Minirhizotron as an In-Situ Tool for Assessing Sugarcane Root System Growth and Distribution
Augusto Yukitaka Pessinatti Ohashi1, Regina Célia de Matos Pires1*, André Luiz Barros de Oliveira Silva1, Leonardo Nazário Silva dos Santos2 and Edson Eiji Matsura3
1Agronomic Institute, Brazil
2Goiano Federal Institute, Brazil
3University of Campinas, Brazil
Submission:June 06, 2019; Published: June 13, 2019
*Corresponding author: Regina Célia de Matos Pires, Scientific researcher, Agronomic Insitute, 1481 Barao de Itapura Ave, Campinas, P.O. Box: 28, Sao Paulo, Brazil
How to cite this article: Augusto Yukitaka Pessinatti Ohashi, Regina Célia de Matos Pires, André Luiz Barros de Oliveira Silva, Leonardo Nazário Silva dos Santos, Edson Eiji Matsura. Minirhizotron as an In-Situ Tool for Assessing Sugarcane Root System Growth and Distribution. Agri Res& Tech: Open Access J. 2019; 22(1): 556182. DOI: 10.19080/ARTOAJ.2019.21.556182
Abstract
Crops root information is necessary in modern agriculture to provide information that promotes water and nutrient use efficiency. The minirhizotron is a non-destructive root method and it allows monitoring root growth along time and seasons. In this context, the aim of this paper is to provide an overview about minirhizotron method use for root growth and distribution assessment in sugarcane crop in irrigated and rainfed areas. We presented information in order to improve minirhizotron methodology and its use by providing highlights concerning critical aspects. Among critical points, the tube installation, tube protection, scanning-schedule and data analysis are addressed. Our information could help future users to acquire high-quality data with proper efficiency
Keywords: Non-destructive method; Root image; Root growth rate; Root method

Introduction
Root-related information, as well as its growth and distribution in soil profile, are fundamental to understand water and nutrients absorption processes by plants [1] and hence improve irrigation and fertigation efficiency.
Irrigation and fertigation practices and its effect on sugarcane root system should be studied case-by-case, because root growth and distribution are both genetic- and environmental-dependent [2]. There are still few data concerning roots, especially when compared to canopy data, probably due to methodological complexity related to roots sampling [3].
The minirhizotron is a method based on root growth visualization by a picture of soil profile obtained by a camera through a glass or acrylic tube inserted into the soil profile. It is considered a non-destructive method and it allows monitoring root growth along time and seasons [4], hence its main advantage is to detect seasons of high root growth rates [5] and root turnover [6]. This method is considered as an alternative for root assessment which could reduce labor and costs compared to other root evaluation methods [3,7]. The aim of this paper is to provide an overview about minirhizotron method use for root growth and distribution assessment in sugarcane crop in irrigated and rainfed areas.
Installing Procedures and Recommendations
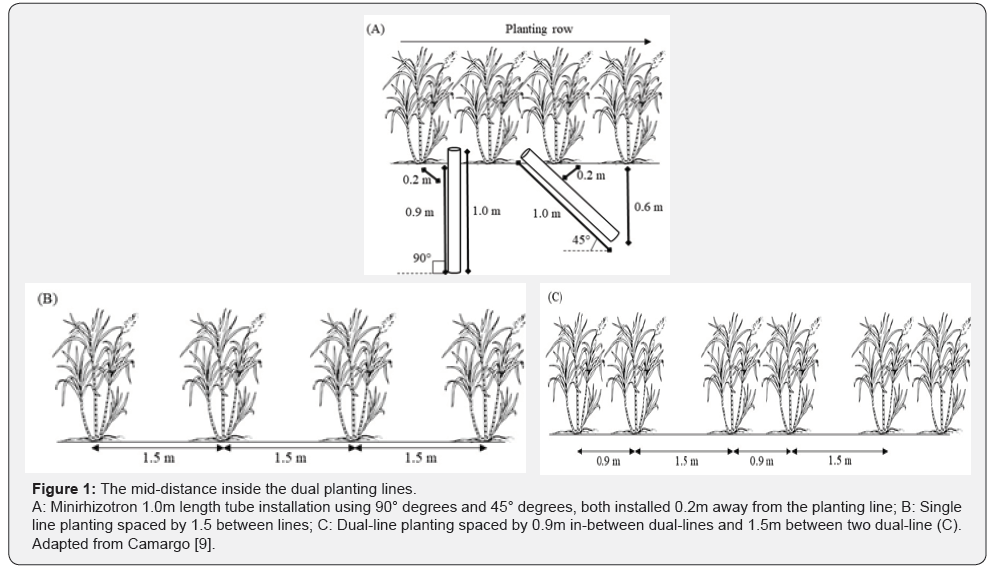
The tube to be used should be relatively resistant and cost-effective, hence the most common material used is the acrylic. Tube diameter depends on the camera or scanner to be used. Usually external diameter is 0.07m and internal diameter is 0.063m, as it fits CID CI-600 In-situ Root Imager®* (CID Bioscience, Inc. Camas, WA - USA). Tube length and its installation angle should be defined depending on the objective of study. Ohashi et al. [5] used 1.0m length tube and it was installed perpendicularly to soil surface in order to achieve 0.8m deep, hence providing four images each 0.2m depth. It is possible, however, to install the tube at 45° angle and laying it along planting line, as well as Santos et al. [8], which resulted in maximum depth of 0.6m and each image obtained represented a 0.15m depth soil layer when using 1.0m length tube. Both studies installed the tube at 0.2m from the sugarcane planting line. Planting lines were single and 1.5m apart from each other. Ohashi et al. [5] and Santos et al. [8] evaluated sugarcane root system in irrigated area using subsurface drip irrigation system, applying water, fertigation and sewage, respectively
Camargo [9] used minirhizotron method in different planting layout and in rainfed cultivation, which was based on dual planting lines, 0.9m apart from each other. Dual planting lines were apart from another by 1.5m from the center of dual lines. Camargo [9] installed the 1.0m length tubes vertically, as well as Ohashi et al. [5], but in the mid-distance inside the dual planting lines (Figure 1).
The drill to open the bore in soil for tube installation should have a diameter slightly smaller than tube diameter, approximately 0.005m smaller. The bore can be done by a specific auger, as used by Ohashi et al. [5], or using a drill coupled to internal combustion engine, as used by Santos et al. [8]. It is important that the bore diameter after drilling be as close as possible the tube diameter and uniform in all depth. The CI-600 In-situ Root Imager®* has an optical reach of 0.008m away from external tube surface. Thus, if soil profile is more than 0.008m of distance from tube external side it will not be possible to high-quality root images.
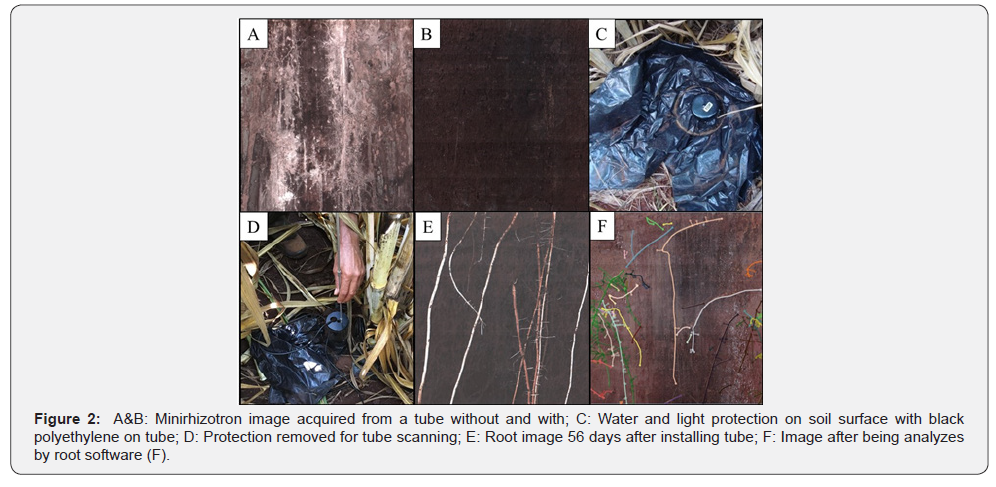
After installed, the tube must be protected at the soil surface and covered in order to prevent light and water to enter inside the tube and between tube surface and soil profile (Figure 2A and 2B). Ohashi et al. [5] and Santos et al. [8] used black polyethylene to cover tube (Figure 2C and 2D) and it successfully prevented from water and light interference on root image (Figure 2B, 2E and 2F).
Root Scanning and Data Analysis
Sugarcane roots grows relatively fast after tube installation. Ohashi et al. [5] observed root growth in the first 30 days after installing tubes in sugarcane second ratoon, although the highest growth rate was observed between 38 and 58 days. Our data regarding first ratoon shows root rapid growth during first 30 days (Figure 3). Thus, it is suggested to acquire root imager at higher frequencies right after installing the tubes, and then image acquisition can be spaced over time along crop cycle.
Camargo [9] monitored root growth along three crop cycles, from plant-cane up to second ratoon and observed that each growing season had different root growth patterns considering higher growth rate. This information shows how dynamic root growth is and how important is to monitor along different crop cycles.
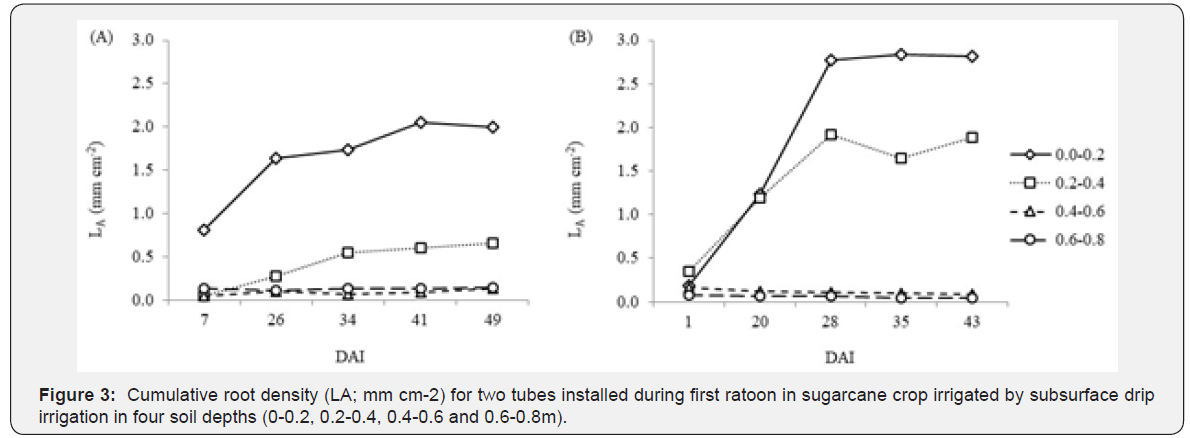
Root analysis are usually made by using an image software. Concerning modern minirhizotrons, the RootSnap!®** software has been used, especially when images were acquired using CI- 600 In-situ Root Imager. It was used by Ohashi et al. [5], Santos et al. [8] and Camargo [9]. Data showed herein (Figure 3) was also analyzed using this software.
Data analysis using RootSnap!®**, however, can become labor intensive depending on the image quality, the amount of roots to be measured (Figure 2F) and the number of images to be analyzed. It is important when designing root experiment to consider having at least three replications in order to reduce the usually high variation coefficient which is observed in root data [10].
Conclusion
Crops root information is necessary in modern agriculture to provide information that promotes water and nutrient use efficiency. It is important to consider that root system depends on different aspects considering the genetic material, cultural practices adopted and physical, chemical and biological of soil and including the climate and water availability. It is also important to evaluate for different cultivars, climate, soil and adopted cultural practices. Information presented herein can improve minirhizotron use by providing information concerning methodology highlighting critical aspects to obtain good results. Among critical points, the tube installation, tube protection, scanning-schedule and data analysis are addressed. Our information could help future users to acquire high-quality data with proper efficiency.
Acknowledgement
This study was supported by grants from São Paulo Research Foundation (FAPESP), grant #2011/16174-2, and FAPESP/ Microsoft Research Connections – BIOEN, grant #2010/52139-4.
Observations
*Use of this equipment does not reflect a recommendation by the authors. **Use of this software does not reflect a recommendation by the authors.
References
- Smith DM, Inman-Bamber NG, Thorburn PJ (2005) Growth and function of the sugarcane root system. Field Crops Research 92(2-3): 169-183.
- Battie-Laclau P, Laclau JP (2009) Growth of the whole root system for a plant crop of sugarcane under rainfed and irrigated environments in Brazil. Field Crops Research 114(3): 351-360.
- Munoz-Romero V, Benítez-Vega J, López-Bellido L, López-Bellido RJ (2010) Monitoring wheat root development in a rainfed vertisol: Tillage effect. European Journal of Agronomy 33(3): 182-187.
- Kirkham MB, Grecu SJ, Kanemasu ET (1998) Comparison of minirhizotrons and the soil-water-depletion method to determine maize and soybean root length and depth. European Journal of Agronomy 8(1-2): 117-125.
- Ohashi AYP, Pires RCDM, Ribeiro RV, Silva ALBDO (2015) Root growth and distribution in sugarcane cultivars fertigated by a subsurface drip system. Bragantia 74(2): 131-138.
- Tierney GL, Fahey TJ (2002) Fine root turnover in a northern hardwood forest: a direct comparison of the radiocarbon and minirhizotron methods. Canadian journal of forest research 32(9): 1692-1697.
- Liedgens M, Richner W (2001) Minirhizotron observations of the spatial distribution of the maize root system. Agronomy Journal 93(5): 1097-1104.
- Santos LNS, Barbosa EAA, Nazário AA, Gonçalves IZ, Ohashi AYP, et al. (2017) Root growth of sugarcane irrigated with wastewater through subsurface drip system. Agricultural Engineering International: CIGR Journal 19(1): 16-25.
- Camargo L (2018) Influência de preparos do solo no sistema radicular da cana-de-acú Masters dissertation. Agronomic Institute, Campinas.
- Vasconcelos ACM, Casagrande AA, Perecin D, Jorge LAC, Landell MGA (2003) Avaliação do sistema radicular da cana-de-açúcar por diferentes métodos. Revista Brasileira de Ciência do Solo 27(5): 849-858.






























