Production of Glucose Oxidase from Aspergillus Niger Using Sugarcane Peels as Carbon Source
Mida M1, Abah M.A1, Idowu N.J2, Audu E1, Gbadeyan F.A3, Ekele J.U4, Agbo O.S5, Michael V.U6, Oladosu M.A7, Adefemi B.D8, Nwali C.T9, Asare B.K10, Adetoyi M.G11, Oyibo O.N12, Ikedionu C.A13, Ogunta C.E14, Adetokunbo O.S15, Asuquo G16, Odoh E.C17, Hassan D.R18, Okani P.C19, Iheanacho C.C20,21 and Ismaila E.O22
1Department of Biochemistry, Faculty of Biosciences, Federal University Wukari, Taraba State, Nigeria
2Department of Chemistry, College of Arts and Sciences, University of Nebraska-Lincoln, Lincoln, Nebraska, USA
3Department of Microbiology, Faculty of Science, Federal University of Lafia, Nasarawa State, Nigeria
4Department of Biological and Environmental Sciences, Liverpool John Moores University, Liverpool, United Kingdom
5Department of Applied Biology and Biotechnology, Faculty of Biological Sciences, Enugu State University of Science and Technology, Nigeria
6Department of Chemistry, Faculty of Natural Sciences, Prince Abubakar Audu University
7Department of Biochemistry, Faculty of Basic Medical Sciences, University of Lagos, Akoka Nigeria
8Department of Chemical Engineering, School of Infrastructure and Processing Engineering Technology, Federal University of Technology, Minna, Niger State, Nigeria,
9Department of Physics, Ahmadu Bello University Zaria, Kaduna State, Nigeria
10Department of Nutritional, Food and Consumer Sciences, Fulda University of Applied Sciences, Germany
11Department of Medical Biochemistry, Faculty of Basic Medical Sciences, University of Ilorin, Nigeria
12Department of Biochemistry, Faculty of Biological Sciences, University of Nigeria, Nigeria
13Department of Manufacturing Engineering, Georgia Southern University, Statesboro Georgia USA
14Department of Applied Biochemistry, Faculty of Bioscience, Nnamdi Azikiwe University, Awka, Anambra State, Nigeria
15Department of Chemical Sciences, Adekunle Ajasin University, Akungba-Akoko, Ondo State, Nigeria
16Department of Biomedical Health, College of Science and Engineering, University of Derby, United Kingdom
17Department of Chemical Engineering, Federal University of Technology Owerri, Imo State, Nigeria
18Department of Biological Sciences, Faculty of Sciences, Federal University Lokoja, P.M.B 1152, Lokoja, kogi State, Nigeria
19Department of Biological Sciences, College of Science, Michael okpara University of Agriculture Umudike, P.M.B, Umuahia, Abia state, Nigeria
20Department of Microbiology, Faculty of Biosciences, Federal University Wukari, Taraba State, Nigeria
21Fujian Key Laboratory of Watershed Ecology, Institute of Urban Environment, Chinese Academy of Sciences, Xiamen 361021, China.
22Department, University: Department of Animal Sciences, Obafemi Awolowo University, Ile Ife, Nigeria
Submission:February 06, 2024; Published: February 24, 2025
*Corresponding author:Moses Adondua Abah, Department of Biochemistry, Faculty of Biosciences, Federal University Wukari, Taraba State, Nigeria.
How to cite this article:Mida M, Moses Adondua A, Nike Jesutofunmi I, Audu E, Femi A G, Ekele J U, et al. Production of Glucose Oxidase from 002 Aspergillus Niger Using Sugarcane Peels as Carbon Source. Adv Biotech & Micro. 2025; 18(5): 555997DOI:10.19080/AIBM.2025.18.555997
Abstract
The growing demand for eco-friendly and sustainable solutions in various industries has fuelled the demand for microbial enzymes. Industries are increasingly seeking enzyme-based alternatives to conventional chemical processes to meet regulatory requirements and consumer preferences. Also, microbial sources are considered more cost-effective than chemical synthesis and enzyme isolation from biological sources. This research aimed at producing Glucose Oxidase from Aspergillus Niger using sugarcane peels as carbon source. Soil samples were collected randomly from a sugar cane market, at a bagasse dumping site in Donga Local Government Area, Taraba State, Nigeria, during the month of October 2023. Isolation of fungi was performed by soil dilution plate technique using potato dextrose agar (PDA) medium. All isolates were subjected to submerged fermentation in 250ml Erlenmeyer flasks. Morphological techniques and biochemical testing were used to confirm the desired strain for this study. The strain was cultured on PDA and incubated at 30°C for 72hours. The cultures were then preserved at 4°C and sub-cultured after every 7 days to maintain their viability. The fungi strain that produced glucose oxidase was screened with the O-dianisidine method. The optimal pH for glucose oxidase production was determined by measuring the enzyme activity at each of the following pH: 4-9. The optimal temperature for glucose oxidase activity was also determined using different temperatures ranging from 30°C to 600°C. Substrate concentration of the enzyme was studied. The effect of incubation days and protein concentration on glucose oxidase production and activity respectively were also investigated. The results obtained in this study showed that optimal conditions for glucose oxidase production appeared to be at day 5 of fermentation (1.658 μmolmin-1), with peak activity observed at a temperature of approximately 40°C (0.117 μmolmin-1) and a pH of 5 (0.161μmolmin-1). Glucose oxidase activity was found to increase proportionately with glucose concentration with the highest activity of glucose oxidase being 0.743μm/min at 10% glucose concentration. The evidence presented in this study revealed that glucose oxidase can be produced from isolates of Aspergillus Niger. By carrying out the aim and objectives of this research, it was revealed that it is possible to produce mass quantity of crude glucose oxidase from Aspergillus Niger using sugarcane peels as carbon source. This was achieved under the following conditions: incubation period of seven days, pH of 5, temperature of 40°C and substrate concentration of 10%.
Keywords: Glucose Oxidase; Isolation; Sugarcane Peels; Aspergillus Niger; Concentration; Substrate; Temperature
Abbreviations: PDA: Potato Dextrose Agar; FAD: Flavin Adenine Dinucleotide.
Introduction
The trend of producing enzymes from microorganisms has been steadily increasing over the years due to several factors that make microbial enzymes highly desirable for industrial applications [1]. Microorganisms, including bacteria, fungi, and yeast, have the inherent ability to produce large quantities of enzymes through fermentation processes. This high yield makes them cost-effective and efficient sources of enzymes for industrial-scale production [2]. Microorganisms exhibit vast genetic diversity, allowing to produce a wide range of enzymes with diverse catalytic activities [3]. This versatility enables the production of enzymes tailored to specific industrial processes and applications. Advances in genetic engineering and biotechnology have enabled the manipulation and optimization of microbial strains for enhanced enzyme production. Genetic modification techniques allow researchers to engineer microorganisms with improved enzyme yields, stability, and specificity [4]. Microbial enzyme production is often considered more environmentally sustainable compared to traditional chemical synthesis methods. Microorganisms can utilize renewable carbon sources, such as agricultural residues and waste materials, reducing the dependence on fossil fuels and minimizing environmental impact. Enzymes produced from microorganisms find diverse applications across various industries, including food and beverage, pharmaceuticals, textiles, biofuels, and bioremediation. Their catalytic properties make them indispensable tools for processes such as food processing, biocatalysis, and waste treatment [5].
Glucose Oxidase which is among enzymes that could be obtained from microorganisms, plays a crucial role in the oxidation of glucose molecules. It belongs to the oxidoreductase class of enzymes and catalyzes the oxidation of β-D-glucose to form gluconic acid and hydrogen peroxide, the enzyme commission number is EC 1.1.3.4. [6]. Glucose Oxidase is widely distributed in nature and can be found in various organisms, including fungi, bacteria, yeast, and certain insects. The enzyme consists of two identical subunits (~80 kDa each), forming a homodimeric structure [7]. Each subunit contains a noncovalently bound Flavin Adenine Dinucleotide (FAD) cofactor, which is essential for its catalytic activity. FAD serves as an electron carrier, facilitating the transfer of electrons during the oxidation reaction. Glucose Oxidase is a versatile enzyme with widespread applications, owing to its ability to catalyze the oxidation of glucose and its compatibility with various industrial processes and diagnostic assays [8]. The enzyme finds extensive applications in various industries, including food and beverage, pharmaceuticals, diagnostics, biotechnology, and textile. In the food industry, it is used for glucose determination, biosensors, and food preservation. In medical diagnostics Glucose Oxidasebased biosensors are employed for blood glucose monitoring in diabetic patients [9]. Additionally, Glucose Oxidase is utilized in biotechnological processes to produce gluconic acid, lactobionic acid, and other biochemicals [10].
Aspergillus niger is a filamentous fungus known for its significant industrial importance due to its ability to produce a variety of enzymes, including Glucose Oxidase. This fungus belongs to the genus Aspergillus, which comprises a diverse group of fungi commonly found in soil, decaying organic matter, and various agricultural products [11]. Aspergillus niger has been extensively studied and utilized in biotechnological applications, particularly in the production of enzymes through fermentation processes. It is known for its high enzyme yields and robust growth characteristics under a wide range of environmental conditions. Glucose Oxidase produced by Aspergillus niger is derived from the fungus’s metabolic processes [12]. During solid-state or submerged fermentation, Aspergillus niger secretes Glucose Oxidase into the surrounding medium, where it can be harvested and purified for various industrial applications. Glucose Oxidase produced by Aspergillus niger shares similar properties with Glucose Oxidase from other microbial sources, including its ability to catalyze the oxidation of glucose to produce gluconic acid and hydrogen peroxide [13]. However, specific characteristics such as optimal pH, temperature stability, and enzyme yield may vary depending on the fermentation conditions and strain of Aspergillus niger used.
Despite the widespread industrial applications of Glucose Oxidase, there remain a need for cost-effective and efficient methods of production [14]. While microbial sources, particularly filamentous fungi like Aspergillus niger are known producers of Glucose Oxidase, there is a lack of comprehensive research into optimizing the production process. This research was set up to address this gap by investigating the factors influencing Glucose Oxidase production from Aspergillus niger [15]. By optimizing the production process, this study further sought to enhance the yield, purity, and stability of Glucose Oxidase, thereby contributing to the development of sustainable and economically viable enzyme production methods.
Materials and Method
Sample collection
Soil samples were collected randomly from a sugar cane market, at the bagasse dumping site, Donga Local Government, Taraba State Nigeria, during the month of October 2023. Enough sugarcane peels was also collected randomly from the same market.
Isolation of fungi
Isolation of fungi was performed by soil dilution plate technique using Potato Dextrose Agar (PDA) medium [16]. The medium was prepared 50% distilled water and sterilized at 121°C for 15 min. The medium was then supplemented with streptomycin at 2μg/ml to prevent the bacterial contamination.
The medium was poured into the sterile petri plates. The collected soil samples were prepared serial dilution of 10-3 to10-5 with sterilized water (1g of the sample was dissolved in 9ml of distilled water). 0.1 ml of the diluted sample was spread over the agar plates. The inoculated plates were incubated at 28 2°C ± 2°C for 48h-72h. After incubation, the colonies were observed on the plates as mycelium color, spore arrangement and the appearance of colonies. The isolated colonies were preserved on Potato Dextrose Agar at 4°C until further use.
Screening for glucose oxidase producing fungi
The method described by Khattab and Brazraa (2005) was used to achieve this. The fungi that produced Glucose Oxidase were screened based on their activity with the O-dianisidine method. Colonies with distinct morphologies were tested for Glucose Oxidase activity. The purified fungal cultures were inoculated on Czapek dox agar medium that contained 100 μl of O-dianisidine and 100 μl of horseradish peroxidase. After incubation, the medium developed a brown zone around the colonies that were positive for glucose oxidase activity.
Submerged fermentation and enzyme extraction
The method described by [17] was used for the enzyme extraction process. For submerged fermentation, a medium consisting of 8% glucose, 0.3% peptone, 0.0388% (NH4)2HPO4, 0.0188% KH2PO4, 0.0156% MgSO4.7H2O, and 3.5% CaCO3 was used. The medium was sterilized and inoculated with a spore suspension, a 5:3 fermentation medium was set up where 50 ml of the basal medium was poured into fermentation conical flasks then 30g of the sugar peel was supplemented into the conical flask to make for a moist environment then allowed to ferment for 8 days. The fermented broth was then filtered, and the mycelium was crushed and homogenized in 0.1M citrate phosphate buffer with a pH of 7.0. The extract was then centrifuged and used for the determination of glucose oxidase activity and total protein content.
Determination of protein concentration
Determination of protein concentration was carried out using the method described by [11]. 2% sodium carbonate was prepared by dissolving the 2g of sodium carbonate weighed in 100ml of distilled water. 0.1m sodium hydroxide was prepared by weighing 4g of sodium hydroxide in 100ml of distilled water. 2% sodium carbonate and 0.1m sodium hydroxide were mixed. This mixture formed reagent A. 0.5% Copper sulphate was prepared by dissolving 0.5g of the Copper sulphate chemical in 100ml of distilled water. 1% potassium sodium tartarate was prepared by dissolving 1g of the chemical in 100ml of distilled water. 0.5% Copper sulphate and 1% potassium sodium tartarate were mixed to give reagent B. Reagent C was prepared by mixing 50ml of reagent A and 1ml of reagent B. Reagent D was prepared by mixing equal volume of 0.1m NaOH with folin-Ciocalteu reagent. Protein concentration was then determined by the following. 1ml of Bovine serum Albumin + 5ml of reagent C+0.2ml of enzyme, then incubated at room temperature for 10 minutes after which 0.5ml of reagent D was added and incubated at dark cupboard for 30 minutes. After this process, the absorbance for protein content was determined at 660nm.This procedure was repeated for 7 days to determine the protein concentration.
pH optimization
This was done using 3 buffers namely: sodium phosphate buffer, sodium acetate buffer and tris HCl buffer.0.1m sodium phosphate buffer was prepared by diluting 10ml of the commercial buffer in 100ml of distilled water.0.1m sodium acetate buffer was prepared by diluting 10ml of the commercial buffer in 100ml of distilled water.0.1m tris HCl buffer was prepared by diluting 10ml of the commercial buffer in 100ml of distilled water. pH optimization was carried out in six test tubes each having 2.0ml of GOX enzyme and 1.0ml of glucose then using a pH meter, the pH of the test tube was adjusted to 4, 5, 6, 7, 8 and 9 using HCl and NaOH. Then the activity is determined at 510nm.
Temperature optimization
The optimization of temperature was done using 8 test tubes, 8% glucose solution. In each test tube, 2ml of distilled water and 1ml of glucose solution was transferred, then 2ml of the GOX enzyme after which one test tube was kept at room temperature (37°C). The next at ice temperature (4°C) and the other 6 test tubes, water bath was used to equilibrate its temperatures at 30, 35, 40, 45, 50, 55 degrees Celsius respectively. After 5 minutes, the activity was taken at 510nm.
Substrate concentration optimization
Substrate concentration optimization was carried out using the method described by [11]. This was done using different glucose concentrations in 7 test tubes as described below. These 7 test tubes contained glucose of different concentrations (2mls each of the glucose concentration in the test tube). Then 3ml of distilled water and 2ml of enzyme were transferred into all test tubes and the absorbance was read at 510nm for activity.
Test tube 1: Glucose 4% = 4g in 100ml distilled water
Test tube 2: Glucose 5% = 5g in 100ml distilled water
Test tube 3: Glucose 6% = 6g in 100ml distilled water
Test tube 4: Glucose 7% = 7g in 100ml distilled water
Test tube 5: Glucose 8% = 8g in 100ml distilled water
Test tube 6: Glucose 9% = 9g in 100ml distilled water
Test tube 7: Glucose 10% = 10g in 100ml distilled water
Results
Screening of fungal isolates
The screening result for Glucose Oxidase production which was aseptically done on a plate by inoculating the fermented medium is presented in Figure 1 below. The screening result revealed that the primary screened fungal isolates upon inoculation at room temperature showed the appearance of a clear zone which is an indication of glucose oxidase production The result also revealed some morphological characteristics of A. niger as seen in (Figure 1) presented below.
Production of glucose oxidase
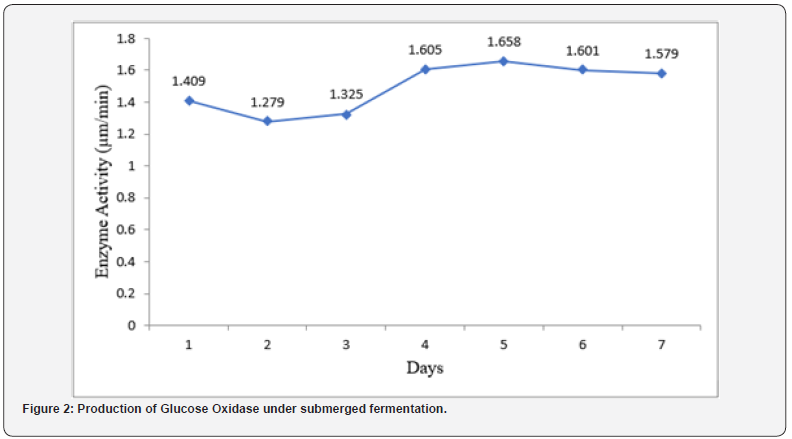
(Figure 2) presents the result of production of Glucose Oxidase under submerged fermentation. The selected isolates from the primary screening method were subjected to submerged fermentation in a suitable medium and observed. The highest enzyme activity was recorded on day 5 (1.658 μm/ min) of fermentation. The isolates were found to be able to utilize the available carbon source to produce Glucose Oxidase. Glucose Oxidase production increased gradually over 24hours of incubation until optimum glucose oxidase production was achieved on day 5. After the optimum incubation period, Glucose Oxidase production began to decrease. The least production of Glucose Oxidase was recorded on day 2 (1.279 μm/min).
Determination of protein concentration
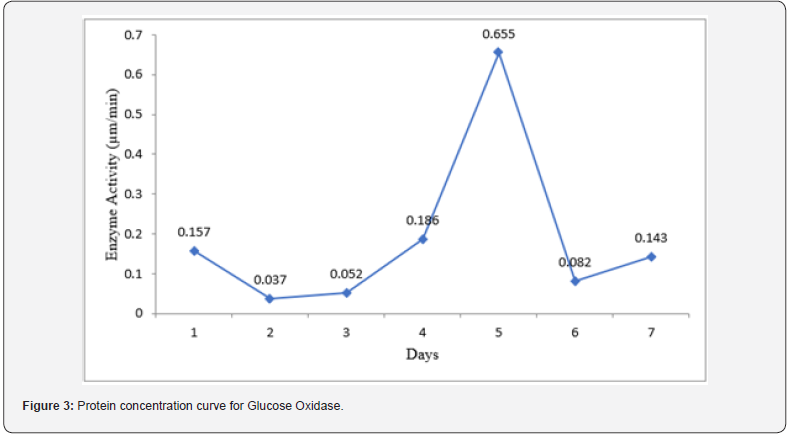
(Figure 3) reveals the result of protein concentration during the incubation period for Glucose Oxidase. The result showed that the highest protein concentration was obtained on day 5 of incubation (0.655 μm/min). Meanwhile, the least protein concentration was achieved on day 2 (0.037 μm/min) incubation period for Glucose Oxidase. A sharp decrease in the concentration of protein was noticed after it peaked at day 5 of incubation. Protein concentration began to increase again after day 6.
Effect of pH on glucose oxidase activity
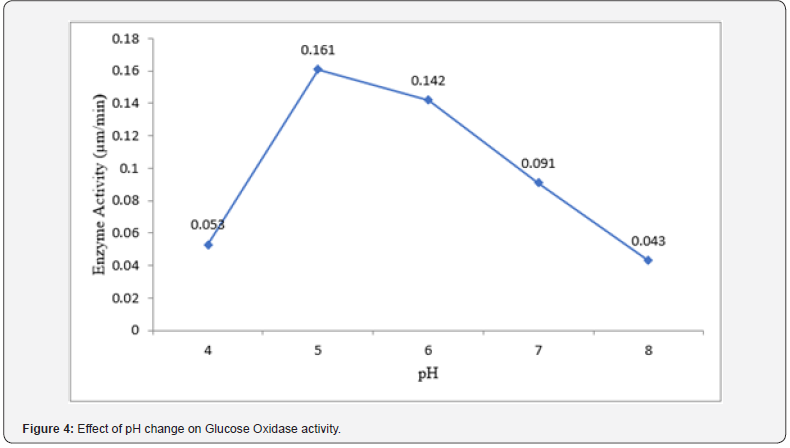
The result of the effect of pH on glucose oxidase activity is presented in (Figure 4) below. The optimum pH for Glucose Oxidase activity was found to be pH 5 (0.161 μm/min) while the lowest activity exhibited by Glucose Oxidase was at was at pH 8 (0.043 μm/min). Following the peak of Glucose Oxidase activity at pH 5, there was a stable decrease in the enzyme’s activity.
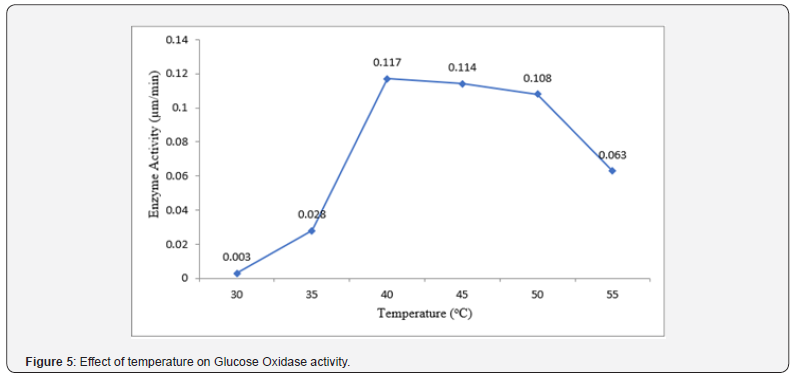
Effect of temperature on glucose oxidase activity
The result of the effect of temperature on Glucose Oxidase activity is presented in (Figure 5) below. The optimum temperature for Glucose Oxidase activity was found to be 40°C (0.117 μm/min) Glucose oxidase exhibited its activity the least at 30°C (0.003 μm/ min).
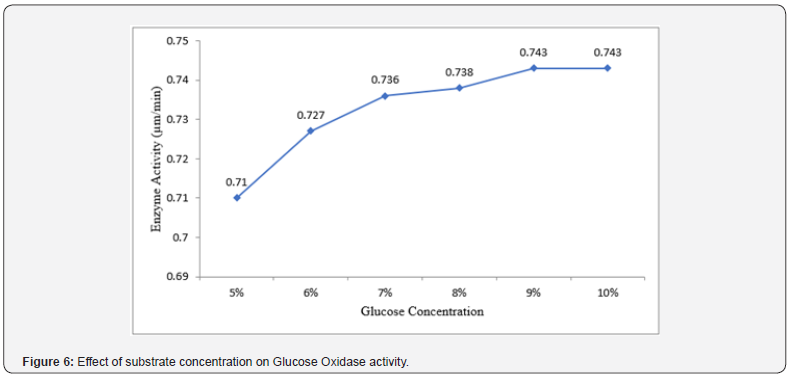
Effect of substrate concentration on glucose oxidase activity
The result of the effect of substrate concentration on Glucose Oxidase activity is presented in (Figure 6) below. The result revealed that Glucose Oxidase activity was found to be optimum at 9% and 10% substrate concentration (0.743 μm/min and 0.743 μm/min respectively) whereas Glucose Oxidase showed the least activity at 5% (0.71 μm/min). Increase in substrate concentration gave a commensurate increase in Glucose Oxidase activity.
Discussion
The trend of producing enzymes from microorganisms has been steadily increasing over the years due to several factors that make microbial enzymes highly desirable for industrial applications. Microorganisms, including bacteria, fungi, and yeast, have the inherent ability to produce large quantities of enzymes through fermentation processes [4]. This high yield makes them cost-effective and efficient sources of enzymes for industrialscale production. Microorganisms exhibit vast genetic diversity, allowing to produce a wide range of enzymes with diverse catalytic activities [3]. This versatility enables the production of enzymes tailored to specific industrial processes and applications. Advances in genetic engineering and biotechnology have enabled the manipulation and optimization of microbial strains for enhanced enzyme production.
Our study investigated the production of glucose oxidase under submerged fermentation. The selected isolates from the primary screening method were subjected to submerged fermentation in a suitable medium and observed. The highest enzyme activity was recorded on day 5 (1.658 μm/min) of fermentation. The isolates were found to be able to utilize the available carbon source to produce Glucose Oxidase. Glucose Oxidase production increased gradually over 24 hours of incubation until optimum glucose oxidase production was achieved on day 5. After the optimum incubation period, Glucose Oxidase production began to decrease. The least production of Glucose Oxidase was recorded on day 2 (1.279 μm/min). The peak in the activity of Glucose Oxidase on day 5 suggests that the culture reached its maximum capacity for enzyme production as explained by [1], who also got similar result however, yam peels were the source of carbon in his study. Beyond the peak, factors such as nutrient depletion or accumulation of inhibitory metabolites may start to impede further enzyme synthesis, leading to a decline in activity [11].
The present study also examined the variation in protein concentration during the incubation period for Glucose Oxidase. The result showed that the highest protein concentration was obtained on day 5 of incubation (0.655 μm/min) which was preceded by an early increase in the increase of protein concentration. This steady increase in protein concentration may signify increase in the rate of protein synthesis, reflecting the initial phase of microbial growth and enzyme production, where the culture rapidly synthesizes proteins to meet metabolic demands [1].
pH optimization studies carried out revealed that the optimum pH for Glucose Oxidase activity was found to be pH 5 (0.161 μm/min) while the lowest activity exhibited by Glucose Oxidase was at was at pH 8 (0.043 μm/min). Following the peak of Glucose Oxidase activity at pH 5, there was a stable decrease in the enzyme’s activity. This suggests that there is an optimal pH range for glucose oxidase to exhibit its activity as reported by [1]. The lowest glucose oxidase activity observed at pH 8 could be attributed to the acidophilic nature of the fungi involved in the fermentation process. Fungi typically thrive in acidic environments, but excessively low pH values may adversely affect their cellular metabolism, hence, production of Glucose Oxidase. Our finding is somewhat in tandem with the study conducted by [18], who reported maximum glucose oxidase activity between pH 5-6, and with the studies of [1] who also reported closely similar value with that of our finding. The pH of the culture medium plays a crucial role in modulating enzyme activity by affecting the conformational stability and catalytic efficiency of the enzyme. Glucose oxidase exhibits pH-dependent activity due to its sensitivity to changes in protonation states and interactions with the surrounding environment.
Temperature has been reported by many researchers to play an important role in the activity of Glucose Oxidase. Upon investigating the effect of temperature on Glucose Oxidase, it was found out that the optimum temperature for Glucose Oxidase activity was found to be 40°C (0.117 μm/min) Glucose oxidase exhibited its activity the least at 30°C (0.003 μm/min). Our findings suggest a temperature-dependent response for Glucose Oxidase activity. This finding does not correlate with the report of [18], who reported optimum temperature for glucose oxidase activity at 27.5°C. The report of [10] is somewhat close to the findings of this study. He and his team recorded a temperature of 35°C for maximum Glucose Oxidase activity. However, the result obtained in this study is like findings of [1], who also depicted that Glucose Oxidase activity is optimum at 40°C. Enzyme activity is highly sensitive to changes in temperature due to its impact on the enzyme’s structural stability, conformational dynamics, and catalytic efficiency. Within a certain temperature range, increasing temperature can enhance molecular motion and substrate binding, leading to increased enzyme activity. At lower temperatures, decreased molecular motion and substrate binding may limit enzyme activity, while at higher temperatures, denaturation and loss of enzyme structure can occur, leading to a decline in activity.
The study also examined the effect of substrate concentration on Glucose Oxidase activity. The result revealed that Glucose Oxidase activity was found to be optimum at 9% and 10% substrate concentration (0.743 μm/min and 0.743 μm/min respectively) whereas Glucose Oxidase showed the least activity at 5% (0.71 μm/min). Increase in substrate concentration gave a commensurate increase in Glucose Oxidase activity. The findings made by our investigation indicates that Glucose Oxidase activity exhibited a trend of increasing activity with increase in substrate concentrations, reaching a peak at 9% and 10%. This suggests that higher concentration of glucose provided more substrate for the enzyme to act upon, resulting in enhanced enzymatic activity. It was also observed that at lower substrate concentration like 5%, the data showed a decrease in Glucose Oxidase activity. This phenomenon can be attributed to the limited availability of substrate molecules for the enzyme to catalyze, resulting in reduced enzymatic activity. The trend observed in this study is in line with the concept of substrate saturation by Michaelis-Menten kinetics which states that as substrate concentration increases, the rate of enzyme-catalyzed reactions also increases until a point of saturation is reached [19]. However, further increase in substrate concentration may not significantly affect the rate of reaction, as the enzyme becomes saturated with substrate molecules [20,21].
Conclusion
The findings presented in this study showed that Glucose Oxidase can be produced from isolates of Aspergillus niger. By carrying out the specific objectives of this research, the possibility of mass production of crude Glucose Oxidase from Aspergillus niger using sugarcane peels as carbon source under submerged fermentation was demonstrated. These were achieved under the following conditions: incubation period of seven days, pH of 5, temperature of 40°C and substrate concentration of 10%.
Acknowledgment
We want to thank all the researchers who contributed to the success of this research work.
Conflict of Interest
The authors declared that there are no conflicts of interest.
Funding
No funding was received for this research work.
- Mayel M, Abah MA, Ayo GF (2025) Production of glucose oxidase from Aspergillus Niger using yam peels as carbon source. Int J Mol Biol: 8(1): 1-
- Timothy M, Abah MA, Ayo VI, Ahmed MU, Oyibo ON, et al. (2024) Production and Characterisation of Peroxidase from Aspergillus terrens Isolated from Water Sample in Wukari, Taraba State, Nigeria. African Journal of Biochemistry and Molecular Biology Research 1(1): 597-611.
- García-Cano I, Rocha-Mendoza D, Kosmerl E, Zhang L, Jiménez-Flores R (2020) Technically relevant enzymes and proteins produced by LAB suitable for industrial and biological activity. Applied microbiology and biotechnology 104: 1401-1422.
- Shaibu C, Abah MA (2024) Production and extraction of pectinase from Penicillum citrinum using tomatoes peel as carbon source. J Appl Biotechnol Bioeng 11(6): 186-
- Batool R, Kazmi SAR, Khurshid S, Saeed M, Ali S, et al. (2022) Postharvest shelflife enhancement of peach fruit treated with glucose oxidase immobilized on ZnO nanoparticles. Food Chemistry 366: 130591.
- Suja SK, Kalaivani GJ (2024) Macromolecular-Enzyme-Triggered Electrochemical Biosensing. In Foundation and Growth of Macromolecular Science Ppp: 209-236.
- Khatami SH, Vakili O, Ahmadi N, Soltani Fard E, Mousavi P, et al. (2022) Glucose oxidase: Applications, sources, and recombinant production. Biotechnology and Applied Biochemistry 69(3): 939-950.
- Akbari B, Baghaei‐Yazdi N, Bahmaie M, Mahdavi Abhari F (2022) The role of plant‐derived natural antioxidants in reduction of oxidative stress. BioFactors 48(3): 611-633.
- Mandpe P, Prabhakar B, Gupta H, Shende P (2020) Glucose oxidase-based biosensor for glucose detection from biological fluids. Sensor Review 40(4): 497-511.
- Bauer JA, Zámocká M, Majtán J, Bauerová-Hlinková V (2022) Glucose oxidase, an enzyme “Ferrari”: Its structure, function, production and properties in the light of various industrial and biotechnological applications. Biomolecules 12(3): 472.
- Timothy M, Mayel MH, Yohanna ER, Adondua MA, Chinekwu UK, et al. (2022) Pectinase production from a local isolate of Aspergillus niger using orange bagasse as a carbon source. Asian J Nat Prod Biochem 19(2): 81-86.
- Liu J, Zhang Q, Liang X, Zhang R, Huang X, et al. (2024) Improving glucose oxidase catalysis in Aspergillus niger via Vitreoscilla hemoglobin fusion protein. Applied Microbiology and Biotechnology 108(1): 1-15.
- Baruch-Shpigler Y, Avnir D (2022) Glucose oxidase converted into a general sugar-oxidase. Scientific Reports 12(1): 10716.
- Bollella P, Katz E (2020) Enzyme-based biosensors: tackling electron transfer issues. Sensors 20(12): 3517.
- Coluccia M, Parisse V, Guglielmi P, Giannini G, Secci D (2022) Metal-organic frameworks (MOFs) as biomolecules drug delivery systems for anticancer purposes. European Journal of Medicinal Chemistry 244: 114801.
- Waksman SA, Tenney FG (1927) The composition of natural organic materials and their decomposition in the soil: II. Influence of age of plant upon the rapidity and nature of its decomposition-rye plants. Soil Science 24(5): 317-334.
- Khatami SH, Vakili O, Ahmadi N, Soltani Fard E, Mousavi P, et al. (2022) Glucose oxidase: Applications, sources, and recombinant production. Biotechnology and Applied Biochemistry 69(3): 939-950.
- Jagathy K, Kalpana K, Rajeshkumar S (2017) Production, optimization, Characterization and Immobilization of Glucose oxidase from Aspergillus species. Research Journal of Pharmacy and Technology 10(6): 1924-1928.
- Yohanna ER, Emochone R, Shaibu C, Abah MA, Mgbede T, et al. (2023) Isolation, partial purification andcharacterization of proteases from Aspergillus niger under solid-state fermentation. Asian Journal of Research in Biosciences 5(1): 27-34.
- Chansaenpak K, Kamkaew A, Lisnund S, Prachai P, Ratwirunkit P, et al. (2021) Development of a sensitive self-powered glucose biosensor based on an enzymatic biofuel cell. Biosensors 11(1): 16.
- Kornecki JF, Carballares D, Tardioli PW, Rodrigues RC, Berenguer-Murcia Á, et al. (2020) Enzyme production of D-gluconic acid and glucose oxidase: successful tales of cascade reactions. Catalysis Science & Technology 10(17): 5740-5771.






























