Factor-Kappa B, in Molecular Interventions in Lifestyle Related Diseases. Houston pp. 244.
- Bharat B, Aggarwal ABK, Kuzhuvelil B, Harikumar, Sheeja T, et al. (2008) Potential of spice-derived phytochemicals for cancer prevention. Planta Med 74(13): 1560-1569.
- Rajput S, Mandal, Mahitosh (2012) Antitumor promoting potential of selected phytochemicals derived from spices: a review. European Journal of Cancer Prevention 21(2): 205-215.
- Shan BC, Yizhong Z Sun, Mei Corke, Harold (2005) Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. Journal of agricultural and food chemistry 53(20): 7749-7759.
- Nurain Aziman, Noriham Abdullah, Zainon Mohd Noor, Khairusy Syakirah Zulkifli, Wan Saidatul Syida Wan Kamarudin (2012) Phytochemical Constituents and In Vitro Bioactivity of Ethanolic Aromatic Herb Extracts. Sains Malaysiana 41(11): 1437–1444.
- Murugan T, Albino Wins J, Murugan M (2013), Antimicrobial Activity and Phytochemical Constituents of Leaf Extracts of Cassia auriculate. Indian J Pharm Sci 75(1): 122-125.
- Baljeet SY, Simmy G, Ritika Y, Roshanlal Y (2015) Antimicrobial activity of individual and combined extracts of selected spices against some pathogenic and food spoilage microorganisms. International Food Research Journal 22(6): 2594-2600.
- Gobalakrishnan R, Kulandaivelu M, Bhuvaneswari R, Kandavel D, Kannan L (2013) Screening of wild plant species for antibacterial activity and phytochemical analysis of Tragia involucrata L. Journal of Pharmaceutical Analysis 3(6): 460-465.
- Kiah J, Farr, Yuri Nakagomi, Nobuko Hongu (2015) Season for Health: A Guide for Using Herbs and Spices for Your Home Cooking. College of agriculture and life sciences cooperative extension 1-8.
- Daniel Ikhane, Kolawole Banwo, Ogunremi Omotade, Abiodun Sanni (2014) Phytochemical and Antimicrobial Activities of Methanolic Extract of Paullinia pinnata Leaves on Some Selected Bacterial Pathogens. Journal of Herbs, Spices & Medicinal Plants 21: 59-74.
- Vedhanarayanan P, Unnikannan P, Sundaramoorthy P (2013) Antimicrobial activity and phytochemical screening of Wrightia tinctoria (Roxb.) R.Br. Journal of Pharmacognosy and Phytochemistry 2(4): 123-125.






























