Examining the Inhibitory Impact of Pyrimidine and Certain Derivatives on the Enzyme Glutathione Reductase
Hatice Esra Duran*
Department of Medical Biochemistry, Faculty of Medicine, Kafkas University, Kars, Turkey
Submission:May 30, 2024; Published: June 14, 2024
*Corresponding author: Hatice Esra Duran, Department of Medical Biochemistry, Faculty of Medicine, Kafkas University, Kars, Turkey
How to cite this article: Hatice Esra Duran*. Examining the Inhibitory Impact of Pyrimidine and Certain Derivatives on the Enzyme Glutathione Reductase. Adv Biotech & Micro. 2024; 18(2):555982.DOI:10.19080/AIBM.2024.18.555982
Abstract
Glutathione Reductase (GR, EC 1.6.4.2), a member of the oxidoreductase family of enzymes, is a NADPH dependent enzyme. In addition to neutralizing free radicals and reactive oxygen species (ROS), GR acts by maintaining the GSH / GSSG ratio in the regulation of many vital cellular functions, such as protein and DNA synthesis. GR inhibitors are known to be agents used in the treatment of cancer and malaria. The aim of this research is to investigate the in vitro inhibition effects of pyrimidine and some derivative compounds; pyrimidine(a), 4-amino-2-chloropyrimidine(b), 4-amino-6-chloropyrimidine(c) and 4-amino-2,6-dichloropyrimidine (d) on the GR enzyme. The activity measurement of the GR enzyme was conducted according to the Carlberg and Mannervik method, and the effects of pyrimidine and its derivatives on this enzyme were examined through inhibition studies. The obtained results were used to plot Lineweaver-Burk curves were drawn for each derivative compound to examine the inhibition effects of pyrimidine derivatives on the GR enzyme. The types of inhibition and KI values were determined with the help of these graphs. KI values were found between 0.979±0.23 - 2.984±0.83 μM. 4-amino-2,6-dichloropyrimidine (c) has been shown to be the most effective inhibitory property with its noncompetitive inhibition type. This study reports an effective protocol for pyrimidines. The inhibition effects of a total of four compounds, pyrimidine and three different derivatives, were investigated. These compounds were monitored in vitro for their inhibitory potential against the GR enzyme. It is reported that these compounds, which have potential inhibitory properties for GR, reduce the enzyme activity at low concentrations. Finally, all derivatives in this series can be evaluated as outstanding multi-target inhibitors for further investigation in the treatments of various diseases caused by free radicals.
Keywords: Artificial Intelligence; Biotechnology; Dentistry; Deep Learning algorithms; Diagnostics
Abbreviations: GR: Glutathione Reductase; NADPH: β-Nicotinamide adenine dinucleotide 2′-phosphate; ROS: Reactive Oxygen Species; GSH: L-Glutathione reduced; GSSG: L-Glutathione oxidized; KI: Inhibition Constant; IC50: Concentration of inhibitor that cuts half of the activity of the enzyme; μM: Micromolar.
Introduction
Cellular metabolism encompasses the series of reactions necessary to facilitate the movements of living organisms. All of these reaction series occurring within the cell are referred to as metabolic pathways. Metabolic pathways are the functional units of the metabolic network present in an organism [1]. From this perspective, any disruption in a pathway leads to an increasing domino effect throughout metabolism. Nearly all metabolic reactions that occur in metabolism are catalyzed by various enzymes to ensure the rapid and efficient execution of reactions. Enzyme deficiency, inhibition, or activation affects not only the metabolic pathway performed by the enzyme but also the entire metabolic system associated with it. Therefore, studies on enzyme-drug interactions are becoming increasingly important. The mechanisms of drugs, chemicals, or their metabolites are carried out by affecting enzyme activities within metabolism because enzymes are directly or indirectly affected by xenobiotics [2].
Free radicals carry many electrons in molecular orbitals. Free radicals such as alkoxyl, hydroxyl, superoxide, peroxyl, nitrogen dioxide, and nitric oxide are derived from oxygen [3]. These compounds are formed in organisms through external influences or metabolic pathways and irreversibly bind to DNA and proteins [4]. Additionally, they cause the degradation of structural coenzymes, nucleotides, and DNA in tissues and cell [5,6]. Moreover, they can covalently bind to enzymes, proteins, and lipids; disrupt cell membranes; damage transport systems; and alter enzyme activities, leading to the emergence of various metabolic disorders [7,8].
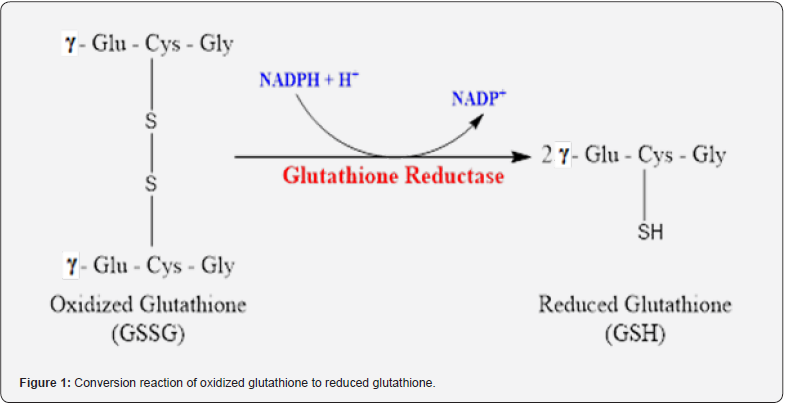
Glutathione Reductase (GR, EC 1.6.4.2) is an important antioxidant enzyme involved in protecting the cell against oxidative stress [2]. Glutathione is believed to be a major contributor to oxidative stress-related diseases. Glutathione serves as a major regulator of intracellular redox reactions. Oxidized and reduced glutathione are the best indicators of oxidative stress. In conditions of increased oxidative stress, reduced glutathione (GSH) decreases while oxidized glutathione (GSSG) increases. GSH is responsible for reducing reactive oxygen species (ROS) that cause oxidative damage. GR converts oxidized glutathione into its reduced form using NADPH obtained through the hexose monophosphate pathway and/or the NADP+ dependent malate dehydrogenase enzyme involved in the transport of acetyl CoA from the mitochondria to the cytosol (Figure 1) [9,10].
00Throughout history, cancer has remained one of the most significant causes of death worldwide, with limited progress made in reducing its causative factors. Natural products have been utilized to treat various illnesses since ancient times. As a result, numerous important anticancer agents have been derived from natural sources. These anticancer agents can be obtained directly from plants or through the modification of natural compounds, offering not only inspiring new compounds but also modifications of existing ones. Despite the discovery of many anticancer agents from natural sources, the exploration of new agents remains a necessity. This is crucial for the improvement and advancement of cancer treatment, highlighting the importance of ongoing research in this field [11].
Reactive oxygen species (ROS) are physiologically generated by aerobic cells. Their production increases during cell necrosis. Physiological levels of ROS serve as mediators in critical intracellular signaling pathways and are essential for cell survival. However, excessive ROS production can cause damage to cells and lead to cell death. It has been known for many years that oxidative stress plays a significant role in the development and progression of various cancer types. Therefore, antioxidant therapy is considered a potential avenue for cancer prevention [12]. Increased levels of GSH are observed in various tumor types, making neoplastic tissues more resistant to chemotherapy. Consequently, GSH system has become one of the most important targets in medical intervention, both in preventing cancer progression and combating chemotherapy resistance. Therefore, the goal in cancer treatment research is to reduce GSH levels by inhibiting GSH biosynthesis. Inhibition of GR leads to the accumulation of oxidized glutathione while depleting reduced glutathione. As a result, inhibition of GR, an antioxidant enzyme, has played a significant role in the development of anticancer and antimalarial drugs in recent years [13].
Heterocyclic compounds play a significant role in medicinal chemistry, agricultural chemicals, polymer materials, and various industrial products. Nitrogen-containing heterocycles are abundant in nature and are found in many natural products such as alkaloids, vitamin derivatives, antibiotics, and hormones. About 60% of approved drugs contain nitrogen-containing heterocycles in their structure. Pyrimidine and its analogs have garnered considerable attention due to their diverse biological activities. Pyrimidine is a crucial class of heterocyclic compounds, valued for its medicinal properties and its role as the fundamental molecule in the pyrimidine base, which comprises uracil, cytosine, and thymine, forming the structures of DNA and RNA [14].
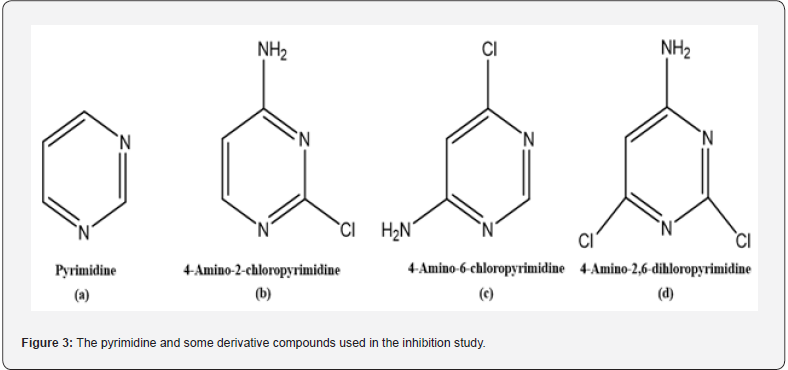
Pyrimidines are indeed vital constituents of all cells, essential for the functioning of biological organisms. These molecules, depicted in Figure 2, belong to the class of heterocyclic compounds and represent a crucial group known for their significant pharmacological activities [15,16].
In this study, the effects of pyrimidine and some derivative compounds (Figure 3) on GR enzyme activity have been investigated.

Materials and Methods
Chemicals
All chemicals used in the study were obtained from Sigma- Aldrich.
GR Enzyme Activity Measurement
GR enzyme activity measurement was performed according to the Carlberg and Mannervik method [17].
In vitro Inhibition Studies
The inhibitory effects of pyrimidine (a), 4-amino-2- chloropyrimidine (b), 4-amino-6-chloropyrimidine (c) and 4-amino-2,6-dichloropyrimidine (d) compounds on the GR enzyme were investigated. Enzyme activity was measured in the presence of different GSSH concentrations. The control activity was assumed to be 100% in the absence of inhibitors. Percentage activity graphs against inhibitor concentration were plotted for each inhibitor. Subsequently, Lineweaver-Burk [18] curves for the GR enzyme were obtained using three different concentrations of pyrimidine derivative compounds and five different concentrations of substrate (GSSG). Thus, KI values and inhibition types for each inhibitor were determined for the GR enzyme.
Results
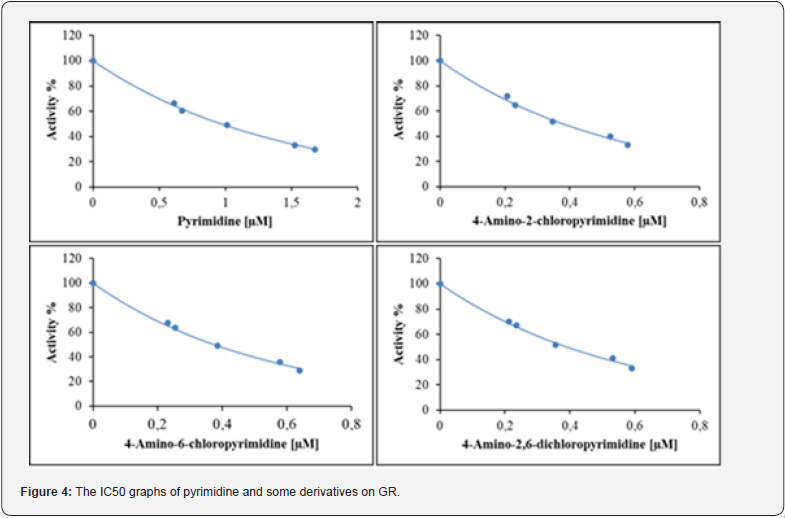
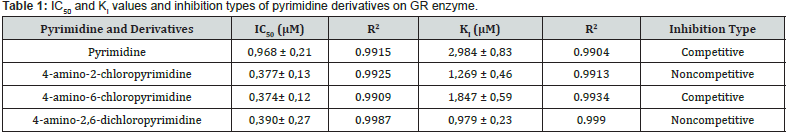
The IC50 values for pyrimidine (a), 4-amino-2- chloropyrimidine (b), 4-amino-6-chloropyrimidine (c) and 4-amino-2,6-dichloropyrimidine (d) were found to be 0.968 μM, 0.377 μM, 0.374 μM, and 0.390 μM, respectively (Figure 4). According to the results of the inhibition studies, 4-amino-2,6- dichloropyrimidine (d) (KI: 0.979 μM) exhibited a high level of inhibition on the GR enzyme.
It is observed that the amino and chloro groups present in 4-amino-2,6-dichloropyrimidine contribute to an effective inhibitory effect. Despite the chemical structures of the pyrimidine and derivative compounds being entirely the same apart from the groups and positions dependent on the ring structure, significantly different inhibition rates were observed. When comparing based on IC50 values, while 4-amino-2-chloropyrimidine showed three times more inhibition than pyrimidine (a), 4-amino-6- chloropyrimidine (c) and 4-amino-2,6-dichloropyrimidine (d) exhibited similar levels of inhibition. Therefore, it can be concluded that the presence of amino and chloro groups attached to the pyrimidine ring provides effective inhibition for the GR enzyme.
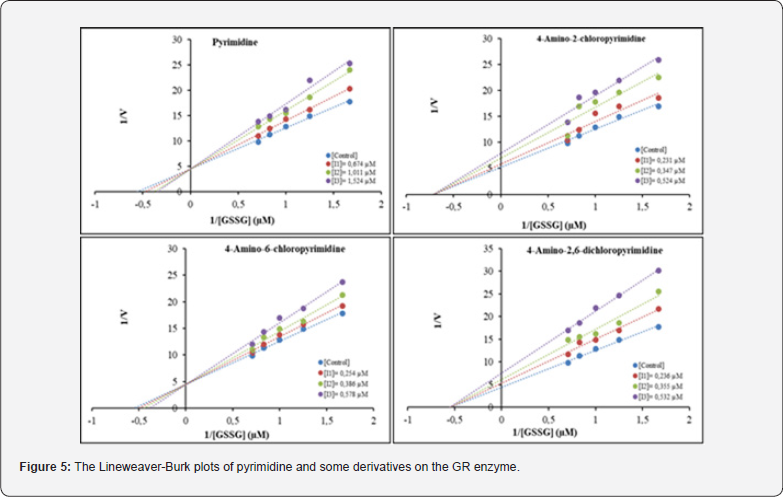
In the study, KI values were determined for each inhibitor. The KI values were found to be as follows: pyrimidine (a) (2.984 μM) > 4-amino-6-chloropyrimidine (c) (1.269 μM) > 4-amino-2-chloropyrimidine (b) (1.847 μM) > 4-amino-2,6- dichloropyrimidine (d) (0.979 μM) (Figure 5).
These compounds, showing potential inhibitor properties for GR, were reported to decrease enzyme activity at low concentrations (Table 1).
Discussion
The excessive production of free radicals results in oxidative stress. Oxidants disrupt homeostasis in the body, leading to a significant imbalance. This condition arises from various lifestyle or disease conditions such as chronic hyperglycemia or ketoacidosis attacks, excessive nutrient intake, and sleep disorders [19].
The imbalance between antioxidants and oxidants affects lipids, DNA, and proteins, leading to cellular necrosis and impaired physiological function of cells. Additionally, oxidative stress is believed to play a significant role in cardiovascular diseases, as well as neurodegenerative diseases such as Alzheimer’s and epilepsy [20-22].
Glutathione reductase (GR), glutathione S-transferase (GST), catalase (CAT), glutathione peroxidase (GPx), and superoxide dismutase (SOD) are a series of enzyme systems that constitute the enzymatic defense system, which are DNA repair enzymes [23]. GR catalyzes the reduction of oxidized glutathione in the presence of NADPH. GSH is utilized in preserving the thiol groups of intracellular proteins, preventing oxidative events, detoxifying xenobiotics, and neutralizing H2O2 and other organic peroxides [24].
Metabolic events occurring in living organisms are associated with the catalytic activity of enzymes involved in metabolism. Changes in enzyme activities are linked to various diseases. Therefore, compounds that inhibit enzyme activity are used as beneficial therapeutic agents [25]. Hence, in this study, the in vitro inhibition effects of pyrimidine and some derivatives on GR enzyme activity have been investigated.
Various drugs and chemicals have been studied for their effects on the GR enzyme in different studies. For example, in a study conducted by Şentürk et al. [26], the GR enzyme was purified from human erythrocytes, and the inhibitory effects of some antibiotics were investigated. Sulfanilamide, rifamycin, imipenem, ceftazidime, ceftriaxone, chloramphenicol, vancomycin, ornidazole, and cefuroxime showed inhibitory effects on the enzyme, while lincomycin, amikacin, clindamycin, and amoxicillin exhibited activation effects on enzyme activity. According to this study, imipenem showed the most potent inhibitory effect [26].
In another study, the inhibitory effects of methotrexate, dacarbazine, pantoprazole sodium, 5-fluorouracil thiocolchicoside, and olanzapine on GR activity were investigated, and dacarbazine exhibited the most potent inhibition effect on the enzyme [27]. A group of researchers has investigated the effects of Schiff base derivatives on baker’s yeast GR. These compounds exhibited effective inhibitory properties [28]. In a study conducted by Demir [21], the effects of the anti-epileptic drugs phenytoin, gabapentin, and primidone on the GR enzyme were investigated. Phenytoin exhibited the most effective inhibitory property with competitive inhibition type [2]. Çalışkan et al. [29] investigated the effects of brimonidine and proparacaine on the GR enzyme. They found the KI values for brimonidine and proparacaine to be 144.10 ± 2.01 and 1,654.00 ± 26.29 μM, respectively. They stated that proparacaine inhibited the GR enzyme at high concentrations [29].
With the increasing level of knowledge in fields such as medicine, genetics, and proteins, we are approaching a new era in understanding the mechanisms of various diseases, making accurate diagnoses, and developing necessary treatment approaches. This subject has been researched by scientists for many years. Currently, numerous studies have been conducted and are ongoing on antibodies, vaccines, natural interferons, and various metabolic enzymes, which are used in the diagnosis and treatment of many diseases. These products are utilized in various fields, especially in medicine [30].
Conclusion
This study presents a highly effective protocol for pyrimidines. The inhibitory effects of pyrimidine and its derivatives on GR enzyme activity, which is known as an important target in the treatment of cancer, were investigated. According to inhibition, in studies conducted, KI values for GR were found to be in the range of 0.979±0.23 - 2.984±0.83 μM. 4-amino-2,6-dichloropyrimidine exhibited the most effective inhibition on GR enzyme activity. The findings obtained from this study contribute significantly to the literature. This work focuses on new approaches to assessing cancer progression by GR, which may then open new, exciting drug discovery opportunities and target identification opportunities. The compounds investigated in the study and their inhibitory effects on the GR enzyme could shed light on further biological research.
- Schilling CH, Letscher D, Palsson BØ (2000) Theory for the systemic definition of metabolic pathways and their use in interpreting metabolic function from a pathway oriented perspective. J Theor Biol 203: 229-248.
- Demir Y (2019) Purification of Glutathione Reductase from Human Erythrocytes: Inhibition Profile of Some Anti-Epileptic Drugs. J Institute Sci Technol 9(4): 2140-2147.
- Ozaslan MS, Demir Y, Kufrevioglu OI, Ciftci M (2017) Some metals inhibit the Glutathione Stransferase from Van Lake fish gills. J Biochem Mol Toxicol 31(11): e21967.
- Ozaslan MS, Demir Y, Aslan HE, Beydemir S, Kufrevioglu OI (2018) Evaluation of chalcones as inhibitors of glutathione S-transferase. J Biochem Mol Toxicol 32(5): e22047.
- Ceylan H, Demir Y, Beydemir Ş (2019) Inhibitory effects of usnic and carnosic acid on some metabolic enzymes: an in vitro study. Protein Peptide Letters 26(5): 364-370.
- Demir Y, Köksal Z (2019) The inhibition Effects of Some Sulfonamides on Human Serum Paraoxonase-1 (hPON1), Pharmacol Rep 71(3): 545-549.
- Demir Y, Isık M, Gulcin I, Beydemir S (2017) Phenolic compounds inhibit the aldose reductase enzyme from the sheep kidney. J Biochem Mol Toxicol 31(9): e21935.
- Demir Y, Taslimi P, Ozaslan MS, Oztaskin N, Çetinkaya Y, et al. (2018) Antidiabetic potential: In vitro inhibition effects of bromophenol and diarylmethanones derivatives on metabolic enzymes. Archiv der Pharmazie 351(12): e1800263.
- Trachootham D, Lu W, Ogasawara M, Rivera-Del Valle N, Huang P (2008) Redox regulation of cell survival. Antioxid Redox Signal 10: 1343-1375.
- Yu BP (1994) Cellular defenses against damage from reactive oxygen species. Physiol Rev 74(1): 139-162.
- Hwang GH, Ryu JM, Jeon YJ, Choi J, Han J, et al. (2015) The role of thioredoxin reductase and glutathione reductase in plumbagin-induced, reactive oxygen species-mediated apoptosis in cancer cell lines. Eur J Pharmacol 765: 384-393.
- Traverso N, Ricciarelli R, Nitti M, Marengo B, Furfaro AL, et al. (2013) Role of glutathione in cancer progression and chemoresistance, Oxid Med Cell Longev 2013: 1-10.
- Elda Valenti G, Tasso B, Traverso N, Domenicotti C, Marengo B (2023) Glutathione in cancer progression and chemoresistance: an update. Redox Exp Med 1: e220023.
- Merugu R, Garimella S, Balla D, Sambaru K (2015) Synthesis and biological activities of pyrimidines: a review. Synthesis 8: 88-93.
- Sasada T, Kobayashi F, Sakai N, Konakahara T (2009) An unprecedented approach to 4, 5-disubstituted pyrimidine derivatives by a ZnCl2-catalyzed three-component coupling reaction. Org Lett 11(10): 2161-2164.
- Duran HE (2023) Pyrimidines: Molecular docking and inhibition studies on carbonic anhydrase and cholinesterases. Biotech Appl Biochem 70(1): 68-82.
- Carlberg I, Mannervik B (1985) Glutathione reductase. Methods Enzymol 113: 484-490.
- Lineweaver H, Burk D (1934) The determination of enzyme dissociation constants. J Am Chem Soc 56: 658-666.
- Türkeş C, Demir Y, Beydemir S (2019) Anti-diabetic Properties of Calcium Channel Blockers: Inhibition Effects on Aldose Reductase Enzyme activity. Appl Biochem Biotechnol 189(1): 318-329.
- Işık M, Demir Y, Kırıcı M, Demir R, Şimşek F, et al. (2015) Changes in the anti-oxidant system in adult epilepsy patients receiving antiepileptic drugs. Arch Physiol Biochem 121: 97-102.
- Demir Y, Oruç E, Topal A (2016) Carbonic Anhydrase Activity Responses and Histopathological Changes in Gill and Liver Tissues after Acute Exposure to Chromium in Brown Trout Juveniles. Hacettepe J Biol Chem 44(4): 515-523.
- Taslimi P, Aslan HE, Demir Y, Oztaskin N, Maraş A, et al. (2018) Diarylmethanon, bromophenol and diarylmethane compounds: Discovery of potent aldose reductase, α-amylase and α-glycosidase inhibitors as new therapeutic approach in diabetes and functional hyperglycemia. Int J Biol Macromol 119: 857-863.
- Alım Z, Kılıc D, Demir Y (2018) Some indazoles reduced the activity of human serum paraoxonase 1, an antioxidant enzyme: in vitro inhibition and molecular modeling studies. Arch Physiol Biochem 125(5): 387-395.
- Erat M, Sakiroglu H, Ciftci M (2005) Effects of some antibiotics on glutathione reductase activities from human erythrocytes in vitro and from rat erythrocytes in vivo. J Enzyme Inhibition Med Chem 20(1): 69-74.
- Kırıcı M, Kırıcı M, Demir Y, Beydemir Ş, Atamanalp M (2016) The Effect of Al3+ and Hg2+ on Glucose 6-Phosphate Dehydrogenase from Capoetaumbla kidney. Appl Ecol Environ Res 14(2): 253-264.
- Senturk M, Kufrevioglu OI, Ciftci M (2008) Effects of some antibiotics on human erythrocyte glutathione reductase: an in vitro study. J Enzyme Inhib Med Chem 23(1): 144-148.
- Akkemik E, Şenturk M, Özgeriş FB, Taşer, P, Çiftci M (2011) In vitro effects of some drugs on human erythrocyte glutathione reductase. Turkish J Med Sci 41(2): 235-241.
- Balaydın HT, Özil M, Şentürk M (2018) Synthesis and glutathione reductase inhibitory properties of 5- methyl-2,4-dihydro-3H-1,2,4-triazol-3-one's aryl Schiff base derivatives. Arch der Pharmazie 8: e1800086.
- Çalışkan B, Öztürk Kesebir A, Demir Y, Akyol Salman İ (2022) The effect of brimonidine and proparacaine on metabolic enzymes: Glucose-6-phosphate dehydrogenase, 6-phosphogluconate dehydrogenase, and glutathione reductase. Biotechnol and Appl Biochem 69(1): 281-288.
- Leader B, Baca QJ, Golan DE (2008) Protein therapeutics: a summary and pharmacological classification. Nature Rev Drug Discovery 7(1): 21-39.






























