In Vitro Culture Response and Regeneration of Plantlets in H. Sabdariffa
Raju Mondal1,2, Pranit Mukheerje1, Kanti Meena1, Sourav Datta1 and Asit B Mandal 1*
1Central Research Institute for Jute & Allied Fibres (CRIJAF), India
2Department of Botany, India
Submission: March 15, 2018; Published: April 23, 2018
*Corresponding author: Asit B Mandal, Central Research Institute for Jute & Allied Fibres (CRIJAF), Barrackpore, Kolkata 700120, West Bengal, India, Tel: 8348956686; Email: goo555@gmail.com
How to cite this article: Raju M, Pranit M, Kanti M, Sourav D, Asit B. In Vitro Culture Response and Regeneration of Plantlets in H. Sabdariffa Open Access J. 2018; 15(3): 555957. DOI: 10.19080/ARTOAJ.2018.14.555957
Abstract
Cotyledonary and hypocotyl explants of H. sabdariffa cultured on MS medium supplemented with 23 hormone combinations showed the former as the best explant for callus induction. Maximum callus proliferation (97.8%) was observed in var. HS 4288 on MS medium containing 2mg/l 2,4-D, 0.1mg/l NAA and 2mg/l Kinetin with shootlet regeneration on MS with 3.0 mg/l BA and 0.2mg/l IAA. Vigorous rooting was observed on hormone free MS medium.
Keywords: Mesta; H. sabdariffa; In vitro culture; Plantlet regeneration
Mini Review
Mesta is a lingo-cellulosic coarse bast fibre crop of family Malvaceae, grown as an orphan crop mostly on marginal lands. It grows best in well drained sandy loam soil under warm humid climate without excessive rainfall. Only two species are cultivated in India viz. H. sabdariffa (2n=36) and H. cannabinus (2n=72). Its fibre is used as blend to meet the requirements of textile and other ancillary industries. Besides fibre, it is extensively used as an input for paper pulp, seed oil for culinary purposes, calyces for preparation of jam, jellies and pickles. In vitro regeneration protocol and transgenic development in mesta have been reported [1-3], however non-reproducibility of plantlet regeneration is a serious constraint may be due to the genotype specificity or some unknown reasons, compelled us to research on In vitro plant regeneration from cotyledons for H. sabdariffa in our laboratory.
Healthy mature seeds of Hibiscus sabdariffa (var. HS 4288,AMV1 and GR 27) conserved in the Gene Bank, Division of Crop Improvement of Central Research for Jute and Allied Fibres, Nilgunj, Barrackpore were employed for the above study.
Seeds were rinsed thoroughly in tap water with 2 drops of Tween 20 (SRL, Cat. No. P9416) followed by dipping in fungicide Bavistin solution (2%w/v) for 15min. Under aseptic condition seeds were rinsed with sterile water (SW) followed by a single wash with 70% (v/v) ethanol for 30s. Seeds were immersed in0.1% freshly prepared HgCl2 (Sigma, Cat. No. 429724) solution for 10 min followed by sterile water washes (4 times). Those seeds were blot dried and germinated on autoclaved % strength hormone free Murashige-Skoog medium Murashige & Skoog [4] (MS), supplemented with vitamins of B5 and 2 % sucrose (Sigma, Cat. No. 84097), solidified with 0.8 % (w/v) Gelrite (Sigma, Cat. No. G1910). Cultures were incubated for 48 h in dark at 28±1 °C, followed by light under 16/8-h photoperiod for 7 days.
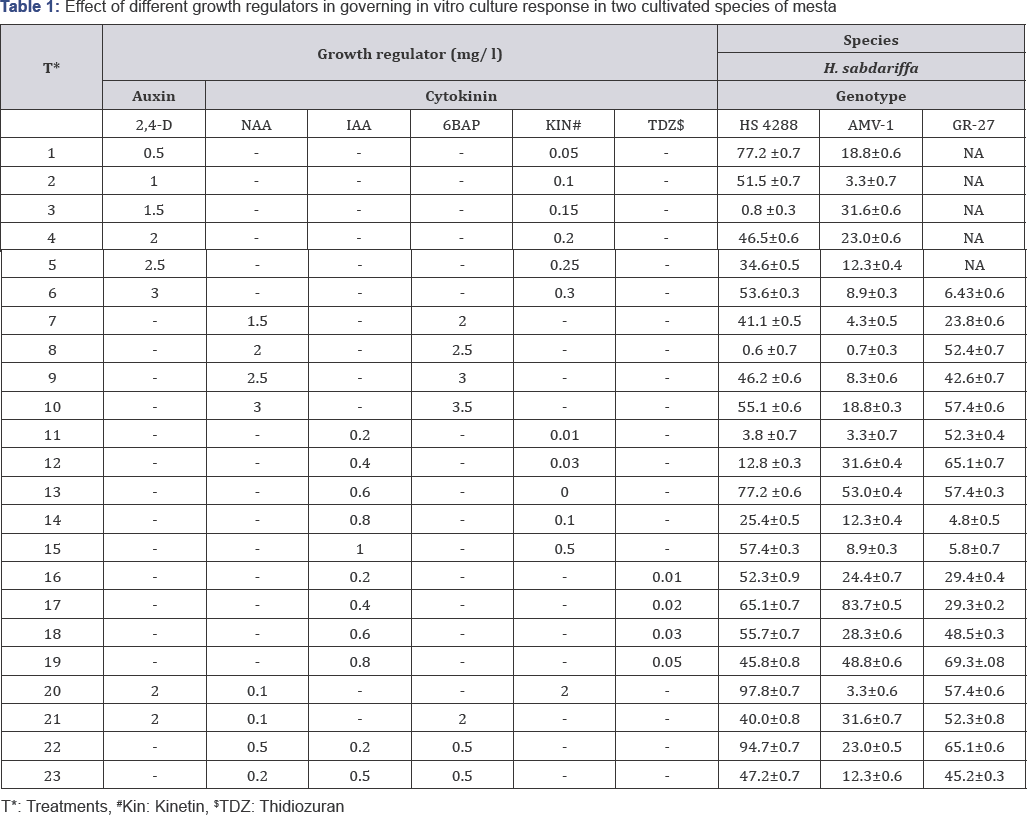
The explants (cotyledon and hypocotyl (1-2cm)) from 10-day old seedlings were cultured in radio sterile 90mm dia. petri plates (Tarson, Cat. No. 460091) containing callus induction medium (CIM) supplemented with 23 different hormone combinations (Table 1). Cultures were incubated in dark at 25±2 °C until callus induction was achieved. After 21 days of callus induction data on callus health (1-9 scale) (data not shown) was recorded. Among the 23 treatments only four combinations (T20 , T23, T and T7) responded well to callus induction (Table 1).
Healthy calli were sub culture (same CIM with % strength of hormone used for callus induction) for 3 weeks. The maximum callus proliferation (97.8 %) was observed in var. HS 4288 followed by 83.7% in var. AMV1 and 69.3% in GR 27 in H.sabdariffa. To the contrary, in H. cannabinus maximum callus proliferation (66.8%) was observed in var. MT 150 followed by 65.1% in var. JBM 2004 and 64.8% in var. HC 583 (data not shown).
Friable, green callus more or less globular or round shaped were considered as putative embryogenic calli, which were transferred onto regeneration medium containing MS medium supplemented with different concentrations of 1AA (0.2 to 0.5mg/l) and BA (2.0 to 5.0mg/l).
Substantial differential response was observed for shoot formation by enhancing the concentration of BAP, which was found to be reverse with 1AA. Only var. HS 4288 showed highest number of shootlets on MS containing 3.0mg/l with 0.2mg/l 1AA.
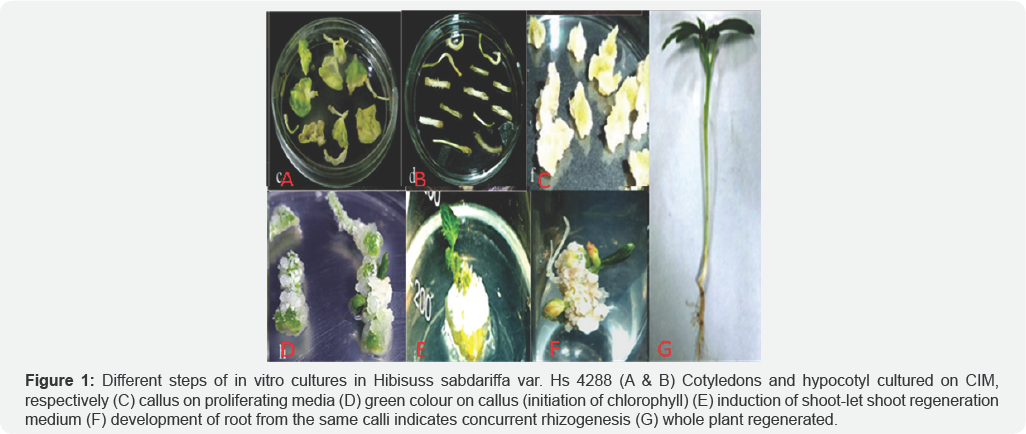
Individual shootlets (5-6cm) responded for rooting within 10 days after transfer to rooting medium (MS supplemented with 1.0mg/l NAA or 2mg/l IBA), however, root developed within 7 days only on hormone free MS medium. Theelongated shootlets with roots (8-10cm) were detached, washed and grown in Hoagland solution under culture room condition followed by transfer to soil, sand and vermiculite (1:1:1, v/v/v) mixture in glasshouse (Figure 1).
In vitro regeneration from H. sabdariffa (HS 4288) seemed to be more successful from10 day old cotyledons. The highest number of shootlets was found with 3.0mg/l BAP and 0.2mg/l IAA. None of the other two varieties or concentrations of hormone combinations responded positively. High number of roots was obtained on hormone free media. Both root as well as shoot initiation was observed from different locations of same calli, indicating high endogenous level of auxins in mesta, which is sufficient for root induction and there is no need of application of exogenous synthetic hormone in the medium for rooting.
Conclusion
In conclusion H. sabdariffa (var. HS 4288) was the best variety to be executed for In vitro culture and cotyledons as best explant. Shoot regeneration was best on MS medium with 3.0mg/l BAP and 0.2mg/l IAA and rooting on hormone free MS medium. However the efficiency of plantlet regeneration was found to be very low, which need to be enhanced further.
Acknowledgement
The authors express sincere thanks to Indian Council for Agriculture Research (ICAR) for providing facilities and fund to carry out this work.
References
- Samanthi PH, Takayuki S, Hattori K (2004) Multiple shoot regeneration from young shoots of Kenaf (Hibiscus cannabinus). Plant Cell Tiss Organ Cult 77(1): 49-53.
- Chen P, LiR, Zhou R, Zhao Y (2010) Direct shoot organogenesis and plant regeneration from cotyledonary node of kenaf (Hibiscus cannabinus L.). Afr J Biotechnol 9(50): 8693-8697.
- Ayadi R, Hamrouni L, Hanana M, Bouzid S, Trifi M, et al. (2011) In vitro propagation and regeneration of an industrial plant kenaf (Hibiscus cannabinus L.). Indus Crops Prod 33(2): 474-480.
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15: 473-479.






























