Evaluation of Larvacidal Activity of Olea europaea Extract against Anopheles Mosquito in In vitro, Fogera Woreda, North Western Ethiopia
Hirpasa Teressa1*, Wondimu Ersino2 and Tadele Alemayo3
1Department of Biology, Debre Tabor University, Ethiopia
2Department of Biology, Wachemo University, Ethiopia
3Department of Wildlife and Ecotourism management, Mekelle University, Ethiopia
Submission:June 08, 2019; Published: June 27, 2019
*Corresponding author: Hirpasa Teressa, Faculty of Natural and Computational Sciences, Department of Biology, Debre Tabor University, P.O. Box 272, Ethiopia
How to cite this article: Hirpasa Teressa, Wondimu Ersino, Tadele Alemayo. Evaluation of Larvacidal Activity of Olea europaea Extract against Anopheles Mosquito in In vitro, Fogera Woreda, North Western Ethiopia. Agri Res& Tech: Open Access J. 2019; 22(1): 556184. DOI: 10.19080/ARTOAJ.2019.21.556184
Abstract
Mosquitoes are the major public health threat as they are the vectors of malaria. Since mosquitoes developed resistances towards chemical insecticides, researchers are searching for alternative control measures. Secondary metabolites and bioactive chemicals present in plants serve as a defense mechanism against insects’ larvae. Therefore, the aim of this study was to evaluate larvacidal activities of Olea europaea (O. europaea) extracts In vitro against 3rd-4th instars larvae of Anopheles arabiensis. The fresh leaves of the plant were collected from Fogera Woreda and brought to Bahir Dar University to be extracted by petroleum ether, chloroform, acetone and ethanol. The extract was filtrated using Buchner funnel with Whatman filter paper; undergone rotary evaporator and stored at -4 C until larvicidal bioassay test. Furthermore, the phytochemical screening was carried out and appropriate solution was prepared. Larvae of A. arabiensis were collected from Woreta town and bioassay was performed on 20 larvae at room temperature. The percentage mortality at 24, 48 and 72 hours was computed. The result showed that petroleum ether, chloroform, acetone and ethanol extracts of O. europaea has resulted in significant mortality of larvae (P=0.00). All solvent extracts showed increased mortality rate with time and concentrations. Based on the current result, we recommend that further studies are needed to characterize active ingredients of screened phytochemicals and evaluate their toxicity, and to study their impacts on human health and non-target organisms in mosquito habitats.
Keywords:Anopheles arabiensis; Larvicidal activity; Olea europaea; Plant extract
Abbrevations: FMoH: Federal Ministry of Health; RDBOA: Rural Development Bureau of Agriculture; IPMS: Improving Productivity and Market Success

Introduction
Malaria is a tropical and subtropical disease caused by protozoan parasites of the genus Plasmodium and transmitted by a bite of female Anopheles mosquitoes [1]. It causes a shocking disease with high morbidity and mortality that demands rapid and comprehensive efforts [2]. According to WHO report, malaria causes about 863, 000 annual deaths worldwide [3]. Globally 3.2 billion people are at risk of malaria each year and, more than 90% death occurs in Sub-Saharan Africa [4]. One of the factors for malaria prevalence is the emergence of Plasmodium falciparum resistance to antimalarial drugs and increasing of insecticide resistant Anopheles mosquito [5].
Ethiopia is one of the tropical Sub-Saharan countries where malaria is a major public health problem. Two Plasmodium spe cies namely P. falciparum and Plasmodium vivax contribute to nearly 60% and 40% respectively of all malaria diseases [6]. In addition, in Ethiopia over 68% of the population lives in areas at risk of malaria infection [7,8].
Traditionally plant products have been used by societies for many centuries to control insects. Several eco-friend secondary metabolites and bioactive chemicals present in plants serve as a defense mechanism against insect attacks [9]. These bioactive chemicals are used as insecticides, repellents and growth inhibitors of insects. Moreover, plant-based pesticides are less toxic and easily biodegradable in the environment [10]. Recently researches have proved larvicidal effectiveness of plant derived compounds such as saponine [11], steroids, bioflavonoid, essential oils, alkaloids and tannins and provided them as alternative in the control of mosquito [12]. Therefore, the aim of the present study was to evaluate larvacidal activities of O. europaea extracts In vitro against 3rd-4th instars larvae of A. arabiensis.
Materials and Methods
Description of the study area
This study was conducted in Fogera Woreda, North western Ethiopia. Fogera Woreda is one of the Woredas in South Gondar Zone of Amhara Regional State and is situated at 11058ꞌ latitude and 37041ꞌ longitude [13]. Woreta is the capital of the Woreda and is found at 625km northwest of Addis Ababa and 60km north of the Regional capital, Bahir Dar. The total area of the Woreda is 117,414 ha and its land use pattern include 44% cultivated land, 24% pastureland, 20% water bodies and the rest for others. The total population of the Woreda is 251,714 with rural population estimated to be 220,421. The mean annual temperature and rainfall of the Woreda is 20 C and 1216.3mm respectively. Its altitude ranges from 1774 up to 2410masl allowing a favorable opportunity for wider agricultural activities [14].
Fogera Woreda is known by its wetland where mixed farming agricultural activity is the means of income. Sales from crops and animal products are the major income sources for the people. They grow cash crops like teff, corn, sorghum, cotton and sesame. Fogera Woreda is also known for its breed of cattle, which is one of the best native milk cows in the country. Recently rice is becoming the dominant cereal crop in the Woreda [15]. Malaria is the most serious disease being number one from the top ten diseases that cause high morbidity in the Woreda. It is highly prevalent during the rainy season, particularly between May and July, and October and December when the rains have ended [16].
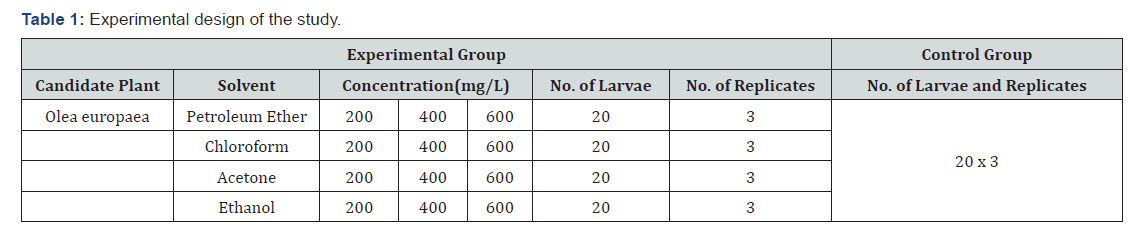
Experimental design of the research
The overall experimental design of the study with the treatments was arranged in 4x3 factorial arrangements in randomized completely block design (RCBD) in three replications and concentrations. Distilled water was used for both groups.
Data collection
Plant collection: The leaves of O. europea, were collected from different areas of Fogera Woreda and brought to Bahir Dar University, Department of Chemistry. The leaves were washed thoroughly by tap water 3 times to remove the adhering dusts. Then it was air dried in dark room at room temperature. Finally, the dried specimens were crushed to powder form using electrical grind mill and the powder was stored at room temperature [17].
Preparation of plant extracts: Four solvents namely petroleum ether, chloroform, acetone and ethanol were used for plant extraction by increasing polarity gradient method [17]. 200g of powder was soaked in 1000ml petroleum ether in separate Erlenmeyer flasks for maceration extraction and the soaked crude extract was placed on orbital shaker at 120rpm for 48hours. The mixture was first filtered by cotton and then passed through Buchner funnel with Whatman filter paper. Before extracting the residue with the next solvent, adhering solvents was removed using rotary evaporator. The macerated residue from petroleum ether was extracted sequentially by the same amount of chloroform, acetone and Ethanol using polarity gradient as done above for the petroleum. All extracts were filtrated using Buchner funnel with Whatman filter paper. Then, the Phytochemical screening was carried out for the extract as per the standard [18-20].
From the filtered extracts, appropriate solution was prepared in individual cups by dissolving the extract in distilled water. From all solutions of solvent extracts, 200mg/L, 400mg/L and 600mg/L solutions were prepared and introduced into separate labeled bowels. Finally, the solutions were stored in refrigerator at -4 C until larvacidal bioassay and general toxicity test according to WHO standard [18].
Collection and identification of mosquito larvae: 3rd-4th instar larvae of A. arabiensis (malaria vector) were collected from the stagnant water present near Woreta town and morphologically identified [21]. The collected mosquito larvae were brought to Woreta Agriculture College where larvacidal bioassay was tasted. Finally, they were put in small room enclosed with a mosquito net and then transferred to a molder with clear tap water.
Larvicidal bioassay at laboratory: Standard method of WHO was used to test larvacidal efficacy of the plant extract against Anopheles mosquito larvae [4]. The bioassay was performed at room temperature, relative humidity of 70-85%, and pH 7.0 of distilled water. For experimental group, twenty 3rd-4th instar Anopheles larvae were taken by pipette and placed in separate flask containing 1000ml distilled water. Then larvae were treated with 200mg/L, 400mg/L, and 600mg/L of all solvent extracts of O. europaea. Each treatment was conducted in three replicates as designed on Table 1 above. All larvae were feed an equal amount of sucrose every 24 hours. For the control group, twenty 3rd-4th instar larvae were also placed in 1000ml distilled water and the same
amount of sucrose (as in experimental group) was feed every 24 hours. Finally, the effect of the plant extracts on Anopheles larvae was examined through counting the number of dead larvae after 24, 48 and 72 hours of treatment and the percentage mortality was computed. The computed mortality was corrected using Abbott’s formula:
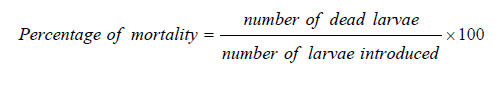
Data analysis
Data was analyzed by using SPSS version 16 Statistical Software. Type of solvents, difference in concentrations and time exposures were used as independent factors and mortality of larvae was used as dependent factor. ANOVA was used to determine the significant difference on the mortality of A. arabiensis larvae between the control and the experimental group and 0.05 was taken as significance level.
Results and Discussion
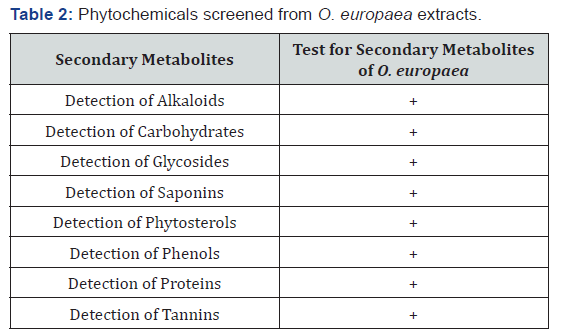
The present study showed that O. europaea extracts that were performed in different tests consist of considerable secondary metabolites (Table 2). Accordingly, from O. europaea extracted by petroleum ether, chloroform, acetone and ethanol, phytochemicals namely alkaloids, carbohydrates, proteins, glycosides, saponins, phytosterols, phenols, and tannins were screened. In agreement with our finding, researchers have revealed that olive leaves are a source of phytochemicals like glycosides, phenols and flavonoids in which alkaloids and phenolics have been proved to have insecticidal activities [22,23]. Furthermore, study identified that plants offer great promise as source of phytochemicals with proven potential as insecticides which can play an important role in the control of mosquitoes [24]. Therefore, screening active compounds from O. europaea may have remarkable contribution in efforts to develop new alternative drugs in malaria control
Acetone, ethanol, petroleum ether and chloroform extracts of O. europaea at concentration of 200mg/L, 400mg/L and 600mg/L showed significant mean A. arabiensis 3rd-4th instars larvae mortality at different time exposure compared to the control group (P=0.00). For almost all solvent extracts, mortality rate increased with time and concentrations. However, no mortality of larvae was recorded for the control group within the same time intervals (Table 3). In agreement with the present study, researchers have proved that plant derived compounds have larvacidal activities and can be effective in insect control [11,12]. Therefore, O. europea which has been identified by the current work to contain considerable secondary metabolites including alkaloids, glycosides, saponins, phytosterols, phenols, and tannins can be the best alternative in the control of mosquito. Such plant derived compounds are preferable than others since they are less toxic and easily biodegradable in the environment [10].
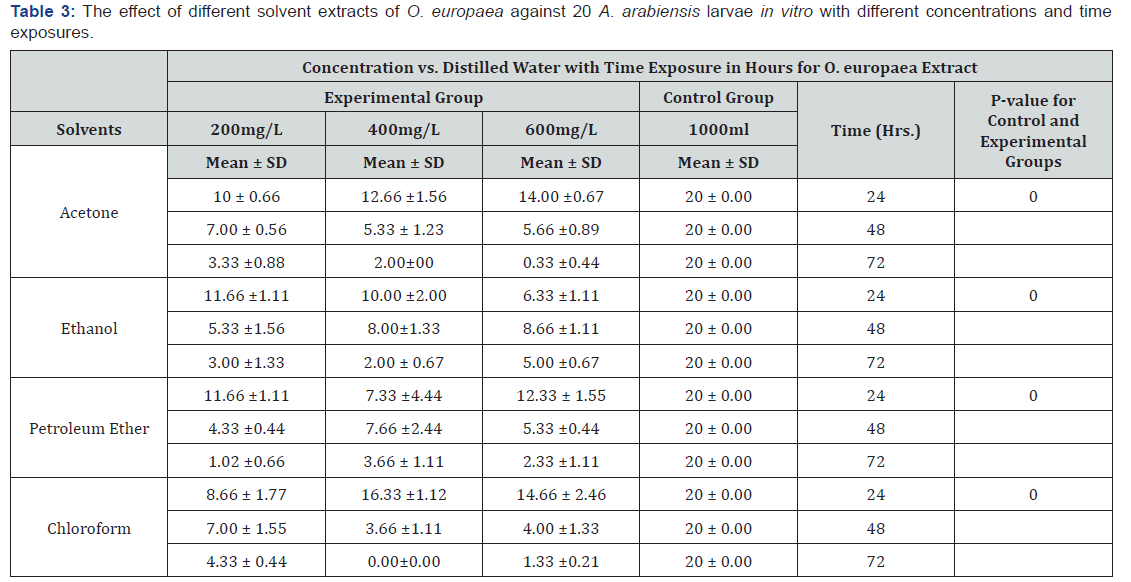
According to the current result, although insecticidal effects of plant extracts can vary due to the polarity of different solvents used during extraction [25], all solvents extract of O. europaea showed almost similar mortality rate for A. arabiensis larvae. This also disagrees with the report of Sukumar et al. [26], which depicted variations in toxicity of phytochemical compounds against the larvae that varied with the solvent used in extraction. However, mortality rate increased with time and concentrations. Among all concentrations and solvents, maximum mean mortality was recorded for 600mg/L. As concentration increased from 200 to 600mg/L, the mean mortality was observed to increase. Only acetone and chloroform extracts showed low mortality rate with increasing concentration within 24 hours for unknown reason to the current study. In agreement with Ali et al. [27], total mortality was also positively correlated with increasing concentrations and duration of exposure.
Conclusion and Recommendation
Phytochemical screening of O. europaea extracted with different solvents showed positive result for alkaloids, carbohydrates, glycosides, phenols, flavonoids, proteins and tannins. Petroleum ether, chloroform, acetone and ethanol extracts of O. europaea showed significant mean mortality of A. arabiensis 3rd-4th instars larvae within 24, 48 and 72 hours at different concentration (200mg/L, 400mg/L and 600mg/L). For all solvent extracts, as concentration and time increased, mortality rate also increased.
Based on the current findings, we recommend that further studies are needed to determine the active ingredients in those phytochemicals and to identify their mode of action and toxicity. Furthermore, intensive study is needed to determine their impacts on human health and non-target organisms in mosquito habitats.
Acknowledgement
We would like to thank Debre Tabor University for granting fund to undertake this study. Furthermore, we would also thank Bahirdar University, College of Natural and Computational Sciences, Department of Chemistry for laboratory materials and chemicals support. Finally, we thank Woreta Agriculture College for giving suitable laboratory room.
References
- Eve W, Suprotik B, Kara H (2005) Is malaria a disease of poverty? Trop Med Int Health 10(10): 1047-1059.
- Bloland PB (2001) Drug resistance in malaria. Geneva: World Health Organization. (WHO/CDS/CSR/DRS/4-25). p. 29.
- World Health Organization (2009) World malaria report. Geneva, Switzerland, p: 13-78.
- World Health Organization (2005) Malaria Control Today. Roll Back Malaria Department, World Health Organization, Geneva, Switzerland, p: 81-91.
- Muregi FW, Chhabra SC, Njagi ENM, Lang‟at-Thoruwa CC, Njue WM, et al. (2004) Antiplasmodial activity of some Kenyan medicinal plant extracts singly and in combination with chloroquine. Phytother Res 18(5): 379-384.
- Deressa T, Mekonnen Y, Animut A (2010) In vivo anti-malarial activities of Clerodendrum myricoides, Dodonea angustifolia and Aloe debrana against Plasmodium berghei. Ethiopian Journal of Health Development 24(1): 25-29.
- Deressa W, Chibsa S, Olana D (2003) Treatment seeking of malaria patients in East Shewa zone of Oromia, Ethiopia. Ethiopian Journal of Health Development 17(2): 9-15.
- FMoH (2007) Africa Malaria Day 2007 Celebrations in Ethiopia. Joint Press Release for the Commemoration of the Africa Malaria Day. pp: 1-98.
- Raghavendra K, Singh SP, Subbarao SK, Dash AP (2009) Laboratory studies on mosquito larvicidal efficacy of aqueous and hexane extracts of dried fruit of Solanum nigrum Linn. Indian J Med Res 130(1): 74-77.
- Kamaraj C, Rahuman AA (2010) Larvacidal and adulticide potential of medicinal plant extracts from south India against vectors. Asian Pacific Journal of Tropical Medicine 3(12): 948-953.
- Vatandoost H, Hanafi-Bojd AA (2008) Laboratory evaluation of 3 repellents against Anopheles stephensi in the Islamic Republic of Iran. East Mediterr Health J 14(2): 260-267.
- Akhtar P, Ali M, Sharma PM, Farooqi H, Khan NH (2010) Phytochemical investigation of fruits of Corylus colurna Journal of Phytology 2(3): 89-100.
- RDBOA (2007) Rural Development Bureau of Agriculture, Fogera Woreda Report, Ethiopia.
- IPMS (2005) Fogera Pilot Learning, Woreda Diagnosis and Program Design and atlas, Ethiopia.
- Fogera Woreda (2007) Fogera Pilot Learning Site, Diagnosis and Program Design, Ethiopia.
- Bake-Migongo C, Catacutan D, Namirembe S (2012) Assessment of the headwaters of the Blue Nile in Ethiopia. ICRAF Working Paper No 149, Nairobi: World Agroforestry Centre, Kenya. p. 72.
- Ncube NS, Afolayan MG, Okoh AI (2008) Assessment techniques of antimicrobial properties of natural compounds of plant origin: current methods and future trends. African Journal of Biotechnology 7(12): 1797-1806.
- Das K, Tiwari RKS, Shrivastava DK (2010) Techniques for evaluation of medicinal plant products as antimicrobial agent: Current methods and future trends. Journal of Medicinal Plants Research 4(2): 104-111.
- Obasi NL, Egbuonu ACC, Ukoha PO, Ejikeme PM (2010) Comparative photochemical and antimicrobial screening of some solvent extracts of Samanea saman pods. African journal of pure and applied chemistry 4(9): 206-212.
- Audu SA, Mohammed I, Kaita HA (2007) Phytochemical screening of the leaves of Lophira lanceolata (Ochanaceae). Life Science Journal 4(4): 75-79.
- Theodore G, Andreadis TG, Thomas MC, Shepard JJ (2005) Identification guide to the mosquitoes of Connecticut: The Connecticut Agricultural Experiment Station, 123 Huntington Street, New Haven, CT 06511, Bulletin No. 966.
- Le Toutour B, Guedon D (1992) Antioxidative activities of Olea europaea leaves and related phenolic compounds. Phytochemistry 31(4): 1173-1178.
- Shaalan EAS, Canyonb D, Younesc MWF, Abdel-Wahaba H, Mansoura AH (2005) A review of botanical phytochemicals with mosquitocidal potential. Environ Int 31(8): 1149-1166.
- Ebong P, Atangwho I, Eyong E, Egbung G (2008) The antidiabetic efficacy of combined extracts from two continental plants: Azadirachta indica and Vernonia amygdalina. Journal of Biochemical and Biotechnology 4(3): 239-244.
- Ghosh A, Chowdhury N, Chandra G (2012) Plant extracts as potential mosquito larvicides. Indian J Med Res 135(5): 581-598.
- Sukumar K, Perich MJ, Boobar LR (1991) Botanical derivatives in mosquito control: A review. J Am Mosq Control Assoc 7(2): 210-237.
- Ali A, Tabanca N, Kurkcuoglu M, Duran A, Eugene K, et al. (2014) Chemical composition, larvicidal, and biting deterrent activity of essential oils of two subspecies of Tanacetum argenteum (Asterales: Asteraceae) and individual constituents against Aedes aegypti. J Med Entomol 51(4): 824-830.






























