Clinical Efficacy of an Osmotic Polymeric Stable Film for the Treatment of Mild to Moderate Atopic Dermatitis
Rémi Shrivastava1*, Deepti Shrivastava2 and Nathalie Maneby3
1Research scientist, Naturveda Laboratories, France
2Medical Device research scientist, Polytrap Pharma, India
3Vitrobio Pharma, France
Submission: September 28, 2023; Published: October 05, 2023
*Corresponding author: Rémi Shrivastava, Naturveda Laboratories, France, Email: remi.s@naturveda.fr
How to cite this article: Rémi Shrivastava*, Deepti Shrivastava and Nathalie Maneby. Clinical Efficacy of an Osmotic Polymeric Stable Film for the Treatment of Mild to Moderate Atopic Dermatitis. Adv Biotech & Micro. 2023; 17(4):555967.DOI:10.19080/AIBM.2023.17.555967
Abstract
Introduction: Atopic dermatitis is one of the most common multifactorial genetic pathologies with a strong impact on the patient’s quality of life. It involves alteration in skin microflora, excessive microbial growth, skin inflammation, dryness, dysregulation of cell growth, broken skin barrier, with a pathetic environment in the lesion. A good treatment should act on these parameters simultaneously but in the absence of any multi-target therapy, we conceived an osmotic polymeric film capable of attracting and draining all the free-floating contaminants from the lesion. The efficacy of this approach is evaluated clinically.
Materials and Methods: We associated highly osmotic glycerol solution with specific glycerol binding polymers to conceive a stable osmotic film (PED). A water gel was used as a placebo. Products were filled in 50-ml tubes and applied on the lesions as a thin layer, twice a day for a period of 6-weeks. EASI scores (EASI 50 and 75), along with skin lichenification, excoriation, dryness, oozing, oedema, erythema, and impact on quality of life were measured at baseline, weeks 2, 4, and 6.
Results: The test product showed significant improvement of the EASI score at each time point compared to the placebo. Regarding the secondary criteria, all the parameters evaluated showed a significant amelioration compared to the placebo. No adverse effects were recorded in any of the patients.
Conclusion: Clinical results show that PED film can be used as an excellent supportive treatment to improve quality of life of AD/AE patients.
Keywords: Atopic dermatitis, eczema, multitarget treatment, medical device, EASI
Abbreviations: AD: Atopic Dermatitis; AE: Atopic Eczema; Il: Interleukin; TP: Test Product; CP: Comparative Placebo Treatments; EASI: Eczema Area and Severity Index; Sem: Standard Error of The Mean; NS: Not Statistically Significant
Introduction
Atopic dermatitis (AD) or atopic eczema (AE) is a chronic immune-mediated inflammatory disorder that makes the skin itchy, red and dry [1]. It is one of the most common skin disorders, affecting millions of individuals worldwide, including children and adults. The prevalence of atopic dermatitis has been increasing over the past few decades, particularly in industrialized countries [2]. The exact cause of atopic dermatitis is not entirely understood, but it is believed to involve a complex interplay of genetic, environmental, and immunological factors leading to the destruction of the skin barrier [3]. People with atopic dermatitis often have a family history of allergic conditions, such as asthma or hay fever, which suggests a genetic predisposition to the development of the disease. Additionally, various environmental factors, such as allergens, irritants, and climatic conditions, can exacerbate the symptoms [3].
Several mechanisms contribute to damage the skin barrier in these diseases: exposure to environmental triggering factors, alteration of natural skin microflora with increased colonisation of Staphylococcus aureus (SA), S. hominis (SH) and S. epidermidis (SE); alterations of filaggrin gene (FLG), responsible for encoding a crucial structural protein involved in the metabolism of lipids and membrane ceramides, loss of the integrity of tight junctions, dysregulation of the differentiation process of keratinocytes, and consequently disruption of the skin barrier [4]. Opportunist Staphylococcus bacteria easily penetrates across the epidermis and triggers local inflammatory response with extensive and continuous topical release of pro-inflammatory cytokines such as interleukin (IL)-4, IL-13, IL-22, IL-25, IL-33, TSLP (thymic stromal lymphopoietin), EGF (epithelial growth factor), and FGF (fibroblast growth factor), with decreased expression of antimicrobial peptides, contributing to skin barrier damage [5,6]. The chronic interplay in the dermis between abundant presence of immune cells, multiple pro-inflammatory cytokines, broken skin barrier, and change in skin microflora with colonisation by Staphylococcus sp., leads to recurrent skin inflammation, extensive presence of dead and dying cells, opportunist microbial growth, skin drying, sloughing, and disturbed skin functions [7].
What is evident from the physiopathology of AD/AE is the complexity of disease pathology involving multiple factors. Being genetically triggered immune diseases, no curative treatments are currently available for such disorders. Multiple guidelines have been published for the management of AD/AE and skin diseases [8,9]. Almost all these recommendations are based on the use of emollients [10,11], either alone or in association with different moisturisers [12] to keep the skin hydrated, and antiinflammatory drugs to reduce itching and skin irritation and allow reconstitution of the skin barrier [13]. Unfortunately, the duration of these treatments is short lasting, temporary, and little is known about their real efficacy [11]. Many of these topical treatments contain glycerol as a moisturising agent [14] but glycerol gets rapidly diluted due to its osmotic properties limiting the duration of lesion hydrating properties [13].
Topical corticosteroids are the first-line treatment for AD/AE flare-ups as they decrease the inflammatory immune response, but due to their long-term potential adverse effects leading to skin atrophy, low-potency topical steroids are to be used for shortest possible period [15]. Topical calcineurin inhibitors which are steroid-sparing immunomodulators, are also used as a secondline treatment, usually in conjunction with topical steroids [16]. Other commonly suggested treatments include antihistamines to stop pruritus, ultraviolet phototherapy to reduce inflammation, oral or topical antibiotics to minimize microbial growth, systemic immunomodulators such as cyclosporine to limit severe flare-ups [17], and even vitamins and mineral supplements to stimulate cellular functions [18].
Although, multiple new generations of systemic biological drugs (monoclonal antibodies) are under clinical development, all are directed to block only one or two disease-related and inflammatory cytokines and unfortunately, other disease factors are left untouched. Among those drugs, the recent FDA approved Dupilumab (Dupixent) injectable monoclonal antibody, designed to block only IL-4 and IL-13 expression, is presented as the most promising future treatment. In our opinion, no biological mono-target drug can give substantial relief as all other disease parameters remains unchanged [19]. A good symptomatic treatment should be totally safe, long-lasting, should protect the lesion against environmental disease flaring factors, keep the lesion hydrated, drain maximum number of inflammatory cytokines to reduce inflammation along with removing dead cells, cellular debris, and other contaminant particles from the lesion. Only then can it provide optimal conditions for the reconstitution of cellular matrix and natural cellular barrier. As it is impossible to conceive a chemical or biological molecule having these basic, yet essential properties, we envisaged employing a unique, mechanically acting, scientific, and logical approach to conceive a stable, non-irritant, osmotic film, capable of inducing a strong osmotic liquid flow from the AD/AE lesion to protect, clean, and keep the lesion hydrated.
We identified glycerol, a highly osmotic, safe, non-irritant solution having capacity to generate strong outward osmotic liquid flow when applied on any live biological surface [20]. The osmotic liquid outflow keeps the lesion hydrated and removes all types of free surface impurities, including cytokines, which should help minimize irritation, itching, and inflammation [21]. Unfortunately, this liquid flow instantly dilutes glycerol and diminishes its osmotic power within a few minutes. Therefore, we introduced specific glycerol molecule binding polymers such as tannin-rich plant extracts and a few food-grade thickeners in the preparation (PED-gel), to render the film stable and resistant to mechanical pressures [21].
Materials and Methods
A randomized, double blind, placebo controlled clinical trial was conducted over a 6-week period to evaluate the safety and efficacy of PED-gel for the treatment of mild to moderate AD/AE lesions, using a water-gel as placebo.
Study organization
Initially a full clinical trial involving patients with psoriasis, eczema and dermatitis was performed with PED product and the study details were published by Shrivastava et al. [22]. The following analysis comprises only patient population diagnosed with atopic dermatitis, including atopic eczema, in order to better evaluate the efficacy and safety of PED gel in this specific subpopulation. In short, the clinical study was conducted in the dermatology department of the Geeta Bhavan Hospital and Research Centre, Indore, India. The study was registered under number ISSN 23195878 and was approved by relevant ethics committees and institutional review boards. The trial complied with the International Conference on Harmonization Guidelines for Good Clinical Practice, the principles of the Declaration of Helsinki and later amendments, and relevant national and local regulations. As the trial was performed with an EU certified medical device (MD) (CE n° 0459), topically applied, already marketed, and with proven safety, CTR (clinical trial registration) was not required. Only those participants who signed an informed consent form were included in the trial.
Inclusion Criteria: The main inclusion criteria were,
i. patients accepting to participate in the trial and to sign informed consent,
ii. subjects having single or multiple localized skin lesions for at least the last 6-weeks, which were diagnosed with AD (or AE) by the dermatologist during the 1st visit.
iii. male or female above 18 years of age;
iv. having lesions which could be measured and covering a surface area of at least 3 cm².
v. participants agree to blindly use the trial treatment or placebo as per randomization outcome, and
vi. patients ready to visit the hospital at planned intervals.
Exclusion criteria
i. patients having skin lesions diagnosed with psoriasis (excluded in the current analysis).
ii. not able to visit the hospital, or not able to read or write.
iii. under treatment for other serious diseases such as cancer.
iv. sensitive or allergic to any ingredient of the product composition.
v. with a history of tubercular, syphilitic, or viral skin infections.
vi. diagnosed with any serious hepatic, renal or other disease, based on recent (0-16 weeks old) hematological, blood biochemical or urine analyses.
vii. under any systemic or topical therapy for AD/AE for the last 15 days.
Test product (TP)
The TP was a liquid gel containing glycerol, honey, water, xanthan gum, and a natural polymeric mix derived from Vaccinium macrocarpon fruit, Camellia sinensis leaves, & Vitis vinifera seeds, in quantities capable to render the glycerol-honey film stable, as described by Shrivastava et al. [23]. TP was manufactured at Vitrobio France (ISO 13485 certified), filled in 50-ml PET white tubes fitted with a small canula for topical application, and labelled PED-1.
Comparator products (CP)
A water-gel (1% carrageenan) solution in 50-ml white PET plastic tubes, labelled PED-2.
Randomization
After screening, patients satisfying all the inclusion and none of the exclusion criteria were enrolled and randomly allocated in a 1:1 ratio, to either TP or CP group. Randomization was performed using SAS Version 9.1.3 following a randomization schedule. Block Randomization methodology was employed to generate the list and a unique enrolment identification number was given to each patient. The identification number was indicated in the personal diary of each patient. It was decided to stop the study or exclude the patient in case of any critical event or in case of any serious undesirable event.
Study Design
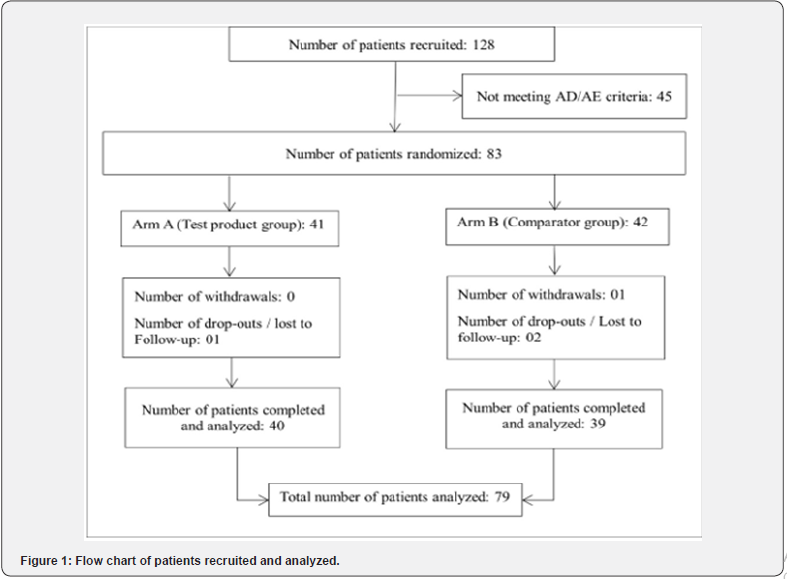
It was suggested to apply a few drops of the allocated treatment directly on the injured skin surface to form a thin product layer on the lesion surface by gentle massage (approximately 1 ml / 5 cm2 surface), twice a day, for a period of 6-weeks. If the lesions were not open (covered with a layer of dead keratinocytes), the lesion surface was gently scrapped with a plastic comb before product application. The flowchart of the patients recruited, randomized and treated, is shown in Figure1.
Parameters Measured
All patients’ personal details and medical history were recorded in the observation file, and patients were asked to come to the hospital at the end of weeks 2, 4, and 6 (±2 days between each visit). For each patient, the dermatologist recorded the distribution of affected area on the body, lower limbs, face, and upper limbs.
The main clinical endpoint was the mean Eczema Area & Severity Index (EASI) which is a widely used tool for assessing severity of AD/AE [24]. The secondary endpoints were individual scores of lichenification indicating thickening, hardening, and lathery skin with exaggerated skin markings compared to normal skin; excoriation, showing the intensity of scratching, rubbing or flaying; skin oozing, indicating exudation of liquid from the lesion surface due to microbial infection; lesion infiltration/papulation, indicating lesion swelling which may be due to inflammation or to accumulation of immune cells in the affected area; erythema, reflecting redness of the skin caused by inflammation and increased blood flow usually accompanied by itching, burning, and/or pain. All the parameters were evaluated for intensity and severity using an arbitrary scoring scale between 0 to 4 where 0 indicated completely normal skin while 4, the highest severity. The mean of all the scored parameters at the end of weeks 2, 4, and 6 were compared to baseline (BL) scores, to assess the change in each parameter at each time point and to determine mean effect on quality of life (QOL) at the end of the study.
The Eczema Area and Severity Index (EASI)
This score was calculated for each body region by multiplying the lesion intensity scores of lichenification, excoriation, erythema, and oedema, due to infiltration of inflammatory mediators by the percentage of surface area affected and by a region-specific weighting factor. The weighting factors were as follows: head & neck: 0.1, upper limbs 0.2, trunk 0.3, lower limbs 0.4.
Total EASI score
To obtain the total EASI score at each time point, the EASI scores for each body region were added together. The total score ranges from 0 (no AD/AE) to 72 (severe AD/AE).
EASI50 and EASI75 scores
EASI50 and EASI75 scores are commonly used criteria in AD/AE clinical trials to assess the efficacy of treatment. EASI50 or EASI75 means a reduction of at least 50% or 75% in the EASI score from the patient’s baseline at a particular time point (weeks 2, 4, and 6). This criterion is used to determine whether a treatment is effective and to compare the efficacy against CP.
Statistical analyses of data
The Student’s t-test was used to compare the mean EASI scores between the TP and CP treatment groups at defined intervals. A two-way analysis of variance ANOVA was then used to evaluate the changes in the two groups over time. For the secondary endpoints, the two-way analysis of variance (ANOVA) test was used to quantify the effect of CP vs TP over a 6-week period. The software used was GraphPad Prism version 9.5.1; * = p<0.05, ** = p<0.01, *** = p< 0.001, and **** = p<0.
Results
Population analyses (Figure 1)
Flow chart of the patients screened and recruited in the study. Most of the patients not meeting AD/AE selection criteria were diagnosed with psoriasis. The drop-out patients (1 in TP and 3 in CP) did not follow the treatment schedule and were excluded. As shown in Figure 1, 128 patients were screened and 45 were rejected mainly because the lesions were diagnosed as psoriasis. Among 83 patients randomized, 41 were allocated to TP group and 42 to the CP group. 1 patient in the CP group was withdrawn while 1 patient in the TP and 2 in the CP group were lost to follow-up because they didn’t complete the treatment schedule. 79 patients completed the study, 39 in the CP group (18M & 21F, mean age 43.2 years) and 40 in the TP group (16M and 24F, mean age 43.1 years). The demographic distribution of the patients in the two groups was comparable (CP vs TP, p=0.989, ns).
Distribution of affected areas
The distribution of AD/AE lesions on body, lower limbs, face, and upper limbs in the CP group was 15%, 62%, 15% and 8% compared to 20%, 50%, 18%, and 13% in the TP group, respectively. The distribution was considered homogenous between the two groups.
Effect on EASI scores
In this study, the initial EASI scores at the start of treatment (BL) was compared between the CP and the TP groups at different time intervals (Figure 2). At BL, the mean score in the CP group was 43.38 (±12.93) and 46.23 (±13.60) in the TP group, showing a mean difference of 2.84 (±2.85). This difference was not significant between the two groups (p=0.34, ns) and corresponds the study criteria.
Just after 2-weeks of treatment, the mean EASI score in the CP group was very slightly reduced (mean 40.14 ±12.57)) but a significant decrease (p<0.001) was observed in the TP group (mean score 26.29 ±13.76) showing a sharp reduction (-13.85 ±2.97) in favour of TP. These results indicate that TP is effective in reducing symptomatic manifestation of AD/AE after 2-weeks of treatment. At 4-weeks, the mean EASI score in the CP group remained stable (38.0±14.03) whereas in TP group (18.40±11.81) a reduction of 51.58% was observed, demonstrating a statistically significant improvement with TP vs CP (p<0.001). At the end of the study period (week 6), the mean EASI scores of the CP and TP groups were 36.0 (±12.14) and 14.73 (±9.18), respectively. The TP treatment showed a significant mean improvement in EASI score compared to the CP group (-59.08% vs CP p<0.001).
It should be noted that compared to BL, after 6 weeks of treatment, the mean EASI score was reduced by 17.01% in CP vs 68.13% in TP; that this reduction was progressive but very slow in the CP compared to TP group, and that the cumulative EASI score in TP vs BL was decreased by 43.14% at the end of week 2, by 61.07% at the end of week 4, and reached 68.14% at the end of the week 6. These results demonstrate that the effects of TP are much stronger during the 1st 2 weeks (-42.14% vs BL) but progressively fade between weeks 2 and 4 (-17.93%) and further between weeks 4 and 6 (-7.07%), suggesting that the TP treatment is effective in reducing the EASI score, particularly during the 1st two weeks of treatment but may not cure the disease even after prolonged treatment.
Effect on EASI50 and EASI75
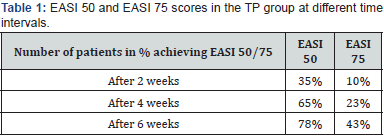
EASI50 was not obtained with the CP treatment but after 2, 4, and 6 weeks of treatment, EASI50 was achieved in 35%, 65% and 78% patients in the TP group, respectively. Similarly, in this group, even EASI75 was achieved in 10%, 23%, and 43% patients after 2, 4, and 6 weeks of treatment, respectively. These results indicate that TP is much more effective in providing symptomatic relief in AD/AE patients Table 1.

Secondary parameters
These parameters included skin/lesion lichenification, excoriation, skin dryness, skin oozing, oedema, and erythema, which were quantified on a scale between 0 (no lesion) to 4 (severe lesion). The mean scores for each lesion at each time point and the statistical significances are summarized.
Mean scores of secondary parameters
Lesion lichenification (Table 2)
Conclusion: At the end of the 2-weeks of treatment, a drop in lichenification score of TP was noticed (-42.92% vs CP). Thereafter, the mean score in the TP group continued decreasing progressively up to week-6 but such slight and progressive reduction was also observed in the CP group from the start of treatment. Compared to BL values, the decrease after 6-weeks of treatment was 34.30% in CP and 67.05% in TP with a statistically significant difference vs CP through the study period. These results show that TP is nearly twice more effective than CP in reducing AD/AE lesion lichenification within 6-weeks of treatment.
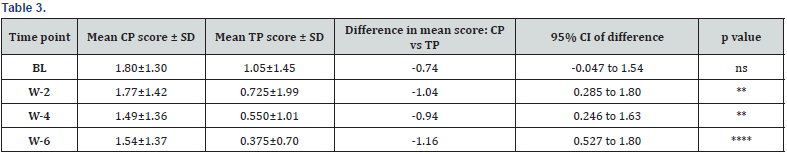
Excoriation (Table 3)
Conclusion: From week-2 onwards, the reduction in TP group was faster and showed a highly significant decrease vs CP at week- 6 (-14.31% in CP vs -64.76% in TP compared to BL mean values).
Skin dryness (Table 4)
Conclusion: The dryness scores were not significantly different between the CP and TP groups before treatment (BL). However, from week-2 onwards, there were significant differences between the groups, with higher dryness scores in the CP group compared to the TP group. This improvement in TP continued and increased further at week-4 and week-6, showing that TP was effective in keeping the skin/lesion hydrated throughout the study period.
Skin oozing (Table 5)
Conclusion: This parameter was not changed in CP up to the end of week-6 but in TP, a decrease was observed during the 1st 2-weeks (p<0.01 vs CP) with further reduction up to the end of the week-6 (p<0.001 vs CP). These results indicate that TP, even if being an osmotic film which should enhance lesion oozing, reduces liquid exudation from AD/AE lesions. This may be related to the fact that cleaning the lesion through osmotic effect continuously reduces the concentration of free-floating inflammatory molecules thereby reducing inflammation-induced lesion oozing.
Lesion infiltration & oedema (Table 6)
Conclusion: The oedema of the lesion is generated when the skin is inflamed due to endless release of proinflammatory cytokines from the epidermal surface [6]. Reduction in inflammation reduces swelling and oedema. As shown are the results the oedema was strong in both CP and TP groups at BL. CP is found to be active as it reduces the oedema by 15.06% compared to BL after 6 weeks of treatment, but the effects of TP were remarkably strong as oedema was reduced by 62.5% vs BL (p=0.001). This shows high efficacy of TP vs CP.
Lesion erythema (Table 7)
Conclusion: At the start of treatment, the AD/AE lesions in patients of both groups showed severe erythema which remained nearly unchanged in the CP group up to the end of the study. On the contrary, at the end of the 2nd week of treatment, a decrease in erythema was seen in the TP group patients (-48.38% vs BL, p<0.001 vs CP). In the TP group, this reduction continued up to the end of the study (W-6, -75.0% vs BL, p<0.001 vs CP).





Tables 2 to 7 Effects of CP and TP treatment on the intensity and severity of mean scores obtained for each secondary parameters at the start of the study (BL), and at weeks 2, 4, and 6. The difference with the CP score, SE of the mean, minimum and maximum 95% confidence interval as well as statistical significance vs CP (p values) at each time point are also indicated. Abbreviations: BL = Baseline T0, W=end of the week, vs = versus, SE = Standard error of the mean; CI = 95% confidence interval (Tables 2 to 7).
Effect on Quality of Life (QOL)
Bonferroni multiple comparisons analysis was used to compare the differences between the mean values of all the scored parameters at BL and at the end of the weeks 2, 4, and 6. Results show no significant differences between the 2 groups at BL (CP 2.35 vs 2.65 TP) and at the end of the Week-2 (CP 2.19 vs 2.08 for TP, p>0.999). But at week 4, the difference between the CP and TP groups was significant (mean difference = 0.6147, 95% confidence interval = 0.1063 to 1.123, adjusted p = 0.0111*). At the end of week 6, a highly significant improvement in QOL was observed in favour of TP. This result indicates that patients in the TP/PED group experienced a significant improvement in quality of life compared to the Placebo group at week 4. The difference between CP vs TP was highly significant at the end of the week 6 (mean score CP 1.97 vs 1.18 for TP, p<0.001) as compared to the BL scores, the reduction was -15.81% in CP compared to -55.85% in TP.
Side effects: No treatment related significant undesired effects were observed in any of the patients of both groups. The physician’s global assessment (PGA) for the efficacy and safety of PED treatment was highly positive.
Discussion
AD/AE is a uncurable, chronic, heterogenous, inflammatory skin disorder, typically starting in childhood and often persisting into adulthood [2]. This disease comprises a highly complex, multi-factorial pathophysiological mechanisms involving interactions between immune dysregulation, epidermal gene mutations, and environmental factors that disrupts the epidermis causing highly infected, damaged, and intensely pruritic skin lesions [25]. Repeated scratching triggers a self-perpetuating itchscratch cycle, which can have a significant impact on the patient’s quality of life. The severity of the lesion may vary depending on the exposure to AD/AE triggering factors, skin dryness, severity of microbial infection, concentration of pro-inflammatory cytokines, epidermal inflammation, presence of dead & dying cells in the lesion, possibilities of cellular matrix & skin-cell regeneration, and the extent of skin barrier disruption. It is therefore comprehensible that a monotargeted treatment focusing only on one of the factors such as protecting the lesion with a cotton bandage [26], reducing inflammation [27], minimizing microbial contamination, keeping the lesions hydrated [28], or blocking the activity of one of the cytokines, can only give temporary symptomatic relief but cannot offer a long-lasting, symptom suppressing effect. Most of such topical therapies are commercialized and presented as drugs or OTC medical devices [29,30].
PED is simple and safe but a scientific and logical global approach to keep the AD/AE lesions protected, clean, and hydrated having low concentration of inflammatory cytokines to reduce inflammation, oedema, oozing and itching. PED forms a non-irritant, stable, safe, osmotic, and non-absorbent topical barrier film on the AD/AE lesion surface which protects the lesions against environmental contaminants. Being osmotic, the film continuously attracts hypotonic liquid from the lesion thereby draining and detaching all the free-floating inflammatory proteins, microorganisms, and other contaminants, allowing reconstitution of the broken skin barrier [21].
In this study, we analyzed the efficacy of TP vs CP on the EASI score and various symptoms associated with AD/AE. The results clearly demonstrate a higher efficacy of TP vs CP on the reduction of the EASI score as well as on the improvement of associated symptoms. Regarding the EASI score, results show that 78% of patients achieved at least 50% improvement (EASI 50), while 43% patients achieved at least 75% improvement (EASI 75) at the end of the 6-week treatment period. This outcome proves that PED effectively limits the progression of eczema and non-severe atopic dermatitis. PED improves skin barrier functions by reducing lesion dryness, oozing, and inflammation as evident by reduction in lichenification, excoriation, and erythema due to continuous osmotic cleaning of the lesion impurities such as inflammatory cytokines. Reduction in inflammation in turn, restores skin barrier and reduces AD/AE lesion exposure to environmental triggers such as allergens [31]. These symptomatic improvements finally reflected in ameliorating the QOL of patients with TP. No side effects were observed in any of the patients which was evident, taking into consideration the mode of action of TP.
Study limitations
The trial duration was short, and the study was conducted with a limited number of patients in India, which may raise concerns about the generalizability of the results to other populations. Evidence suggests the physiopathology of AD/AE remains relatively similar in all ethnic groups which should not affect efficacy of treatments [32]. A part of vulnerable population such as children, breastfeeding and pregnant women and patients under treatment for chronic diseases were excluded but taking into consideration the product composition and the mode of action of PED, it is likely that the treatment should achieve similar results in this population.
Conclusion
In light of the multifaceted nature of AD/AE, the need for an integrated approach to management becomes paramount. Our findings highlight the potential of PED as a supplementary tool in the broader therapeutic landscape for AD/AE. While many treatments aim at symptomatic relief, PED’s holistic approach seeks to address multiple underlying factors, notably by enhancing the skin’s barrier functions and mitigating environmental exposure. The demonstrated efficacy of TP in this study, coupled with its safety profile, reinforces its value as a complementary option. However, it’s crucial to emphasize that in the continuum of care for AD/AE, PED is one piece of a larger puzzle. It can be used as a supplement to existing therapies, offering patients a natural, holistic tool to potentially enhance their quality of life. As the field of dermatology continues to evolve, collaborative approaches that blend traditional treatments with innovative modalities like PED will likely be pivotal in ensuring optimal patient outcomes.
Data Sharing Statement
The data presented in this study are available on request from the corresponding author.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors and was entirely financed by VITROBIO Pharma Research Institute in France.
Conflict of Interest
The author reports no conflicts of interest in this work.
- Weidinger S, Novak N (2016) Atopic Dermatitis. The Lancet 387(10023): 1109-1122.
- Bylund S, Kobyletzki L Svalstedt M, Svensson A (2020) Prevalence and Incidence of Atopic Dermatitis: A Systematic Review. Acta Derm Venereol 100(12).
- Archer C B (2021) Atopic Dermatitis. Medicine (Baltimore) 49(6): 370-373.
- Folster‐Holst R (2022) The role of the skin microbiome in atopic dermatitis – correlations and consequences. JDDG 20(5): 571-577.
- Fania L, Moretta G, Antonelli F, Scala E, Abeni D, Albanesi C, et al. (2022) Multiple Roles for Cytokines in Atopic Dermatitis: From Pathogenic Mediators to Endotype-Specific Biomarkers to Therapeutic Targets. Int J Mol Sci 23(5): 2684.
- Alsabbagh M, Ismaeel A (2022) The Role of Cytokines in Atopic Dermatitis: A Breakthrough in Immunopathogenesis and Treatment. Acta Dermatovenerol. Alp Pannonica Adriat 31(1): 13-31.
- Çetinarslan T, Kümper L, Fölster-Holst R (2023) The Immunological and Structural Epidermal Barrier Dysfunction and Skin Microbiome in Atopic Dermatitis-an Update. Front Mol Biosci 10: 1159404.
- Eichenfield L F, Tom W L, Chamlin S L, Feldman S R, Hanifin J M, Simpson E L, et al. (2014) Guidelines of Care for the Management of Atopic Dermatitis. J Am Acad Dermatol 70(2): 338-351.
- Thyssen J P, Vestergaard C, Deleuran M, De Bruin‐Weller, M S Bieber, T Taieb A, et al. (2020) European Task Force on Atopic Dermatitis (ETFAD): Treatment Targets and Treatable Traits in Atopic Dermatitis. J Eur Acad Dermatol Venereol 34(12).
- Van Zuuren E J, Fedorowicz Z, Christensen R, Lavrijsen A P, Arents W (2017) Emollients and Moisturisers for Eczema. Cochrane Database Syst. Rev 2(2): CD012119.
- Danby S G, Andrew P V, Taylor R N, Kay L J, Chittock J, et al. (2022) Different Types of Emollient Cream Exhibit Diverse Physiological Effects on the Skin Barrier in Adults with Atopic Dermatitis. Clin Exp Dermatol 47(6): 1154-1164.
- Vanessa V V, Wan Ahmad Kammal W S L, Lai Z W, How K N (2022) A Review of Moisturizing Additives for Atopic Dermatitis. Cosmetics 9(4): 75.
- Breternitz M, Kowatzki D, Langenauer M, Elsner P, Fluhr J W (2008) Placebo-Controlled, Double-Blind, Randomized, Prospective Study of a Glycerol-Based Emollient on Eczematous Skin in Atopic Dermatitis: Biophysical and Clinical Evaluation. Skin Pharmacol. Physiol 1: 39-45.
- W Fluhr, M Gloor, L Lehmann, S Lazzerini (1999) Glycerol Accelerates Recovery of Barrier Function In Vivo. Acta Derm Venereol 79(6): 418-421.
- Axon E, Chalmers J R, Santer M, Ridd M J, Lawton S, et al. (2021) Safety of Topical Corticosteroids in Atopic Eczema: An Umbrella Review. BMJ Open 11(7): e046476.
- Pena J, Zameza P A, Pixley J N, Remitz A, Feldman S R (2023) A Comparison of Topical Corticosteroids and Topical Calcineurin Inhibitors for the Treatment of Atopic Dermatitis. J Allergy Clin Immunol Pract 11(5): 1347-1359.
- Sawangjit R, Dilokthornsakul P, Lloyd Lavery A, Lai N M, Dellavalle R, et al. (2020) Systemic Treatments for Eczema: A Network Meta-Analysis. Cochrane Database Syst Rev 2020 (9).
- Reynolds K A, Juhasz M L W, Mesinkovska N A (2019) The Role of Oral Vitamins and Supplements in the Management of Atopic Dermatitis: A Systematic Review. Int J Dermatol 58(12): 1371-1376.
- Lobefaro F, Gualdi G, Di Nuzzo S, Amerio P (2022) Atopic Dermatitis: Clinical Aspects and Unmet Needs. Biomedicines 10 (11): 2927.
- shrivastava R, Shrivastava L, Shrivastava R (2014) Composition for Topical Application Comprising Glycerol and Tannins.
- Shrivastava L, Shrivastava R, Shrivastava R (2020) Dual Acting Polymers in an Osmotic Film for Topical Application to Treat Inflammatory Diseases and Cytokine Release Syndrome.
- SHRIVASTAVA R (2013) Clinical Efficacy of a Liquid Bandage Compared to Placebo for the Treatment of Psoriasis, Eczema and Dermatitis. Int J Mod Pharm Res.
- Shrivastava R, Shrivastava R, Johansen B, Allain T (2021) Anti-Inflammatory and Antiviral Osmotic Polymeric Film to Treat Covid-19 Early-Stage Infection. J Inflamm Res 14: 1195-1206.
- Norito Katoh, Yukihiro Ohya, Masanori Ikeda, Tamotsu Ebihara, Ichiro Katayama (2019) Clinical practice guidelines for the management of atopic dermatitis. J Dermatol 46(12): 1053-1101.
- Chan C X, Zug K A (2021) Diagnosis and Management of Dermatitis, Including Atopic, Contact, and Hand Eczemas. Med Clin North Am 105 (4): 611-626.
- De Paepe K, De Rop E, Houben E, Adam R, Rogiers V (2008) Effects of Lotioned Disposable Handkerchiefs on Skin Barrier Recovery after Tape Stripping. Skin Res Technol 14(4): 440-447.
- Blanchard G, Walker A, Dendooven E, Aerts O, Goossens A, et al. (2023) Allergic Contact Dermatitis from a Skin‐calming Cream Containing Hydroxyphenyl Propamidobenzoic Acid. Contact Dermatitis 88(1): 68-70.
- Loden M (2005) The Clinical Benefit of Moisturizers. J Eur Acad Dermatol Venereol 19(6): 672-688.
- Butala S, Paller A S (2022) Optimizing Topical Management of Atopic Dermatitis. Ann Allergy Asthma Immunol 128(5): 488-504.
- Korting H C, Schöllmann C (2012) Medical Devices in Dermatology: Topical Semi-Solid Formulations for the Treatment of Skin Diseases: Medical Devices in Dermatology. JDDG 10(2): 103-109.
- Jensen J M, Proksch E (2009) The Skin’s Barrier. G Ital Dermatol E Venereol 144(6): 689-700.
- Kaufman B P, Guttman‐Yassky E, Alexis A F (2018) Atopic Dermatitis in Diverse Racial and Ethnic Groups—Variations in Epidemiology, Genetics, Clinical Presentation and Treatment. Exp Dermatol 27(4): 340- 357.






























