Assessment of Growth, Lipid Peroxidation and Reactive Oxygen Species Scavenging Capacity of Ten Elite Cassava Cultivars Subjected to Heat Stress
Jelili Titilola Opabode*, Omolara Rukayat Ibrahim and Adekemi Francisca Dosumu
Department of Crop Production and Protection, Obafemi Awolowo University, Nigeria
Submission: March 12, 2019; Published: April 04, 2019
*Corresponding author: Jelili Titilola Opabode, Crop Production and Protection Department, Obafemi Awolowo University, Ile-Ife, Nigeria
How to cite this article: Jelili T O, Omolara R I, Adekemi F D. Assessment of Growth, Lipid Peroxidation and Reactive Oxygen Species Scavenging Capacity of Ten Elite Cassava Cultivars Subjected to Heat Stress. Agri Res& Tech: Open Access J. 2019; 21(1): 556151. DOI: 10.19080/ARTOAJ.2019.21.556151
Abstract
Background and objective: Heat stress is a serious threat to cassava growth, development and yield as a result of climate change. The objective of this study was to assess growth, lipid peroxidation and reactive oxygen species removing ability of ten commercial cultivars of cassava.
Methods and materials: Pot-grown, four-week old plants of ten cultivars were subjected to 40 ˚C for 30 minutes using Randomized complete-block design with three replicates. Shoot height, leaf area, number of leaves, number of root and dry weight were recorded four weeks after the heat stress. Lipid peroxidation, proline, phenolic, hydrogen peroxide and activity of ascorbate peroxidase, catalase, peroxidase and superoxide peroxidase were measured a day after heat treatment.
Results: Analysis of variance showed that cultivars differed for all growth traits at 1% level of probability. All cultivars increased their shoot height, number of leaves, leaf area, root number and dry weight at different rates after exposure to heat stress. Physiological responses of the cultivars differed to the heat stress. The highest lipid peroxidation was observed in TMS 4 (2) 1425, TMS 97/3200, TMS 91/02324, TMS 98/0505 and TMS 98/0510 and least in TME 419 and TME 12. Hydrogen peroxide production was highest in TMS 98/0505 and TMS 98/0510 and lowest in TME 12. Cultivars TME 419, TME 12, UMUCASS 36, UMUCASS 37 and UMUCASS 38 had higher ascorbate peroxidase and superoxide dismutase activities than remaining cultivars
Conclusion: All cultivars showed heat tolerance ability and capacity to scavenge reactive oxygen species. However, TME 419 had outstanding ability for heat tolerance.
Keywords: Antioxidants; Heat stress; Manihot esculenta; Physiological responses; Reactive oxygen species
Abbreviations: SDG: Sustainable Development Goals; TBARS: Thiobarbituric Acid Reactive Substances; SOD: Superoxide Dismutase; CAT: Catalase; POX: Peroxidases; GR: Glutathione Reductase; APX: Ascorbate Peroxidase; ROS: Reactive Oxygen Species
Introduction
Cassava is an important source of energy-giving food in the developing countries [1]. Cassava productivity is stable and reliable, making the crop a candidate for reducing food insecurity, hunger and poverty in developing countries [2]. Under normal growth conditions, the crop gives high tuber yield and when the growth conditions are sub-optimal, cassava tuber yield is satisfactory [1]. For these reasons, cassava production in developing countries is expanding, a situation that makes the crop suitable for meeting Sustainable Development Goals (SDG) [2]. However, empirical evidence from climate change studies suggested that most cassava production areas would experience global warming and temperature extremes [3]. Indeed, heat wave (high temperatures) has been reported during growing period of cassava in Africa, Asia and Latin America [3]. As a warm temperate crop, cassava has best shoot and root growth and development at 25-32 ˚C [2]. Tempera tures above the normal optimum are sensed as heat stress. Heat stress upsets cellular equilibrium and lead to severe retardation of growth and development, and even result in plant death [4]. One of the physiological damages of oxidative stress caused by heat stress is lipid peroxidation. Peroxidation results in the breakdown of lipids and membrane functions by causing loss of fluidity, lipid cross-linking, and inactivation of membrane enzymes [5]. The extent of lipid peroxidation can be evaluated by measuring thiobarbituric acid reactive substances (TBARS) content, which is a secondary breakdown product of lipid peroxidation [6]. Hydrogen peroxide is the product of the first detoxification process of superoxide radical by SOD before scavenging by CAT and other peroxidases. Hydrogen peroxide production invariably measures ROS scavenging ability of plants under heat stress. Environmental stresses such as heat stress induce the accumulation of proline in many plant species [4]. Proline plays a role in cellular osmoregulation and also exhibits many protective effects; plants with elevated proline levels were reported to exhibit enhanced tolerance to abiotic stresses [7]. Levels of proline can be increased either by stimulation of its biosynthesis by 1-pyrroline-5-carboxylate synthetase(s) (P5CS) or by inhibition of its degradation by proline dehydrogenases [7].
Heat stress triggered an upsurge in production of reactive oxygen species (ROS) such as superoxide radical (O2 −), singlet oxygen (1O2), hydrogen peroxide (H2O2) and hydroxyl radicals (OH•) [8]. The ROS are produced from different sources in plants. Heat stress causes ROS production in chloroplasts and mitochondria by disturbing membrane stability and biochemical reactions such as the activity of ribulose-1,5-bisphosphate carboxylase/oxygenase [9]. In addition, ROS are produced in mitochondria from membrane instability, resulting in photorespiration and enzymes involved in cellular respiration such as complex I and III in the mitochondrial electron transport chain [10]. Furthermore, ROS are produced from NADPH oxidases (NOX) in the plasma membrane, amine oxidase in the apoplast and xanthine oxidase in peroxisomes, which are all induced by environmental stimuli including heat [11,12]. Excessive production of ROS under heat stress damages plant cells and tissues permanently by oxidation of cellular components such as lipids, proteins and DNA [13]. To remove excessive ROS, plants have developed detoxifying enzymes such as superoxide dismutase (SOD), catalase (CAT), peroxidases (POX), glutathione reductase (GR), ascorbate peroxidase (APX), and non-enzymatic antioxidants such as ascorbate, glutathione, carotene and tocopherols [13,14]. Apart from their destructive effects in cells, ROS can also act as signaling molecules in many biological processes such as stomatal closure, growth, development, and stress signaling [15]. Due to this dual role of ROS, plants are able to fine-tune their concentrations between certain thresholds by means of production and scavenging mechanisms. Since this ROS homeostasis is disrupted under stress in favour of production, constitutive and induced enzymatic antioxidant defenses are considered a crucial component of plant stress tolerance [8,14].
Physiological, antioxidant defence capacity and molecular responses of cassava to drought stress have been reported [16- 18]. In the same vein, the antioxidant defence capacities of wheat [19], rice [20], maize [20] have been investigated in response to heat stress. However, responses and antioxidant defence capacity of cassava to heat stress has not been reported. Equally, genetic improvement of cassava for heat tolerance has not been given adequate research attention. The objective of this study was to assess growth, lipid peroxidation and reactive oxygen species scavenging ability of ten commercial cultivars of cassava.
Materials and Methods
Planting materials and growth conditions
Stem cuttings of cassava cultivars TMS 4 (2) 1425, TMS 97/3200, TMS 91/02324, TMS 98/0505, TMS 98/0510 TME 419, TME 12, UMUCASS 36, UMUCASS 37 and UMUCASS 38. were obtained from the International Institute of Tropical Agriculture (IITA), Ibadan. A stem cutting (10cm long), with more than two nodes, was planted per plastic pot containing 8 kg sterilized sandy loam soil with: рH оf 7.2 аnd саtiоn еxсhаngе сарасitу оf 15.3 сmоlkg-1. Daily, each plant was irrigated manually with 600mL to water holding capacity bу tар wаtеr, рH 6.8. Plants were grown at an average temperature of 26±2 ˚C under 65±5% relative humidity and 7-9 hours of daylight before and after heat treatment.
Heat treatment and experimental design
Four weeks after planting, temperature was raised from 26 ˚C and maintained at 40 ˚C for 30 minutes. The experimental design was randomized complete-block in three replications. Fifteen uniform plants were used per cultivar.
Measurement of growth parameters
At four weeks after planting and before heat treatment, number of leaf, ѕhооt hеight, leaf аrеа, number of root and drу wеight (biomass) of plants were dеtеrminеd. This was repeated four weeks after the heat treatment and the differences recorded as growth after exposure to heat stress. For drу wеight, plants were carefully removed to obtain intact roots. Adhering soil particles on roots were removed by dipping them in water before dried in аn оvеn at 80 ˚C to a constant weight. Lеаf area was mеаѕurеd by a leaf area meter.
Lеаf рrоlinе соntеnt
To examine the osmotic adjustment of plants, proline content of the third fully expanded leaf from the top was determined according to Bates et al. [21] 24 hours after heat treatment. Leaf tissues (3g) were extracted in 2ml of sulphosalicylic acid. The same volume of ninhydrin solution and glacial acetic acid was added. The samples were heated at 100 ˚C for 10 minutes, cooled in an ice bath and 5 ml of toluene was added. At 528 nm, absorbance by toluene was measured.
Phеnоliсѕ соntеnt
The method of Julkunen-Titto [22] was used to determine leaf total phenolics content 24 hours after heat treatment. Briefly, fresh tissues (0.5g) of third fully expanded and matured leaves from shoot tip were ground in 80% acetone and the homogenized mixture collected. Thereafter, a mix of Folin-Ciocalteu reagent (1ml), water (2ml) and the supernatant (0.1ml) were homogenized and vigorously shook for 10 minutes. To the mix was added 5ml of Na- 2CO3 and the volume was brought to 10ml using distilled water. Absorbance was read at 750nm wavelength.
Antioxidant enzyme assays
Enzyme activities were assayed from the fourth fully expanded leaves from the shoot tip 24 hours after heat treatment. After washing with distilled water, leaf sample (0.5g) was ground in cold 0.1mol/l phosphate buffer (pH 7.5) containing 0.5mmol/l EDTA. The homogenized mixture was centrifuged at 4 ˚C for 15 minutes at 15,000 x g. The supernatant served as enzyme assay in this study.
Ascorbate peroxidase
Determination of activity of ascorbate peroxidase (APX) as outlined by Nakano et al. [23] was followed. The 3ml-reaction mixture contained 50mmol/l potassium phosphate (pH 7.0), 0.2mmol/l EDTA, 0.5mmol/l ascorbic acid, 2% H2O2 and 0.1ml of enzyme extact. For one minute, a drop-in absorbance at 290 nm was noted. Oxidation of ascorbate was calculated using the extinction coefficient Ɛ =2.8/mmol/l/cm. One unit of APX activity was defined as one mmol ascorbate oxidized /ml /min at 25 ˚C.
Superoxide dismutase
The method of Dhindsa and Dhindsa [24] was followed for determination of activity of superoxide dismutase (SOD). In this study, a unit of SOD was the enzyme extract that caused photo-reduction of a half of inhibition of nitro-blue tetrazolium and SOD activity expressed as unit/mg protein.
Catalase
Activity of catalase (CAT) was measured as described by Aebi [25]. A 3ml-reaction mixture containing 0.1ml enzyme extract, 50mmol. /l phosphate buffer (pH 7.0 and 30mmol/l hydrogen peroxide was conducted. Activity of CAT was determined by recording absorbance of hydrogen peroxide at 240nm.
Peroxidase
The method of Hemeda and Klein [26] was used to determine activity of реrоxidase (POD) in a reaction mixture that contained enzyme extract, 0.05% guaiacol, 25mmol/l phosphate buffer (pH 7.0), 10mmol/l hydrogen proxide. The POD activity was determined by absorbance at 470nm [ε = 26.6/(mmol/l cm).
Statistical analysis
A one-way analysis of variance was performed on data to determine significance of the treatment effect using Statistical Analysis Systems 9.1.3. At 5% level of probability, treatment means were separated by Ducan’s Multiple Range Test.
Results
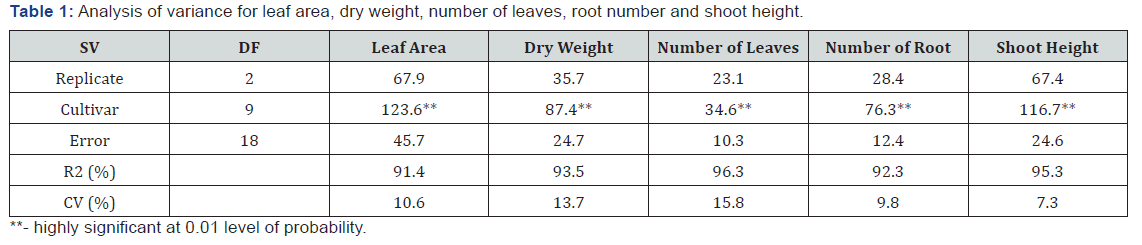
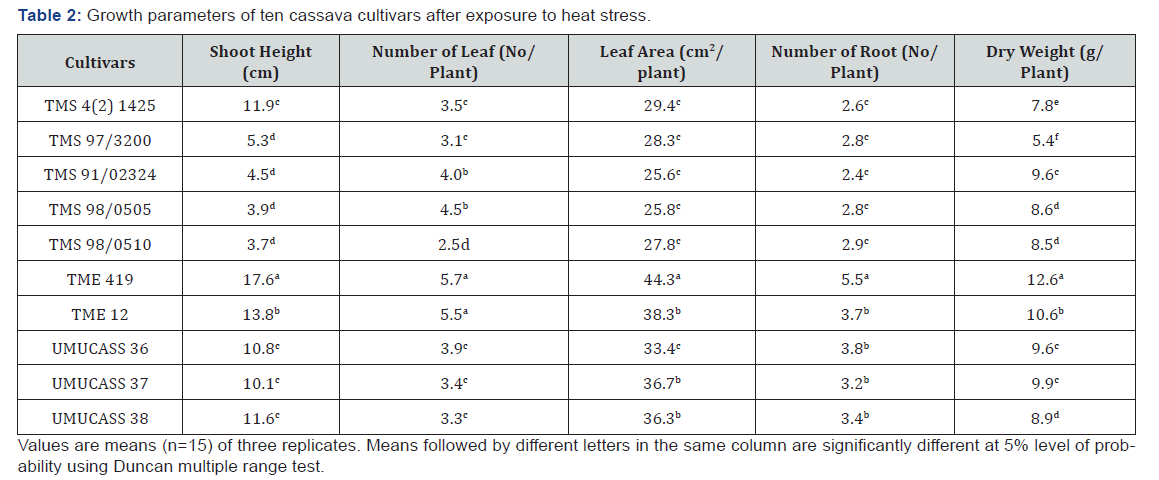
Analysis of variance showed that cultivars differed for all growth traits at 1% level of probability (Table 1). The R2 ranged from 91.4 to 96.3%, while coefficient of variation ranged between 7.3 and 15.8%. All cultivars increased their shoot height, number of leaf, leaf area, root number and dry weight after exposure to heat stress (Table 2). Shoot height ranged from 17.6cm in TME 419 and 3.7cm in TMS 98/0510. The highest shoot (17.6cm) was more than triple the least shoot heights (10.1-11.9cm) observed in TMS 98/0510, TMS 98/0505, TMS 91/02324 and TMS 97/3200. Leave production was ranged from 5.7 per plant in TME 419 and TME 12 to 2.5 per plant in TMS 98/0510 (Table 2). Leaf area varied from 44.3 to 25.6cm2/plant the highest leaf area was observed in TME 419, followed by UMUCASS 37, UMUCASS 38 and TME 419. The number of roots ranged from 5.5 per plant in TME 419 to 2.6 per plant in TMS 4 (2) 1425, TMS 97/3200, TMS 91/02324, TMS 98/0505 and TMS 98/0510. Similarly, TME 419 recorded highest (12.6g per plant) dry weight but TMS 97/3200 had the lowest (Table 2).
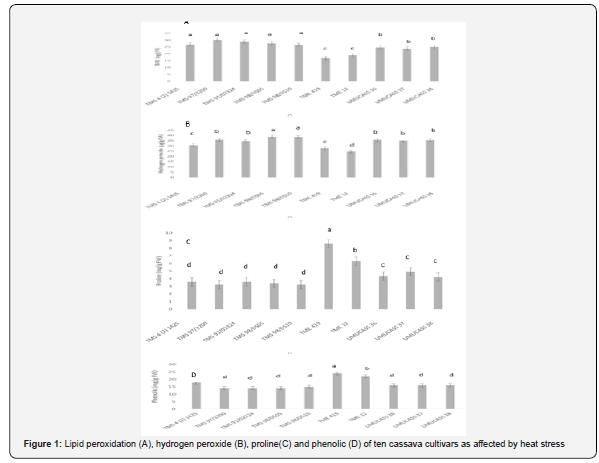
Analysis of variance showed that cultivars differed for all physiological traits measured at 1% level of probability (Table 3). The R2 ranged from 85.4 to 95.1%, while coefficient of variation ranged between 12.3 and 23.1%. Lipid peroxidation ranged from 30.1 to 16.7mg/g of TBARS. The highest lipid peroxidation was observed in TMS 4 (2) 1425, TMS 97/3200, TMS 91/02324, TMS 98/0505 and TMS 98/0510 and least in TME 419 and TME 12 (Figure 1). Hydrogen peroxide production ranged from 24.6 to 38.5μg/g. Hydrogen peroxide production was highest in TMS 98/0505 and TMS 98/0510 and lowest in TME 12. Among the cultivars, proline content ranged 3.2 to 8.6mg/g while phenolic ranged from 14.0 to 24.0mg/g. While the highest proline and phenolic were produced by TME 419, the lowest proline was recorded in TMS 4 (2) 1425, TMS 97/3200, TMS 91/02324, TMS 98/0505 and TMS 98/0510 and lowest phenolic was found in TMS 97/3200, TMS 91/02324, TMS 98/0505, TMS 98/0510, UMUCASS 36, UMUCASS 37 and UMUCASS 38 (Figure 1).
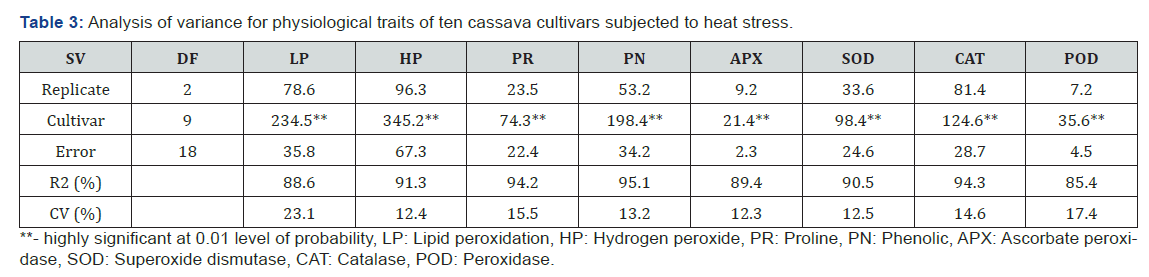
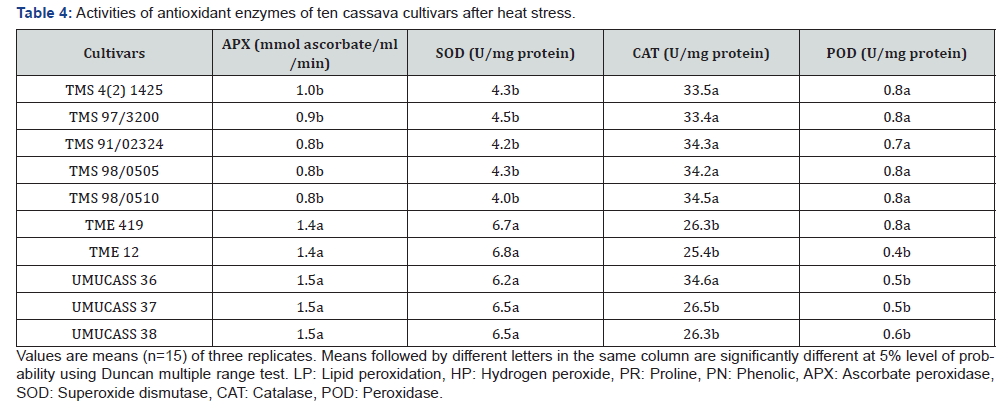
Activity of ascorbate peroxidase ranged between 0.8 and 1.5mmol ascorbate/ ml/min while the activity of superoxide dismutase ranged between 4.0–6.7 unit/mg protein. TME 419, TME 12, UMUCASS 36, UMUCASS 37 and UMUCASS 38 had higher ascorbate peroxidase and superoxide dismutase activities than remaining cultivars (Table 4). In this study, catalase and peroxidase activities ranged from 25.4-34.3 unit/mg protein and 0.4-0.8 unit/mg protein, respectively. However, catalase activity of TMS 4 (2) 1425, TMS 97/3200 TMS 91/02324 TMS 98/0505, UMUCASS 36 and TMS 98/0510 was higher than that of TME 419, TME 12, UMUCASS 37 and UMUCASS 38. Cultivars were grouped into two by peroxidase (POD) activity: the POD activity of the first group (TMS 4 (2) 1425, TMS 97/3200, TMS 91/02324, TMS 98/0505, TMS 98/0510 and TME 419) doubled POD activity of the second group (TME 12, UMUCASS 36, UMUCASS 37 and UMUCASS 38, Table 4).
Discussion
Like other crops, cassava plants experience heat stress on the field at all stages of its life cycle. Heat stress is exacerbated by climate change and long growth cycle of cassava [2,3]. Heat stress elicits molecular reactions in plants which triggers sequences of physiological responses that manifested in morphological alterations and adjustments [3]. In this study, it is noteworthy that exposure of cassava young plants to high temperature (heat) did not lead to total loss of growth in the ten cultivars investigated. Rather, cultivars displayed varying degree of adjustments of morphological traits as observed in shoot height, leaf area, root formation and dry matter accumulation as allowed by their genetic constituent. This implying that heat stress may not markedly reduce cassava productivity of these popular cassava cultivars in Africa as the plant may possess functioning heat tolerance mechanism. Variability in growth responses among cassava cultivars observed in this study agrees with previous report on morphological response to heat stress wheat, maize and rice [19,20]. For instance, after heat stress, cultivar 84-S had relative growth of 0.97g/g/day whereas M-503 had relative growth of 0.101 g/g/day in cotton [27].
Plants have developed several heat stress adaptive responses. In the present study, rapid increase in shoot height, to provide certain physiological and metabolic advantages, could be heat stress adaptive mechanism by TME 419 which is not present in other cultivars. Adjustment in leaf formation is a vital stress-adaptive mechanism in cassava to maintain metabolic processes. In the present study, all cultivars continued production of leaves at varying degree after exposure to heat stress indicating leaf formation cessation was not caused by heat stress in the cultivars. However, four cultivar displayed outstanding leaf production under heat stress suggesting their tolerance to heat stress. Furthermore, roots are essential organ of plants providing anchorage and extracting water and nutrients for plants. All cultivars retain their roots following exposure to heat stress indicating water and nutrient absorption might not be severely disrupted under heat stress in cassava. No plant was lost to heat stress. Dry matter accumulation of most cultivars was impressive, suggesting cassava has heat tolerance mechanism that allows dry matter production under heat stress.
Our data suggested that heat stress caused damage to lipids in cassava at varying magnitude across cultivars. Lipid oxidation by reactive oxygen species has been established to be produced by heat stress. For example, lipid peroxidation in cultivar 84-5 increased by 79.9% by heat stress [27]. Limited lipid peroxidation displayed in TME 419 and TME 12 could have resulted from low quantity of ROS generated by the cultivars or destruction of ROS by antioxidant enzymes. In addition, all cultivars produced hydrogen peroxide, an ROS generated by heat stress indicating negative metabolic machinery of the plants which must be removed to prevent damage of proteins, lipids and DNA. Limited amount of hydrogen peroxide observed in three cultivars (TMS 4 (2) 1425, TME 419, TME 12) indicated that the cultivars have capacity to remove hydrogen peroxide and thus tolerance of heat stress. While heat stress increased hydrogen peroxide production by 50.0% in drought-sensitive cultivar in cotton, heat stress has no effect on hydrogen peroxide release drount-tolerant cultivar [27].
Proline (av. 5mg/g) was detected in all cultivars after exposure to heat stress. Gathering of proline to high concentration is one of the early physiological reactions of plants experiencing abiotic stress to amelioriate its negative effects. After six hours of heat stress, high content (2.8-3.9pmol/g FW) of proline was observed in lower leaves of wild and transgenic tobacco [28]. Drought-sensitive cotton cultivar 84-S increased proline content by 5.9% after exposure to heat stress [27]. We suggest that TME 419 and TME 12 that recorded outstanding quantity (6-7mg/g) of proline to be exhibiting heat tolerance. Phenolics are produced by plants mainly for protection against biotic and abiotic stresses. All cultivars produced high amount of phenolic suggesting that they were capable of protecting themselves against adverse effects of heat stress.
Our results showed that APX, SOD, POD and CAT were active in all cultivars subjected to heat stress in this study. Heat stress has no effect on CAT activity in cotton [27]. Heat stress decreased POD activity in cotton.by 41.3%. APX activity increased by heat stress in some cultivar of cotton while heat stress had no effect on APX in other cultivars of cotton. This is important because toxicity of ROS to plants necessitated their immediate removal before destroying cellular components [29]. Thе ROS аrе rеmоvеd bу these аntiоxidаnt еnzуmеѕ which findings have suggested are involved in stress tolerance in plants.
Conflict of Interest
The authors declare that they have no conflict of interest.
Acknowledgement
The authors thank International Institute of Tropical Agriculture (IITA), Ibadan for supplying stem cuttings of the ten cassava cultivars.
References
- Ospina B, Ceballos H (2012) Cassava in the third millennium: modern production, processing, use and marketing systems. Published by CIAT/CLAYUCA/CTA, pp. 574.
- Opabode JT, Adesina GO (2013) Farming in Nigeria: agricultural development and applicable technology in Africa. Zhejiang Science and Technology Publishing House, Hangzhou. p. 95.
- IPCC (2007) Climate change 2007: the physical science basis. In: Contribution of Working Group I to the Forth Assessment Report of the Intergovernmental Pane on Climate Change. Cambridge University Press, Cambridge, UK, pp. 1009.
- Kotak S, Larkindale J, Lee U, von Koskull-Doring P, Vierling E, et al. (2007) Complexity of heat stress response in plants, Curr Opin Plant Biol 10(3): 310-316.
- Girotti AW (1990) Photodynamic lipid peroxidation in biological systems. Photochem Photobiol 51(4): 497-509.
- Halliwel B, Gutteridge JMC (1989) Lipid peroxidation: a radical chain reaction. In: Halliwell B, Gutteridge JMC (Eds.), Free Radicals in Biology and Medicine. Clarendon Press, Oxford, pp. 188-260.
- Kishor PB, Sangam S, Amrutha RN, Sri Laxmi P, Naidu KR, et al. (2005) Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: its implication in plant growth and abiotic stress tolerance, Curr Sci 88(3): 424-438.
- Miller G, Shulaev V, Mittler R (2008) Reactive oxygen signaling and abiotic stress. Physiol Plant 133(3): 481-489.
- Sharkey TD (2005) Effects of moderate heat stress on photosynthesis: importance of thylakoid reactions, rubisco deactivation, reactive oxygen species, and thermotolerance provided by isoprene. Plant Cell Environ 28(3): 269-277.
- Jaspers P, Kangasjarvi J (2010) Reactive oxygen species in abiotic stress signalling. Physiol Plant 138(4): 405-413.
- Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, et al. (2011) ROS signalling: the new wave? Trends Plant Sci 16(6): 300-309.
- Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48(12): 909-930.
- Suzuki N, Koussevitzky S, Mittler R, Miller G (2012) ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ 35(2): 259-270.
- Demiral T, Turkan I, Sekmen AH (2011) Signalling strategies during drought and salinity, recent news. Adv Bot Res 57: 293-317.
- Vandegeer R, Miller RE, Bain M, Gleadow MR, Cavagnaro TR (2012) Drought adversely affects tuber development and nutritional quality of the staple crop cassava (Manihot esculenta Crantz). Funct Plant Biol 40(2): 195-200.
- Turyagyenda LF, Kizito EB, Ferguson M, Baguma Y, Agaba MIJW, et al. (2013) Physiological and molecular characterization of drought responses and identification of candidate tolerance genes in cassava. AoB Plants 5: 7-24.
- Jolayemi OL, Opabode JT (2018) Responses of cassava (Manihot esculenta Crantz) varieties to in vitro mannitol-induced drought stress. J Crop Improvement 32: 566-578.
- Sairam RK, Srivastava GC, Saxena DC (2000) Increased antioxidant activity under elevated temperatures: a mechanism of heat stress tolerance in wheat genotypes. Biol Plantarum 43(2): 245-251.
- Kumar S, Gupta D, Nayyar H (2012) Comparative response of maize and rice genotypes to heat stress: status of oxidative stress and antioxidants. Acta Physiol Plant 34(1): 75-86.
- Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant and Soil 39(1): 205-207.
- Julkunen-Titto R (1985) Phenolic constituents in the leaves of northern willows: methods for the analysis of certain phenolics. J Agric Food Chem 33(2): 213-217.
- Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22(5): 867-880.
- Dhindsa RS, Dhindsa PP, Thorpe TA (1980) Leaf senescence correlated with increased levels of membrane permeability and lipid-peroxidation and decreased levels of superoxide dismutase and catalase. J Exp Botany 32(1): 93-101.
- Aebi H (1984) Catalase in vitro. Methods Enzym 105: 121-126.
- Hemeda HM, Klein BP (1990) Effects of naturally occurring antioxidants on peroxidase activity of vegetable extracts. J Food Science 55(1): 184-185.
- Sekmen AY, Ozgur R, Uzilday B, Turkan I (2014) Reactive oxygen species scavenging capacities of cotton (Gossypium hirsutum) cultivars under combined drought and heat induced oxidative stress. Environ Exp Botany 99: 141-149.
- Cvikrováa M, Gemperlováa L, Dobráa J, Martincováa O, Prásil IT, et al. (2012) Effect of heat stress on polyamine metabolism in proline- over-producing tobacco plants. Plant Sci 182: 49-58.
- Foyer CH, Noctor G (2012) Managing the cellular redox hub in photosynthetic organisms. Plant Cell Environ 35(2): 199-201.






























