Fungal Organisms Isolated from Rotted White Yam (Dioscorea rotundata) Tubers and Antagonistic Potential of Trichoderma harzianum against Colletotrichum Species
Gwa VI1,2* and Ekefan EJ2
1Department of Crop Production and Protection, Federal University, Nigeria
2Department of Crop and Environmental Protection, Federal University of Agriculture, Nigeria
Submission: July 21, 2017; Published: August 24, 2017
*Corresponding author: Gwa VI, Department of Crop Production and Protection, Faculty of Agriculture and Agricultural Technology, Federal University, Nigeria, Email: igwa@fudutsinma.edu.ng
How to cite this article: Gwa VI, Ekefan EJ.Fungal Organisms Isolated from Rotted White Yam (Dioscorea rotundata) Tubers and Antagonistic Potential of Trichoderma harzianum against Colletotrichum Species. Agri Res & Tech: Open Access J. 2017; 10(3): 555787. DOI: 10.19080/ARTOAJ.2017.10.555787
Summary
Nine fungal organisms were isolated from rotted white yam (Dioscorea rotundata) tubers from Kadarko, Nasarawa, Nigeria between February and May, 2015. Trichoderma harzianum was used as a biological agent to evaluate its potential against Colletotrichum species isolated from rotted white yam tubers in dual culture method at Advanced Plant Pathology Laboratory, Federal University of Agriculture, Makurdi, Nigeria. Percentage frequency of occurrence of the isolated fungal organisms was determined at monthly interval. The test antagonist (T harzianum) was introduced at three different times (same time with Colletotrichum spp, two days before the inoculation of Colletotrichum spp and two days after the inoculation of Colletotrichum spp). The plates were incubated for 192 hours and measurements of mycelial radial growths were recorded at intervals of 24 hours beginning from the third day. The results of the in vitro interactions between T. harzianum and Colletotrichum spp revealed that T. harzianum was able to significantly (P≤0.05) inhibit the growth of Colletotrichum spp. Mean percentage growth inhibition was found to be highest (50.61%) when T. harzianum was introduced 2 days before inoculation of Colletotrichum spp followed by introduction of T. harzianum same with Colletotrichum spp (37.12%) with the least percentage growth inhibition (12.48%) recorded when the antagonist was introduced 2 days after inoculation of the pathogen. Minimum inhibition concentration (MIC) showed slightly effective to effective control indicating that the antagonist has the potential for biological control of Colletotrichum spp at all levels. It is therefore, recommended that the biological agent should be introduced prior to infection in order to be more effective in controlling the pathogenic fungus.
Keywords: Inoculation; Incubation; Inhibition; Effective; Biological agent
Introduction
Yam production is constrained by several factors including those caused by the genus Colletotrichum [1,2], causing about 90% loss in yield. The genus Colletotrichum was recently voted the eighth most important group of plant pathogenic fungi in the world, based on perceived scientific and economic importance [3].
The genus Colletotrichum includes a number of plant pathogens of major importance, causing diseases of a wide variety of woody and herbaceous plants. It has a primarily tropical and subtropical distribution, although there are some high-profile species affecting temperate crops [4].
The genus Colletotrichum affects the leaves, petioles, stems and veins of the plant and even tubers, causing leaf spots, leaf blotches, petiole blights, premature abscission, dieback and eventual death of the entire plant [1,2,5]. The disease usually has a dramatic effect on infected plants, converting a field of initially healthy yam plants from 'green' to 'black' within a few weeks [6]. Other fungal organisms commonly associated with yam rots include Aspergillus flavus, Aspergillus niger, Botryodiplodia theobromae, Colletotrichum spp, Fusarium oxysporum, Penicillium chrysogenum, Pennicillium digitatum [2,5,7,8]. Colletotrichum species are occasionally identified in yam tubers >[9] . Several methods of control of fungal organisms have been used. Chemical control of soil borne pathogens provides certain degree of control but at the same time have adverse effects on environment affecting the beneficial soil microorganisms[10].Therefore, biological control of plant pathogens has been considered as a potential control strategy in recent years and search for these biological agents is increasing. Trichoderma is the most commonly used fungal biological control agent and have long been known as effective antagonists against plant pathogenic fungi [11,12]. The possibility of applying Trichoderma harzianum as a biocontrol agent of Colletotrichum spp. causing yam dry rot in Nigeria has not been reported. This research describes experiments conducted to determine the effect of dual culture of introduced at different intervals on mycelia inhibition of Colletotrichum spp. isolated from rotted yam tubers in Nigeria.
Materials and Methods
Experimental site
The experiment was conducted at the Advanced Plant Pathology Laboratory, Federal University of Agriculture, Makurdi, Nigeria.
Source of T. harzianum isolate
T. harzianum was collected from yam Pathology Unit of University of Ibadan, Oyo State, Nigeria. Stock cultures of the isolate were maintained on slants of acidified potato dextrose agar (PDA) in McCartney bottles for subsequent studies.
Collection of rotted and healthy yam tubers
Deteriorated white yam tuber (Dioscorea rotundata) cultivars of Ghini and Hembankwase showing varying degrees of rots were collected from yam barns in Kadarko, Keana local government area of Nasarawa State, Nigeria, which lies between longitude 8° 30' and 8° 35' E, and on latitudes 8° 10' and 8° 14' N. The rotten yam tubers were packaged in sterile polyethylene bags and taken to the laboratory for isolation and identification of fungal organisms two days after collection. The healthy yam tubers were used for pathogenicity test. Test isolate in this study was Colletotrichum spp.
Preparation of Potato dextrose agar (PDA)
Potato dextrose Agar (PDA) was prepared according to manufacturer's recommendations by dissolving 39g of dehydrated PDA in 1 litre of distilled water and autoclaved at 121 °C for 15min [13] and medium was allowed to cool to 4550 °C. About 0.16g/l streptomycin sulphate powder was added to suppress bacterial contaminations [14]. 15ml of the molten PDA was poured into sterile 9cm glass Petri dishes and were allowed to cool at room temperature before inoculation of the test organisms.
Isolation and identification of fungi organisms
Deteriorated yam tubers were cut approximately 2 x 2mm with sterile scalpel from at the inter-phase between the healthy and rotted portions of the tubers. The cut pieces were first surface sterilized by dipping completely in a concentration of 5% sodium hypochlorite solution for 2min; and rinsed in four successive changes of sterile distilled water (SDW) [15]. Infected yam tissues were later picked onto sterile filter paper using a sterile forceps and then blotted with filter paper for 2-3 minutes in the laminar Air flow cabinet. The dried infected tissues were aseptically plated on Petri dishes containing acidified sterile potato dextrose agar (PDA) and the plates were incu-bated at ambient room temperature (30±5 °C) for 7 days. Fungi organisms were identified following sub-culture of growing fungus after 7 days of incubation [16]. The culture plates obtained were examined and spores were collected from distinct growths. This was inoculated onto sterile PDA plates and incubated. When pure cultures were obtained, the growth pattern was examined for uniformity. Microscopic examination and morphological characteristics were noted and compared with existing authorities [14,17].
Determination of frequency of occurrence of isolates
To determine the frequency of occurrence of the isolates, records of organisms isolated were kept on periodic basis. Since isolation and characterization were carried out at monthly interval, the number of times each fungi pathogen was isolated in a month was expressed as a percentage of the total of all the different organisms over the period [18], which was calculated as follows:

Where,
x = number of times of occurrence of the individual isolates over the period
n = total number of micro organisms isolated in the study over the period
Stock cultures of the isolates were maintained on slant of acidified potato dextrose agar (PDA) in McCartney bottles for subsequent studies.
Pathogenicity test
Some healthy yam tuber (Discorea rotundata) cultivars were washed under running tap water to remove soil (dirt). Surface contaminants were removed by dipping each yam tuber in 5% sodium hypochlorite solution for 2min and rinsed in four successive changes of sterile distilled water (SDW) [15]. A disc of five days old culture of the fungal isolates obtained from rotted yam tubers grown on PDA was the source of inoculum for the pathogenicity test. A five-millimeter diameter cork borer was used to remove 4mm tissue from the healthy D. rotundata tuber surfaces aseptically [19]. A five-millimeter diameter cork borer (Sterilized by dipping in alcohol followed by flaming) was used to cut plugs from the five day old cultures of Colletotrichum spp.to be tested. These fungal plugs were put in the holes created in the yam tubers. Petroleum jelly was used to seal the edges of the replaced yam tissues [12]. The same procedure was used for the control except that discs of uninoculated PDA were placed in the holes created in the tubers [20]. The inoculated yam tubers were placed at room temperature in a completely randomized design [21] for 14 days under sterile condition to determine rot. Rot was determined by using a sterilized and flamed knife to cut open the inoculated yam tubers from the point of inoculation.
Evaluation using dual culture on agar plates
The assay for antagonism was performed on Potato Dextrose Agar (PDA) on Petri dishes by the dual culture method [22]. The mycelial plugs (5mm diameter) of T harzianum used as antagonist and Colletotrichum spp. isolated from rotted yam tubers were both inoculated on the same dish with T. harzianum 6cm from Colletotrichum spp. T. harzianum was plated same time with the pathogen, two days before the inoculation of pathogen and two days after inoculation of pathogen on Petri dishes containing 15ml of PDA. Paired cultures were incubated at room temperature (30±5 °C) for 192 hours. In the control plates, a PDA plug was used instead of the antagonist against the pathogen. Treatments comprised times of introduction of antagonist which were replicated three times for each treatment and arranged in completely randomized design [21].
Measurement of radial mycelia growth
Measurement of radial mycelia growths of the T. harzianum and Colletotrichum spp. in dual culture and Colletotrichum spp. in control plates were done after three days of inoculation on a 24 hour interval beginning from the 72nd hour up to the 192nd hour of incubation at ambient room temperature (30± 5 0C). Percentage Growth Inhibition (PGI) of Colletotrichum spp. was calculated as described by [23].
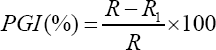
Where,
PGI = Percent Growth Inhibition
R = the distance (measured in mm) from the point of inoculation to the colony margin in control plate,
R1 = the distance of fungal growth from the point of inoculation to the colony margin in treated plate in the direction of the antagonist.
The percent growth inhibition was determined as a guide in selecting the minimum inhibition concentration (MIC) that will be effective in controlling the rot-causing fungus for the three treatments. Antagonist was also rated for inhibitory effects using a scale by Sangoyomi [24] as:
o ≤0% inhibition (not effective),
o >0-20% inhibition (slightly effective)
o >20-50% inhibition (moderately effective),
o >50-<100% inhibition (effective)
o 100% inhibition (highly effective)
Data analysis
Data collected were subjected to Analysis of variance (ANOVA) using GenStat Discovery Edition 12 for ANOVA and means separation, Minitab Release 17 for descriptive statistics and Graph Pad Prism 6 for trend graphs. Statistical F-tests were evaluated at P≤0.05. Differences among treatment means for each measured parameter were separated using Fisher's least significant difference (FLSD) [25].
Results
Sample collection, isolation and identification of Colletotrichum species


Fungi organisms were isolated and identified from the rotted yam tubers. Colletotrichuim spp. was one of the fungi organisms identified as causing dry rot of white yam (D. rotundata) tubers in the study area. Macroscopic examination of pure cultures of this fungus on PDA showed slow growth rate taking more than 7 days to fill the plates (Figure 1). The colour of Colletotrichum spp. was grey, to dark orange while the reverse side of the growth pattern was either circular with the mycelia showing a uniform growth pattern and radial in a ring. Acervuli were rounded or elongate, separate or confluent, superficial, erumpent, with conspicuous multicellular, darkly pigmented setae (Figure 2). Setae were brown with a dark swollen base and a pale rounded tip dark brown colour.
*indicates statistical significance at (P≤0.05)
Percentage frequency of occurrence of fungal isolates in Kadarko
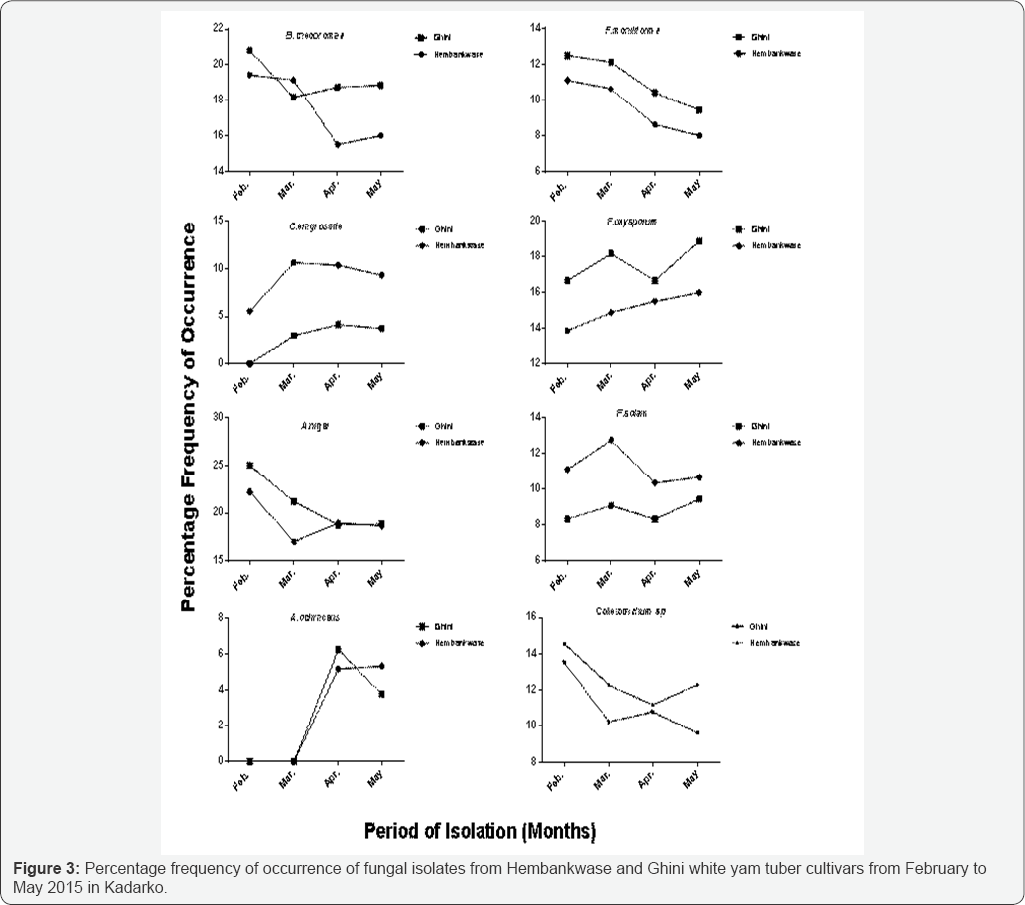
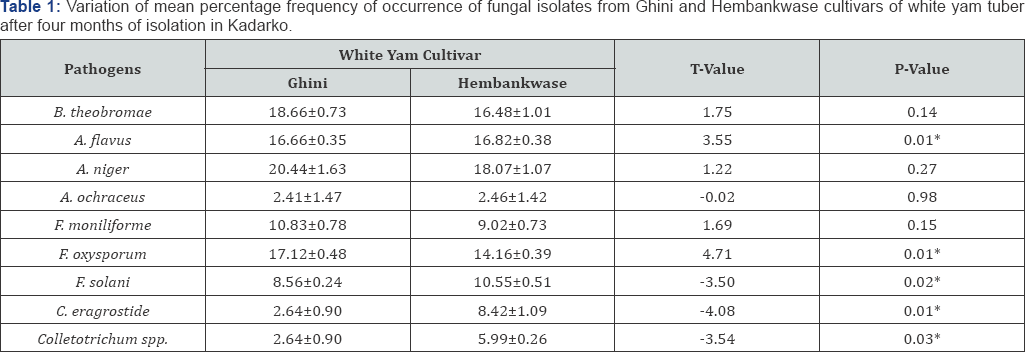
Figure 3 shows fungi organisms isolated and identified on rotted Hembankwase and Ghini cultivars of white yam tuber samples collected from farmers' barns in Kadarko, which is one of the yam producing settlements in Nasarawa State, Nigeria. The fungi organisms include Botryodiplodia theobromae, Aspergillus flavus, A. niger, A. ochraceus, Fusarium moniliforme, F oxysporum, F solani, Curvularia eragrostide and Colletotrichum spp. Percentage frequence of occurrence of A. ochraceus showed that it was same in Hembankwase and Ghini in February and March. The occurrence of the pathogen increased in Ghini compared with Hembankwase in April but decreased in Ghini compared with Hembankwase in May. The occurrence of A. niger in Hembankwase was less than in Ghini in February and March but recorded an increase in Hembankwase slightly above Ghini in April. The difference in frequency of occurrence was nearly the same in Ghini which rose slightly above Hembankwase in May. C. eragrostide, F. solani and Colletotrichum spp. occurred higher in Hembankwase compared with Ghini throughout the period of isolation. In F. oxysporum and F. moniliforme, the frequency was higher in Ghini compared with Hembankwase within the same period. B. theobromae showed the highest level of occurrence in Hembankwase than in Ghini in February, April and May while the pathogen displayed higher frequency of occurrence in Ghini only in March. Occurrence of A. flavus in Ghini and Hembakwase was the same in February but increased in Ghini far above Hembankwase in March and decreased in April and May but was still above Hembankwase. Mean percentage frequency of occurrence of B. theobromae, A. niger, A. ochraceus, F moniliforme, between Hembankwase and Ghini in Kadarko after four months of isolation showed no significant difference (P≤0.05) between the two cultivars. However, there were significant differences (P≤0.05) in mean percentage frequency of occurrence between Ghini and Hembankwase in A. flavus, F. oxysporum, F. solani, C eragrostide and Colletotrichum sp. after four months of isolation (Table 1).
Pathogenicity test
The results of the pathogenicity test of Colletotrichum spp is presented in Figure 4. The pathogenicity test shows that Colletotrichum spp induced rot in the healthy looking yam tubers after 14 days of inoculation. Symptoms of infections were seen on the inoculated yam tubers. The yam tubers that were not inoculated with the test fungi used as control experiments however, did not show any sign of rot indicating absence of reproductive propagules in the bored yam tissues (Figure 5)


Evaluation using dual culture method on agar plates
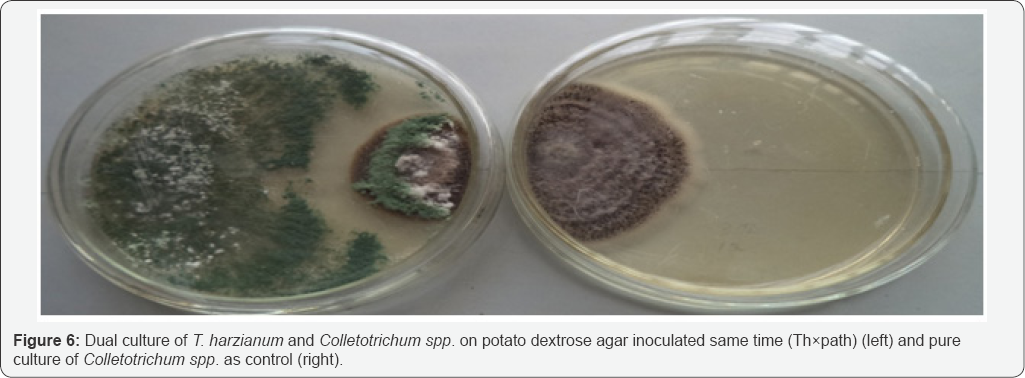
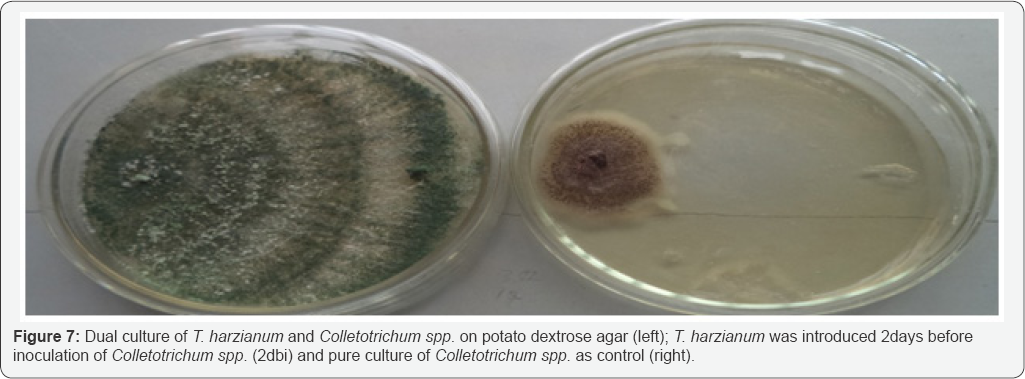
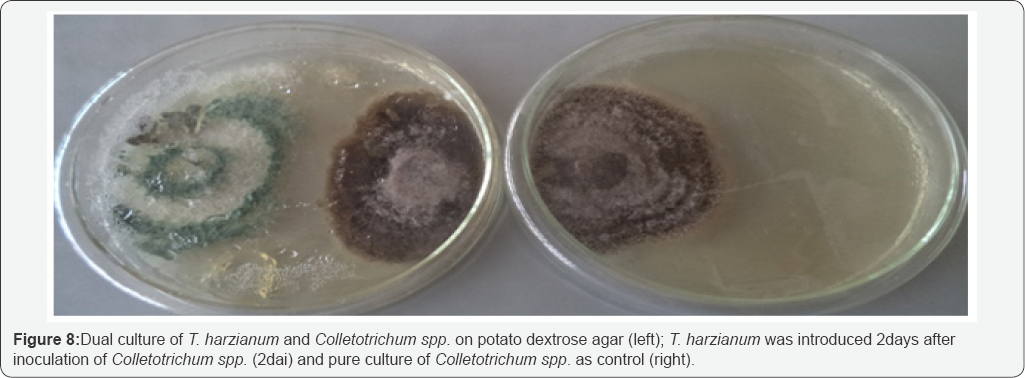
Results of dual culture method used indicated that T harzianum inhibited the growth of Colletotrichum spp at varying degrees. The dual culture interactions showed significant success in biocontrol of Colletotrichum spp., under in vitro conditions. Result of the study revealed that T. harzianum exhibited antagonistic activities on Colletotrichum spp in all the treatments. The potential antagonist was seen to grow faster than the fungus in the dual culture. The control plates also grew much faster when compared with their respective dual cultures. Colletotrichum spp. had its growth stopped either when the antagonist was introduced same time with it (Figure 6) or whenwas introduced 2 days before inoculation of Colletotrichum spp. (Figure 7), in these interactions, the antagonist continued with its growth uninhibited and over grew the pathogen resulting into complete degradation of the fungus and sporulation of the antagonist over the entire plate. When T. harzianum was introduced 2 days after the inoculation of the pathogen, there was a clear zone of inhibition (Figure 8). Table 2 shows the percentage growth inhibition of Colletotrichum spp increases with increase in time of incubation from 72 hours to 192 hours irrespective of the treatment. When T. harzianum was introduced same time with Colletotrichum spp, it was observed that the percentage growth inhibition of the fungus in dual culture with T. harzianum increased steadily from 24.94% at 72 hours to 54.06% at 192 hours respectively. A similar trend was recorded when T. harzianum was introduced two days before inoculation of the pathogenic fungus, with percentage growth inhibition of 27.80% at 72 hours to 80.93% at 192 hours respectively Percentage growth inhibitions rose from 16.03% at 72 hours to 24.04% at 192 hours when T. harzianum was introduced two days after inoculation of Colletotrichum spp. Duration of incubation for each of the treatments showed significant differences at 24 hour interval. Mean variation of percentage growth inhibition of Colletotrichum spp. tested at different times of introduction of T. harzianum significantly (P≤0.05) inhibited the growth of Colletotrichum spp (Table 2).
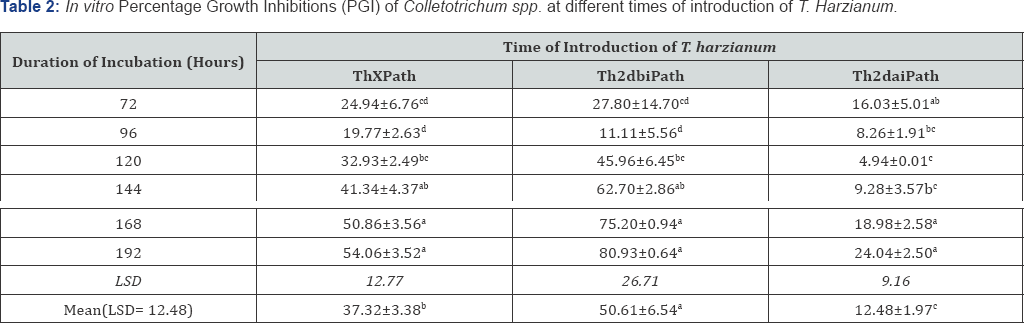
Means on the same column with the same superscript are not statistically significant (P≤0.05). Means on the same row (for Mean) with the same superscript are not statistically significant (P≤0.05) by time of introduction of T. harzianum. Thxpath = T.harzianum introduced same time with pathogen; Th2dbipath = T. harzianum introduced 2 days before inoculation of pathogen; Th2daipat = T. harzianum introduced 2 days after inoculation of pathogen.
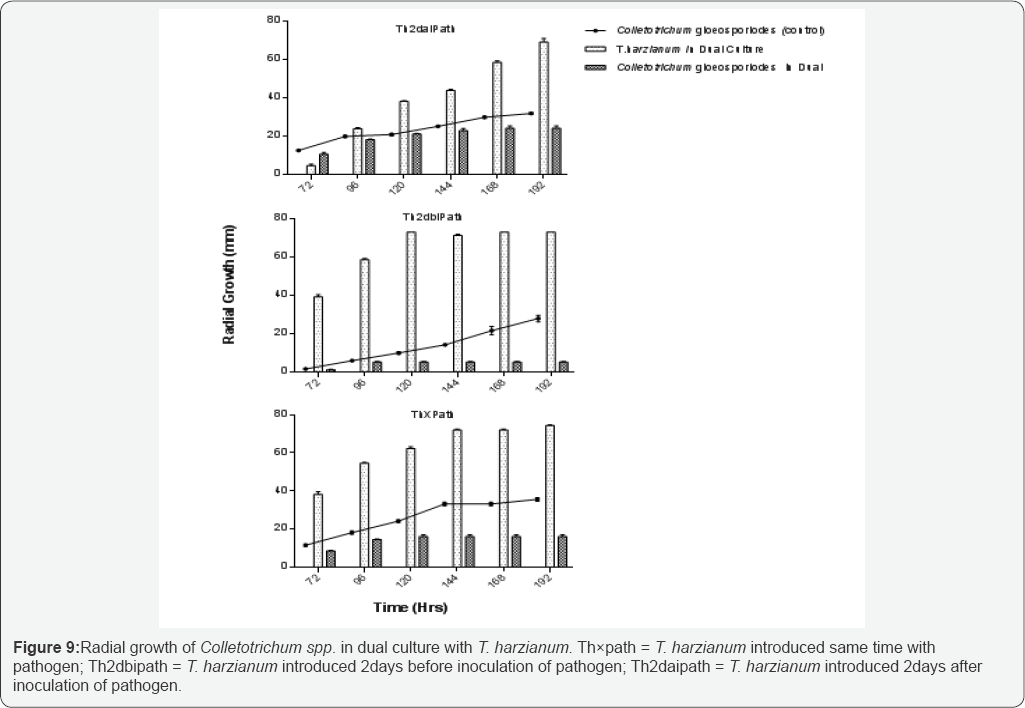
Radial mycelia growth and determination of inhibition
Radial mycelia growth of T. harzianum and Colletotrichum spp in dual culture and Colletotrichum spp. in control plates for each of the treatments were measured from 72 hours to 196 hours of incubation. Figure 9 shows that growth was rapid in the control plates than in the dual culture plates in all the treatments. It was also found that T. harzianum grew much faster than Colletotichum spp in all the treatments in dual culture thereby inhibiting the growth of the fungus.
Effectiveness levels of T. harzianum were found to be slightly effective to effective and significant (P≤0.05) across treatments (Table 3).
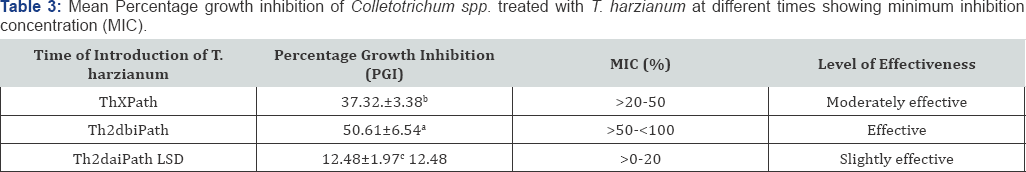
Thxpath = T.harzianum introduced same time with pathogen; Th2dbipath = T.harzianum introduced 2 days before inoculation of pathogen; Th2daipath = T. harzianum introduced 2 days after inoculation of pathogen; MIC = minimum inhibition concentration (%); ≤0% inhibition (not effective); >0-20% inhibition (slightly effective); >20-50% inhibition (moderately effective); >50-<100% inhibition (effective); 100% inhibition (highly effective)
Discussion
Fungal pathogenic organisms are the main causative agents of rot in yam tubers in storage in major yam producing areas reducing the yield and productivity of yam per annum. As a result, the demand for yam tubers has always exceeded its supply [26]. Fungal organisms isolated and identified had been previously linked with post harvest yam tuber rot in different locations in Nigeria [5,8,19,27,28]. Results of inoculation of Colletotrichum spp into the healthy yam tubers shows that the fungus was able to cause rot. This was probably due to the ability of the pathogen to utilize the nutrients of the tubers as substrates for growth and development. The control tubers were however not infected suggesting the absence of inoculum in the tissues. The occurrence of Colletotrichum spp. in yam tubers may probably be due to the fact that Colletotrichum spp. overwinters in leaves, stems and seeds and in infected soil [9]. It has been found that most of these microorganisms infect the tubers in the field reducing their capacity to germinate and affect their survival in the field and then subsequently manifest in storage barns; occurring even when infected tubers do not have any sign of external symptoms [9]. The results of the dual culture revealed the capability of T. harzianum to reduce mycelia growth of Colletotrichum spp irrespective of the time of introduction of the antagonist. This was probably due to the ability of the antagonist to grow much faster than the pathogenic fungus and cover the entire plates thus competing efficiently for space and nutrients. The mechanism of action was mainly competition for limited nutrient and space resulting to starvation and subsequently death of the pathogen [29,30]. Microscopic observations showed the effect of T harzianum and its volatile metabolite substances on mycelia and on spore formation of the pathogenic fungus at different times of the introduction of the antagonist. The interaction between T. harzianum and Colletotrichum spp. showed that spores of Colletotrichum spp. were only seen when T. harzianum was introduced two days after inoculation of pathogen. The result also revealed that T. harzianum mycelia entangled the hyphae fragments of Colletotrichum spp. and eventually plasmolysed and lysed them. As a result, no zones of inhibitions were formed in the interactions between the antagonist and these isolates in the dual culture plates. The conidia of Colletotrichum spp in the presence of T. harzianum mycelia were torrulose [30-33]. The actions of T. harzianum could be due to the possible role of chitinolytic and/or glucanases enzymes in bio-control by Trichoderma [30]. They showed that the enzymes function by breaking down the polysaccharides, chitin, and glucans that are responsible for the rigidity of fungal cell walls, thereby destroying the cell wall integrity and limiting the growth of the pathogen. The growth inhibition of Colletotrichum spp. by T. harzianum in dual culture in this study could be due to its fast growing nature. Trichoderma species have been successfully used as biocontrol agents due to their high reproductive capacity, efficient utilization of nutrients, and strong aggressiveness against other pathogens. It is also reported that T. harzianum isolates suppressed the growth of Colletotrichum capsici eventually overgrowing it within seven days [34]. It was found that the antagonism in vitro of T harzianum against Fusarium oxysporium showed an inhibition on the pathogenic fungus growth with a ratio more than 65%. Moreover, the volatile metabolism substances of the antagonism reduced the pathogenic fungus growth by 63% compared with controls [35]. The dual culture inoculation and the introduction of potential antagonist two days before the pathogen was in agreement with earlier work [36]. The author believed that there are no biocontrol agents that have enough competitive ability to displace an already established pathogen. The time lapse between inoculation of the antagonist and the pathogens contributed to the success recorded with the antagonist against the pathogens. This allows adequate increase in cell concentration and subsequent colonization by antagonist before the arrival of the pathogen [37]. When T. harzianum was introduced 2 days after inoculation of the pathogen even when the pathogen had a significant space and time advantage, it could not compete effectively with the antagonist in culture. T. harzianum has shown to have an antagonistic influence. This effect is produced because of competition for food and space, mycoparasitism and possible antibiosis [38]. Minimum inhibition concentration (MIC) showed that T. harzianum introduced 2 days before the arrival of Colletotrichum spp inhibited the growth of the pathogen at the highest level compared with that introduced same time with the pathogen with least percentage growth inhibition recorded when the antagonist was introduced 2 days after inoculation of the fungus. T harzianum introduced 2 days before inoculation of Colletotrichum spp was therefore considered more effective in controlling the pathogenic fungus in vitro.
Conclusion
Fungal pathogens are the main cause of rot in yam tubers in different parts of Nigeria. The use of biological antagonist which is environmentary safe has the capability of inhibiting the growth and survival of Colletotrichum spp. and can therefore, provide alternative ways of controlling fungal pathogens of yams. This can be mostly achieved when the bioagent (T. harzianum) is introduced on the host (yam tubers) before the arrival of fungal pathogen (Colletotrichum spp.) as this has achieved the highest percentage growth inhibition.
References
- Egesi CN, Onyeka TJ, Asiedu R (2009) Environmental stability of resistance to anthracnose and virus diseases of water yam (Dioscorea alata). African Journal of Agricultural Research 4(2): 113-118
- Aduramigba-Modupe AO, Odebode AC, Asiedu R (2010) Distribution of yam anthracnose disease in Nigeria. African Journal of Root Tuber Crops 8(1): 51-55.
- Dean R, Van Kan JAL, Pretorius ZA, Hammond-Kosack KE, Di Pietro A (2012) The Top 10 fungal pathogens in molecular plant pathology. Molecular Plant Pathology 13(4): 414-430.
- Cannon PF, Buddie AG, Bridge PD, de Neergard E, Lübeck M, et al. (2012) Lectera, a new genus of the Plectosphaerellaceae for the legume pathogen Volutella colletotrichoides. Mycokeys 3: 23-36.
- Gwa IV, Bem AA, Okoro JK (2015) Yams (Dioscorea rotundata Poir and D. Alata Lam.) Fungi Etiology in Katsina-Ala Local Government Area of Benue State, Nigeria. Journal of Phytopathology and Plant Health 3: 38-43
- Green KR, Simons SA (1994) 'Dead Skin' on Yams (Dioscorea alata) caused by Colletotrichum gloeosporioides. Plant Pathology 43(6): 1062-1065.
- Markson AA, Amadioha AC, Omosun G, Madunagu BE, Udo SE, Umana EJ (2012) Control of Botryodiplodia theobromae causing Tissue Rot of White Yam (Dioscorea rotundata Poir). Scholarly Journal of Agricultural Science 2(1): 1-7.
- Ogunleye AO, Ayansola OT (2014) Studies of Some Isolated Rot-Causing Mycoflora of Yams (Dioscorea Spp.). American Journal of Microbiology and Biotechnology 1(1): 9-20.
- Amusa NA, Adegbite AA, Muhammed S, Baiyewu RA (2003) Yam diseases and its management in Nigeria. African Journal of Biotechnology 2(12): 497-502.
- Amin F, Razdan VK, Mohiddin FA, Bhat KA, Saba Banday (2010) Potential of Trichoderma Species as Biocontrol Agents of Soil Borne Fungal Propagules. Journal of Phytopathology 2(10): 38-41
- Chet I, Harman GE, Baker R (1981) Trichoderma hamatum: its hyphal interaction with Rhizoctonia solani and Pythium spp. Microbial Biology 7(1): 29-38.
- Amaeze NJ, Ezeh PA, Dan-kishiya AS (2013) Evaluation of Garlic (Allium Sativum) and uziza (Piper Guineense) on the Control of Tuber Rot Fungi of Potato and carrot. American Journal of Research Communication 1(9): 167-174
- Jawetz M, Adelberg Brooks GF, Butel JS, Morse SA (2004) Medical Microbiology (23rd edn). McGraw Hill Companies Inc., Singapore pp.818.
- Burgess LW, Knight TE, Tesoriero L, Phan HT (2008) Diagnostic Manual for Plant Diseases in Vietnam. ACIAR Monograph 129: 210.
- Ritchie B (1991) Practical Techniques in Plant Pathology CAB. Wallingford. UK.
- Gwa VI, Akombo RA (2016) Studies on the Antimicrobial Potency of Five Crude Plant Extracts and Chemical Fungicide in in vitro Control of Aspergillusflavus, Causal Agent of White Yam (Dioscorea rotundata) Tuber Rot. Journal of Plant Sciences and Agricultural Research 1(1): 1-8.
- Navi SS, Bandyopadhyay R, Hall AJ, Bramel-Cox PJ (1999) A pictorial Guide for the Identification of Mold Fungi on Sorghum Grain. Information Bulletin no. 59 (In En. Summaries in En, Fr). International Crops Research Institute for the Semi-Arid Tropics pp. 118.
- Okigbo RN, Ikediugwu FEO (2000) Studies on Biological Control of Post Harvest Rot in Yams (Dioscorea rotundata) using Trichoderrma viride. Journal of Phytopathology 148(6): 351-355.
- Taiga A (2011) Comparative Studies of the Efficacy of some Selected Fungicidal Aqueous Plant Extracts on Yam Tuber Dry Rot Disease. Annals of Biological Research 2(2): 332-336.
- Amienyo CA, Ataga AE (2006) Post Harvest Fungal Diseases of Sweet Potato (Ipomoea batatas L. Lam) Tubers sold in Selected Markets in Rivers State, Nigeria. Science Africa 5(2): 95-98.
- Gomez KA, Gomez AA (1984) Statistical Procedures for Agricultural Research (2nd edn). John Wiley and sons, USA, pp. 680.
- Evans HC, Hoimes KA, Reid AP (2003) Phylogeny of the Frosty Pod Rot Pathogen of Cocoa. Plant Pathology 52(4): 476-485.
- Korsten L, De Jager ES (1995) Mode of Action of Bacillus subtilis for control of Avocado Post Harvest Pathogens. South African Avocado Growers Association Yearbook 18: 124-130.
- Sangoyomi T (2004) Post-harvest Fungal Deterioration of yam (Dioscorea rotundata. Poir) and its Control. University of Ibadan, Nigeria, pp. 179.
- Cochran GW, Cox GM (1992) Experimental Designs. (2nd edn). John willey and Sons Inc., USA, pp. 611.
- Food and Agricultural Organization (2013) Food and Agricultural Organisation of the United Nations, USA.
- Ogaraku AO, Usman HO (2008) Storage Rot of Some Yams (Dioscorea Spp) in Keffi and Environs, Nasarawa State, Nigeria. Journal of Production Agriculture and Technology 4(2): 22-27.
- Okigbo NR, Enweremadu CE, Agu CK, Irondi RC, Okeke BC, et al. (2015) Control of White Yam (Dioscorea rotundata) Rot Pathogen using Peel Extract of Water Yam (Dioscorea alata). Advances in Applied Science Research 6(10): 7-13.
- Abdollahzadeh J, Goltapeh EM, Rouhani H (2003) Evaluation of Antagonistic Effect of Trichoderma species in Biological Control of Causal Agents of Crown and Root Rot of Sunflower (Sclerotinia minor) in vitro. Agricultural Sciences Tabriz 13(2): 13-23.
- Siameto EN, Okoth S, Amugune NO, Chege NC (2011) Molecular Characterization and Identification of Biocontrol Isolates of Trichoderma harzianum from Embu District, Kenya. Tropical and Subtropical Agroecosystem 13(1): 81-90.
- Manjula K, Mwangi M, Bandyopadhyay R (2005) Potential of some bacteria and fungi as biocontrol agents of cassava and yam tuber rot pathogens under laboratory and green house conditions. African Crop Science Conference Proceedings 7: 1395-1400.
- Mokhtar H, Aid D (2013) Contribution in Isolation and Identification of some Pathogenic Fungi from Wheat Seeds, and Evaluation of Antagonistic Capability of Trichoderma harzianum against those Isolated Fungi in vitro. Agricultural and Biological Journal of North America 4(2): 145-154.
- Singh A, Sharma R (2014) Biocontrol and Environmental Studies on Paper Degrading Mycoflora Isolated from Sanganer Area, Jaipur, India. International Journal of Current Microbiology and Applied Science 3(8): 948-956.
- Ekefan EJ, Jama A, Gowen SR (2009) Potential of Trichoderma harzianum Isolates in Biocontrol of Colletotrichum capsici Causing Anthracnose of Pepper (Capsicum spp.) in Nigeria. Journal of Applied Biosciences 20: 1138-1145.
- Hibar K, Mejda D, Haifa K, Mohamed E (2005) Effect Inhibiteur in vitro et in vivo du Trichoderma harzianum sur Fusarium oxysporium f.sp. Radicislycopersici. Biotechnol Agron Soc Environment 9(5):163-171.
- Campbell RB (1988) Biological Control of Microbial Plant Pathogens. Cambridge University Press, Cambridge, USA pp. 218.
- Janisienwicz JWJ (1998) Biocontrol of post-harvest disease of Apples with antagonistic mixtures. Journal of Phytopathology 78: 194-198.
- Sempere F, Santamarina M (2007) In vitro biological analysis of Alternaria alternate (Fr.) Keissler under different environmental conditions. Mycopathollogia (163)3: 183-190.






























