Integrated Diseased Management on Coffee Wilt Disease Caused by Fusarium Xylarioides and its distribution in Ethiopian Review
Asmamaw Kassahun Wassie*
Bihar Agricultural University, Sabour, India
Submission: July 11, 2019 Published: November 12, 2019
*Corresponding author:Asmamaw Kasahen, Debre Tabor University, Department of Plant sciences, Plant pathologist Lecturer, Agriculture and Environmental Sciences Faculty, Director of research and publication, Email:-asmamawk7@gmail.com , Tell: +251911065399, Debre Tabor University, Po Box 272, Debre Tabor, Ethiopia
How to cite this article: Asmamaw Kasahen. Integrated Diseased Management on Coffee Wilt Disease Caused by Fusarium Xylarioides and its Mangement Option Review. Agri Res& Tech: Open Access J. 2019; 22(4): 556232. DOI: 10.19080/ARTOAJ.2019.22.556232
Abstract
Coffee belongs to the family Rubiaceae, which is widely distributed throughout the tropical region. Ethiopia the center of origin for Coffea arabica. Coffea arabica originates from Southwest Ethiopia Keffa. Coffea arabica very important for local people as a cash crop for the local and international market. Coffee arabica is attack by several diseases among these major diseases are coffee berry disease, coffee wilt disease, coffee leaf rust are the major fungal diseases to reduced yield of coffee in the country. Coffee wilt disease caused by fungal pathogen that affect vascular wilt disease, it was the main factor of coffee tree death in different coffee growing regions, with national average disease incidence 28% and severity 5%. Coffee wilt disease is a soil-borne pathogen, and this presents difficulties in the application of chemical treatments; affected fields to be left as fallow for some years or other crops planted. Currently in coffee trees are less productive because of variable factors, among that diseases are the major constraints which threatening coffee genetic resources. These diseases are occurring with varying degree of infestation and distribution in the Ethiopia. Thus, the coffee conservation strategies should take the disease into account and apply the recommended principles and practices of disease management. Integrated disease management for coffee Fusarium wilts, one of the most devastating and challenging type of diseases impairing agricultural production worldwide, based on the IPM, (i) use of pathogen-free planting material; (ii) site selection to avoid planting into high risk soils; (iii) reduction or elimination of F. oxysporum inoculum in soil; (iv) use of biocontrol agents for protection of healthy planting material from infection. Based on our review findings suggest resistant coffee varieties through large-scale collection and screening against the pathogen would be inevitable for increase coffee yield.
Keywords:IPM; Leaf rust; Wilt disease; Wilt diseases
Introduction
Coffee belongs to the family Rubiaceae, which is widely distributed throughout the tropical region. Although there are many species of coffee, the two commercially important ones are Coffea arabica and Coffea robusta Pieters & Van der Graff [1]. Both species can grow best on deep, free- draining, loamy soils, with a good water holding capacity and a slightly acid soil (pH 5-6) and soil fertility is important for good production of coffee Lewis Ivey [2]. Coffee has rapidly become one of the prominent commodity crops in global transactions, and it stands first in earning foreign currency for many countries including Ethiopia. Ethiopia has the longest tradition of coffee production and consumption in the world with a traditional way of cultivation and the performance of inimitable ‘coffee ceremony’. Coffee is crucial to the Ethiopian economy because it contributes 10% of the country’s gross domestic product and generates more than 40% foreign exchange earnings. Coffee remains crucial to the biological, social and economic values of the country, but despite being the birthplace of coffee, Ethiopia has not exploited and benefited from the crop to the best of its genetic and ecological potential.
Coffee production systems remain predominantly traditional, and diseases and insect pests greatly reduce the productivity and quality of the produce. Historically, Coffee Wilt Disease (CWD) on C. arabica was first observed in Ethiopia by Stewart [3], who described the wilting symptom and also identified the causal organism to be Fusarium oxysporum f.sp. coffeae. Later, based on comparative studies of the isolates collected from dying Arabica coffee trees from different origins and different Coffea spp., the causal was confirmed to be Gibberella xylarioides Heim & Saccas, of which Fusarium xylarioides Steyaert is the imperfect (conidial) state Kranz & Mogk [4]. Van der Graaff & Pieters [5] reported that this pathogen caused a typical vascular wilt disease and was the main factor of coffee tree death in Ethiopia. During recent years, the prevalence and importance of CWD have been markedly increasing throughout coffee producing areas of the country Girma & Oduor [6,7]. Besides drastic reduction in average yield, the crop is attacked by several diseases, among that coffee leaf rust; coffee berry disease and coffee wilt disease are the major fungal diseases contributing to reduced yield in the country.
Objective of the Study
• To review the main impact of coffee wilt disease and distributions in Ethiopia. • To review the status of coffee wilt disease including its occurrence and its management options.
Experimental Verification
Coffee in Ethiopia
Coffee is the most important cash crop for Africa as a whole, contributing some 10% of the total foreign exchange earnings in the continent. A number of coffee-producing countries in Ethiopia depend on the export of this commodity for more than half of their foreign exchange earnings Phiri. Arabica coffee has become a major global commodity which accounts for 66 percent of the world coffee market. Coffee production in Ethiopia is broadly grouped into four systems on the basis of biological diversity of the species and level of management, namely, forest, semi-forest, garden and plantation coffee Meyer & Paulos [8,9]. More importantly, majority of the coffee farmers in the producing countries are small scale growers who primarily depend on coffee for their livelihood. Its cultivation, processing and transportation provide employment for millions of people. The average yield in Ethiopia is low (about 700kg/ha per year) which is half of that achieved.
Coffee wilt disease distribution
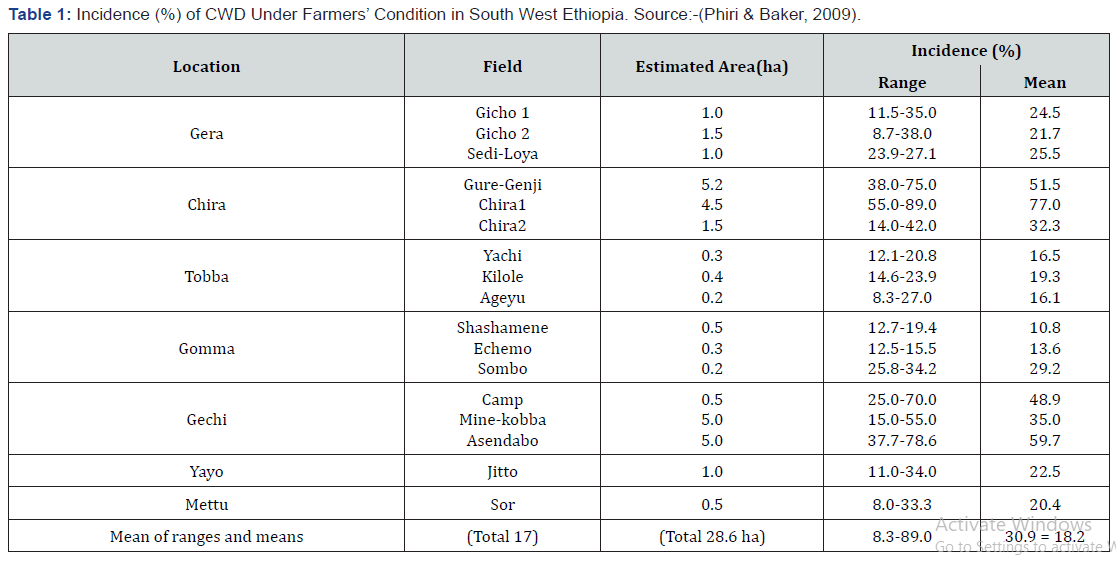
Trachemys’s or vascular wilt of coffee historically was first observed in 1927 on Coffea excelsa in Central Africa Republic and first reported in 1946 and the causal agent was identified as (Fusarium xylarioides) by Steyaert, Girma Adugna [6]. Coffee wilt disease was first observed in Ethiopia in the Kaff Province by Stewart [3], who described a wilting of C. arabica and mistakenly classified it as Fusarium oxysporum f. sp. coffeae. Lejeune also noted the presence of this disease on Arabica coffee. Later, the causative agent of the disease was confirmed to be Fusarium xylarioides Kranz & Mogk [4]. The pathogen also attacks Coffea arabica and is endemic in all coffee growing areas of Ethiopia. During the 1950s and 1960s, it was considered to be the most serious disease of coffee in Africa and destroyed millions of coffee trees Oduor [7]. Coffee Wilt Disease (CWD), which is caused by Fusarium xylaroides Steyaert, the conidial stage of Gibberella xylarioides Hem. & Saccas is the most serious problem of Robusta coffee (Coffea canephora) production in Ethiopian. Coffee wilt disease the main symptoms are yellowing and collapsing. The disease enters through a low surface wound or a shallow root. Afterwards the connecting vascular strands in the stem are discoloured violet brown to black in a broad, hardened band. CWD occurs in all of the above coffee production systems to varying extent of damage among and within coffee fields and districts (Woredas) depending on different interacting factors, mainly susceptibility of coffee trees, intensity of cultural practices and environmental conditions Merdassa [10]. CWD, for many years remained as an endemic disease of Coffee arabica but has gained importance over time in almost all coffee-growing regions. The nationwide biological survey of CWD showed that on average, 27.9% of 1607 sample coffee farms were affected, with disease incidence ranging from 15% to 34.0% and disease severities varying between 1.3% and 5.0% Oduor [7].
Survival and spread of the pathogen
The pathogen survives in the soil in the form of microconidia, macroconidia, chlamydospores and perithecium with ascospores. The pathogen appears to be a soil inhabiting fungus which can penetrate through wounds either above or below ground. Inside the coffee the fungus invades the water conducting system (xylem) and blocks the movement of water upwards from the roots to the rest of the plant. The timing from first symptoms to death of the tree varies from days in young plants to eight months in trees more than ten years old. Once the fungus infects the coffee tree, all affected trees eventually die Girma Adugna. When seedlings with healthy roots are transplanted into either naturally or artificially infested soils, no wilting symptoms appeared. Infection exhibits when the tap roots are injured and transplanted into naturally or artificially infested soils, and also only on those seedlings inoculated by stem wounding through ditching with F. xylarioides infested scalps or by injecting the conidial suspensions with needles Lewis Ivey. The stem nicking or root drenching inoculation methods also elaborate the roles of contaminated farm implements in cross inoculating coffee trees as well as disseminating the coffee wilt pathogen in the field CABI [11].
Principles of disease management coffee wilt disease
Coffee suffers from a range of co-evolved diseases including Coffee Berry Disease (CBD), Coffee Wilt Disease (CWD) and Coffee Leaf Rust (CLR) caused by Colletotrichum kahawae, Gibberella xylarioides and Hemileia vastatrix, respectively. From those Disease Coffee Wilt Disease is sever affect the growth of coffee, its nature soil-borne pathogen and this presents difficulties in the application of chemical treatments; affected fields may need to be left as fallow for some years or other crops planted. For diseases caused by soil-borne pathogens, such as Fusarium wilts, which are mainly monocyclic in nature, this disease continued reliance on unproductive coffee varieties, the widespread and prevalence of pests and diseases. Integrated Disease Management programs are based on the following principles of disease control Coffee Wilt Disease: those method are the control principles
a) Exclusion of the pathogen or
b) Eradication, of the pathogen;
c) Escape from infection;
d) Development and use of genetic resistance against the pathogen;
e) Protection of the plant from infection; and
f) Reduction of disease in infected plants.
They can be applied by biological, chemicals, cultural, physical, and regulatory methods, depending of the nature of the agents employed and methods should be targeted to excluding the pathogen, as well as reducing the amount and/or efficiency of the initial inoculum.
Therefore, IDM strategies of those Coffee Wilt diseases within the framework of sustainable agriculture would include:
(i) Use of pathogen-free planting material;
(ii) Site selection to avoid planting into high risk soils;
(iii) Reduction or elimination of F. exospore inoculum in soil;
(iv) Use of biocontrol agents for protection of healthy planting material from infection by resident or incoming inoculum subsequent to planting;
(v) Use of resistant cultivars regardless the level of resistance; and
(vi) Choice of cropping practices to avoid conditions favoring infection of the plant.
Use of pathogen-free planting material:
Many wilt-inducing Fusaria can be transmitted in infected seeds, vegetative propagated planting material (e.g., bulbs, cuttings, rootstocks, scions, etc.), or transplants developed from them. Use of infected propagating material can lead to introducing the pathogen or its pathogenic variants into pathogen-free production areas or pathogen-free soils in areas where the pathogen occurs already. Therefore, the importance of checking the health of that material through certification programs, phytosanitary inspection, and quarantine legislation cannot be too strongly emphasized. Failure in this pursue may lead to the establishment of new pathogens in a country, as it recently happened in Spain with Fusarium circinatum, F. oxysporum f. sp. basilici, F. oxysporum f. sp. radicis-lycopercisi, Fusarium solani f. sp. cucurbitae race 1, etc. More importantly, introduced exotic pathogens can potentially be invasive and give rise to devastation in cultivated as well as natural plant communities. The Plant Protection Organization (EPPO), being well aware of such a risk, has placed a warning on quarantine fungal pathogens, of which 21 species are already present in member states and 39 are currently absent from them One of most important difficulties for the detection and identification of Fusarium wilt pathogens concerns the similarity in morphology between pathogenic and non-pathogenic strains of F. oxysporum.
Site selection to avoid sowing/planting into high risk soils:
Proper selection of the planting site optimizes the use of F.oxysporum ff. spp.-free planting material in non-infested soils. For that purpose, accurate information on the disease history of the field with regard to production of susceptible crops is of utmost importance. Disease risk assessment based on Inoculum Density (ID) Disease Incidence (DI) relationships would be most useful if the inoculum density in soil at planting sites could be estimated to avoid those with high risk for severe disease. Populations of F. oxysporum in soil can be assessed by soil dilution plating using selective media. However, this does not allow inferring ID of pathogenic strains because of their morphological similarity with non-pathogenic ones. For example, De Vay et al. assessed the ID of F. oxysporum in cotton soils by agar dilution plating but identified colonies belonging to F. oxysporum f. sp. vasinfectumby further pathogenicity assay to cotton seedlings. That allowed estimating the number of F. oxysporum f. sp. vasinfectum Colony Forming Units (CFU) g-1 soil and relating a range of 1,100 to 2,608 CFU g-1 soil to increase of Fusarium wilt incidence over physiological time in degree days and effects on crop growth and yield. Ben Yephet used a similar approach for Fusarium wilt in carnations and found that 6, 25, 120, 770, and 3,500 CFU g-1soil of F. oxysporum f. sp. dianthi determined a final DI of 2, 5, 13, 34, and 57 %, respectively; the flower yield being related inversely to ID of the pathogen. Conversely, disease risk can be made by directly bio-assaying the planting soil with susceptible and resistant host cultivars.
Use of CWD resistant cultivars:
These are undoubtedly the most feasible option for controlling CWD in all affected countries. Use of resistant cultivars was found to be highly effective when combined with other control measures during the previous outbreak of the disease. The combined use of selected cultivars and biocontrol agents can provide better disease control than the use of any of them alone. It has been reported that varietal differences in resistance to the pathogen and suggested the use of resistant varieties as a means of control. However, developing resistant varieties is long-term and requires considerable resources (human, facilities and financial). Megan reported that Uganda has advanced further with its CWD breeding programme, using single-tree selection, and some of the more promising selections are currently being evaluated on-farm. The DRC is also trying to select varieties for CWD-resistance. This method was very successful in controlling outbreaks of the disease in 1950s and 1960s in West and Central Africa, where affected coffee is uprooted and destroyed and the fields replanted with resistant cultivars of C. canephora such as cultivar `robust`, but recently resistance is broken down due to emergence of a new form of the fungus Meseret Wondimu also reported apparent differences for the same materials planted in different areas of the region, i.e. certain C. liberica and C. canephora varieties showing resistance in Ivory Coast were completely susceptible in CAR, suggesting the resistance was either being influenced by environmental conditions or there were different physiological races of the pathogen in different localities of this region. Vander Graaff & Pieters [12] reported that coffee lines of C. arabica in Ethiopia showed differences in resistance to the CWD pathogen, thus providing potential for controlling CWD using resistant varieties in Arabica coffee. They suggested that resistance in C. arabica was quantitative in nature and horizontal, and there was no evidence of single-gene (vertical) resistance that could be readily overcome by pathogen adaptation.
Coffee wilt disease management practices
Cultural control:
Unlike with other coffee diseases, namely, CBD and CLR, coffee trees infected by CWD cannot be saved. Successful control of the disease depends on the principles of disease prevention (avoid wounding of any part of the plant) and phytosanitation. The conventional phytosanitary approach of uprooting and burning the whole infected coffee tree on the spot is strongly recommended to coffee farmers to contain the disease as soon as symptoms are seen, but this relies on early diagnosis. Use of CWD-infected trees for any purpose is prohibited and replanting with susceptible coffee seedlings should be delayed at least for 2 years Girma [4]. Cultural weed control activities like slashing and digging should be avoided in CWD-prone coffee fields, and agronomic practices (pruning and stumping) that bring about wounding in coffee trees should be done with efficiently disinfected tools. Disinfection of farm implements such as machetes, bow saws and pruning shears with potent disinfectants (>75% alcohol) followed by intense heating with fire is strongly recommended to farmers whenever pruning, rejuvenating old coffee trees and thinning newly suckers. Farmers’ field schools recommend growing cover crops such as Desmodium sp. and haricot bean, which are very efficient in suppressing weeds (so reducing the need for slashing) and as legumes, promote the growth of coffee trees. Applying ash, mulch and slashing between plots with hand weeding around coffee trees were also promising treatments in CWD control trials CABI [11].
Immediate intervention:
The first step taken in this direction was training of both extension staff and farmers in disease recognition, followed by sensitization of fanners and civic leaders. Sanitary control measures were then implemented which include:
a) Regular inspection and destruction of affected trees by cutting trees at ground level, chopping and burning in situ.
b) Uprooting and burning of the entire plant produces best results. Neighboring trees should be cut back and thick mulch applied.
c) Diseased trees when left standing in the field continue to discharge spores to neighboring or distant trees for several months.
d) Destruction of dead or severely diseased trees proceeded by superficial burning of the upper parts before any handling/ uprooting trees assists in reducing dispersal of spores.
e) Wounding trees should be avoided, since wounds provide entry points for the pathogen.
f) Restriction of movement of infected plants as firewood, coffee husks and kiboko from infected areas to other areas.
g) Restriction of movement of planting materials from infected to non-infected areas.
h) Banning the use of coffee husks as mulch in coffee fields, as a precaution.
i) Vse of volunteer seedlings from forests on infected coffee plantations is discouraged. Seedlings from infested plantations may harbours the disease without showing obvious symptoms.
j) Milling coffee should be done in the district of production.
k) Continuous surveillance of disease in all coffee growing areas to keep track of spread and ascertain the effectiveness of control measures.
l) Training and sensitization of all stakeholders on dangers of the disease etc.
Biological control:
Biological control is the reduction of inoculums density or disease producing activities of a pathogen or parasite in its active or dormant state, by one or more organisms accomplishing naturally or through manipulation of the environment, host or antagonists, or by mass introduction of one or more antagonist. Biological control is the strategy for reducing disease incidence or severity by direct or indirect manipulation of microorganisms (Tesfaye and Kapoor, 2004). Antagonists that produce antibiotics kill pathogens and eradicate or control them from substrate. Some microorganisms occupy the niches and exclude pathogens from becoming established, thereby protecting plants from infection. Biological control has attracted great interest because of increasing regulation and restriction of fungicides or unnecessary control attempts by other means [13]. It is especially attractive for soil borne diseases because it needs critical evaluation of economics of the country and the pathogens that are difficult to reach with specific fungicides. The result of a recent in vitro study conducted by Muleta & Negash on antagonistic effects of some rhizobacteria and Tricoderma isolates against the F. xylarioides were promising. Of 23 bacterial isolates obtained from rhizospheres of arabica coffee trees in south-west Ethiopia, 21 significantly inhibited the mycelial spread of F.xylarioides. Bacillus subtilis, designated as isolate ‘AUBB20’, was the most antagonistic to this pathogen. T. viride and T.harzianum has shown good potential in inhibiting the mycelial growth of F. xylarioides
Fungicide spray:
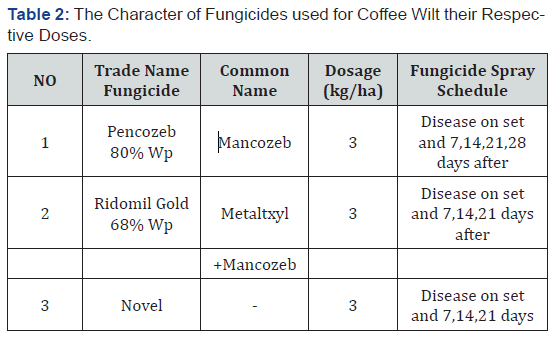
Fungal diseases of coffee are the major constraints to reduce coffee production and quality in major coffee producing countries of Africa. Next to Coffee Berry Disease (CBD) the most limiting factors for coffee production in Central and East African countries is tracheomycosis or vascular wilt disease of coffee caused by Fusarium xylarioides Steyaert imperfect stage (Gibberella xylarioides Heim and Saccas Perfect stage). The major difference between tracheomycosis and many other coffee diseases is that it kills all affected trees at all stages of development. The fungicides were sprayed at the rates, schedules and combinations indicated in (Table 1) [14]. Three fungicides, Ridomil Gold (metalxyl 8%+ Mancozeb 64%) 68% Wp 2.5kg/ha with protective and curative action and Pencase 80% WP (Mancozeb) at the rate of 2.5kg/ha (Cornell University, 1988) with protective action and Novel was used systemic at the rate of 2.5kg/ha coffee wilt disease effectively control. Spraying was performed by using manually pumped knapsack sprayers of 20 litter’s capacity [15]. During fungicide spraying using surrounded by plastic sheet in order to avoid spray drift to adjacent area. Fungicide spray intervals weekly (7day), biweekly (14day), three weekly (21) and 28day intervals until maturity. The intervals between successive sprays were constant week when diseased symptoms occurred. The data on disease severity recorded one day before spray and 7 days after spraying using, sprayed at early flowering and fruit setting (Table 2). The Spraying started soon after the appearance of coffee wilt symptoms.
Research strategies
Prospects for long term control measures depend on research activities, which will be implemented to generate information on: -
I. Epidemiology and biology of the pathogen, to cover mode of spread and transmission, survival, presence of alternate hosts particularly, major food crops generally intercropped with coffee
II. Host plant resistance/tolerance is being explored by inoculation of all available germplasm, breeders’ materials and current recommended arabica and Robusta varieties. The same materials are also planted out on farmers’ fields where coffee has been destroyed by wilt. Before release to farmers, it is essential to screen all materials for resistance to dermatomycosis.
III. The effects of production systems (intercropping, soil fertility management and cultivation on non-host crops for 5 or more years followed by coffee) on wilt incidence and severity will be elucidated. The role of weather factors e.g. rainfall, temperature. etc. as well as soil types, effect of organic manures, are also to be assessed and correlated to wilt incidence.
IV. The role played by other Fusarium species found associated with F. xylarioides needs to be clearly spelt out.
V. Factors responsible for the appearance and disappearance of wilt disease are to be investigated
VI. The use of biocontrol agents e.g. a strain of Fusarium oxysporum to suppress Fusarium and use of systemic fungicides as drench in planting holes etc. are to be investigated. These could find use for spot treatments on large plantations where the fanners have invested a lot of money.
VII. Collaboration in research at regional and international levels needs to be strengthened in order to speed up progress through exchange of intonation, resistant materials etc. These wiII eventually provide information required for formulation of an integrated control procedure
Conclusion and Suggestion
Currently in Ethiopia most coffee trees are less productive because of variable factors, among that diseases are the major constraints which threatening coffee genetic resources. These major diseases are coffee leaf rust, coffee wilt disease and coffee berry diseases are becoming important and that occurs with varying degree of infestation and distribution in the main area of Ethiopia. Thus, the forest coffee conservation strategies should take the disease into account and apply the recommended principles and practices of disease management. This economic loss coupled with difficulty to manage the disease indicates that CWD is the second leading disease of coffee, after CBD in Ethiopia and the most distractive coffee production threat without any solution till now. The soil-borne nature of the pathogen and perennial character of coffee have made management of the disease difficult through the conventional control approach of ‘uproot and burn infected trees at the spot.
Development of resistant coffee varieties through large-scale collection and screening against the pathogen would be inevitable, although it seems that most coffee trees exhibit susceptibility in the forest coffee populations. Investigation in the area of developing resistant varieties, biocontrol agents, use of proper cultural practices and screening effective chemicals as a last resort or use of these techniques in integrated pest and disease management strategy could minimize the damage caused by diseases and would definitely conserve coffee genetic pools and facilitate utilization of coffee genetic resources at their place of origin in natural rainforests for global benefits. It is important that prior to their release to coffee growers, resistant coffee types should also be tested for their level of resistance to those diseases particularly coffee wilt and coffee leaf rust. Therefore, employing effective, easily applicable, environmentally sound and economically feasible control approaches should be exercised for disease management of Arabica coffee in forest populations of Ethiopia.
References
- Pieters R, Vander-Graff N (1980) Resistance to Gibberella xylarioides in Coffee Arabica: Resistance to Gibberella xylarioides in Coffea arabica: Evaluation of screening methods and evidence for the horizontal nature of the resistance. Netherlands Journal of Plant Pathology 86(1): 37-43.
- Lewis Ivey M, Miller S, Hakiza G, Geiser D (2003) Characterization of the coffee wilt.
- Stewart RB (1957) Some plant diseases occurring in Kaffa province, Ethiopia. Imperial Ethiopian College of Agriculture and Mechanical Arts Alemaya Ethiopia.
- Kranz J, Mogk M (1973) Gibberella xylarioides Heim et Saccas on Arabica coffee in Ethiopia. Journal of Phytopathology 78(4): 365-366.
- Van der Graaff NA, Pieters R (1978) Resistance levels in Coffea arabica to Gibberella xylarioides and distribution pattern of the disease. Netherlands Journal of Plant Pathology 84(4): 117-120.
- Girma A (1997) Status and economic importance of Fusarium wilt disease of Arabica coffee in Ethiopia.
- Oduor (2005) Surveys to establish the spread of coffee wilt disease, Fusarium (Gibberella) xylarioides, in Africa. In: Proceedings of the 20th International Scientific Conference on Coffee Science (ASIC), pp. 1252-1255.
- Meyer FG (1965) Notes on wild Coffea arabica from southwestern Ethiopia, with some historical considerations. Economic Botany 19(2): 136-151.
- Paulos D, Demel T (2000) The need for forest germplasm conservation in Ethiopia and its significance in the control of coffee diseases. In: Proceedings of the Workshop on Control of Coffee Berry Disease (CBD) in Ethiopia, pp. 125-135.
- Merdassa E (1986) A review of coffee diseases and their control in Ethiopia. In: Abate, T. (Edt.), Proceedings of the First Ethiopian Crop Protection Symposium. Institute of Agricultural Research Addis Ababa Ethiopia, pp. 187-195.
- (2003) CABI (CAB International) Surveys to assess the extent and impact of coffee wilt disease in East and Central Africa. Final technical report. CABI Regional Centre Nairobi Kenya, pp. 149.
- Pieters R, Vander-Graff N (1980) Resistance to Gibberella xylarioides in Coffee Arabica.
- Hakiza GJ, Birkunzira B, Musoli P Proceedings of the First Regional Workshop on Coffee Wilt Disease (tracheomycosis). International Conference Centre Kampala Uganda, pp. 53-61.
- Evaluation of screening methods and evidence for horizontal Nature of the resistance. Netherlands journal of Plant pathol 86: 37-43.
- Pathogen in Uganda. Phytopathol 93: 550.






























