Bacteriological Assessment of Some Raw, Chilled Chicken Meat Cuts in Benha City
Fahim A Shaltout1*, Shimaa N Edris1, Mohamed E Nabil2 and Soha TTaha3
1Food Hygiene and Control Deptarment, Faculty of Veterinary Medicine, Benha University, Egypt
2Food Hygiene Deptarment, Animal Health Research Institute, Egypt
3Master of Veterinary Medicine, Egypt
Submission: August 23, 2023; Published: September 13, 2023
*Corresponding author: Fahim A Shaltout, Food Hygiene and Control Deptarment, Faculty of Veterinary Medicine, Benha University, Egypt, Email: fahimshaltout@hotmail.com
How to cite this article: Fahim A Shaltout*, Shimaa N Edris, Mohamed E Nabil and Soha TTaha. Bacteriological Assessment of Some Raw, Chilled Chicken Meat Cuts in Benha City. Adv Biotech & Micro. 2023; 17(4):555966.DOI:10.19080/AIBM.2023.17.555966
Abstract
Chicken meat is a popular, highly nutritious, and easily digestible source of protein. Chicken meat is a desirable target for direct or indirect bacterial contamination at each stage of production, from rearing to ready-to-eat meal. Therefore, the present study was conducted to evaluate the bacteriological quality of 120 random samples of raw, chilled chicken cuts (breast, thigh, drumstick, and wings,30 of each) sold in Benha city’s local markets and their risk to public health. The obtained results indicated that the examined chicken cuts meat samples exhibited the lowest safety with the highest bacterial counts; where aerobic plate count (APC), coliform count (CC), S. aureus and C. perfringens counts (CFU/g) were 1.9×104, 18x102, 7.2x102 and 1.1x103 for breast samples; 63x104, 22x102, 9.1x102 and 1.8x103 for drumstick samples; 85x104, 28x102, 12x102 an 2.8x103 for thigh samples; 8.6x104, 20x102, 10x102 and 1.5x103 for wing samples, respectively. The thigh samples also had a significantly higher rate of E. coli and salmonella than the other chicken samples (50 and 10 %, respectively). In addition, eight of the isolated S. aureus strains demonstrated an affinity for producing enterotoxins that were typed as SEA, SEC, and SED with a prevalence of 62.5%, 12.5%, and 25%, respectively. Samples were evaluated in accordance with Egyptian standards and their suitability for human consumption was documented. Therefore, strict hygienic measures should be implemented to reduce the affinity and dangers posed by bacteria that cause food poisoning.
Keywords: Food safety; Poultry meat; Egypt; Food poisoning; Water bacteria
Introduction
Chicken accounts for approximately two thirds of the world’s total production of animal protein, which helps alleviate the problem of lack of animal meat [1,2]. The widespread consumption of poultry meat can be attributed to its high quality, easily digestible proteins, which include essential amino acids; its low fat and cholesterol content; and its considerable content of minerals and vitamins [3,4].Poultry meat is considered perishable because it contains animal proteins that are easily degraded, a favorable PH, and physicochemical characteristics that promote the growth of microorganisms [5] Furthermore, poultry meat has been easily contaminated during evisceration from gut bacteria as salmonella and/or personal cross contamination, or by the surrounding environment from air or water bacteria increasing the incidence of foodborne microorganisms such as Salmonella, S. aureus, E. coli, and C. perfringens which remain a public health issue with zoonotic importance [6,7].
Among most prevalent bacteria contaminants poultry meat products, contamination with Enterobacteriaceae, which includes E. coli and salmonella and is a common resident of the gastrointestinal tract (GIT) of chicken, occurs not only during slaughtering but also in wet markets [8]. Salmonella and E. coli infections are typically accompanied by clinical symptoms of gastroenteritis, including vomiting, abdominal pain nausea, headache, and fever [9]. In addition, Shiga toxin- producing E. coli (STEC) can cause advanced persistent diarrhea as well as hemolytic uremic syndrome (HUS) [10]. Additionally, gram-positive bacteria, particularly Staphylococcus aureus and C. perfringens, are one of the main contaminants of meat and meat products. One of the most common types of bacteria found on people’s skin and in their environments (dust, water, air, feces, or on utensils) that can contaminate food is Staphylococcus aureus [11]. Staphylococcal enterotoxins encoded as SEA, SEB, SEC, SED, SEE, are primarily associated with S. aureus food poisoning and are responsible for emesis, nausea, diarrhea, and abdominal cramps for about 24-48h [12].
On the other hand, Clostridium perfringens, which is typically present in the GIT of food animal, can contaminate meat and meat products through improper practices that occurred during slaughtering and evisceration and may be linked to fecal contamination [13]. It is classified as a pathogenic bacterium that causes food poisoning because many vegetative cells can survive the acidic PH of the stomach and produce enterotoxin in the small intestine [14]. causing acute diarrhea and severe abdominal pain 8-24 hours after ingestion of the contaminated meat products [15]. As the result, the current study aimed to assess the bacteriological quality of chicken cuts (breast, thigh, drumsticks, and wings) and their suitability for human consumption in relation to Egyptian standards.
Materials and methods
Collection of samples
A total of 120 random samples of different raw, chilled chicken meat cuts represented by breast, thigh, wing, and drumstick (30 of each) were collected from different poultry butchers located in Benha city. Each sample was presented to the following steps for evaluation of their bacteriological quality:
i. Preparation of samples [16]
tenth fold serial dilutions were prepared on sterile peptone water (0.1%); from which the following parameters were examined:
ii. Aerobic plate count “APC” according to ISO 4833-1 [17]
APC agar and incubated at 30±1OC for 72h. The Aerobic Plate Count (APC) per gram was calculated on plates containing 15 – 300 colonies and each count was recorded separately.
iii. Coliform count “CC” according to ISO 4832, [18]
Violet red bile agar and incubated at 37±1OC for 24h. Suspected colonies, which showed purplish - red colonies surrounded by a red zone of precipitated bile acid, were enumerated to obtain coliforms count /gram.
iv. Prevalence and enumeration of Enteropathogenic Escherichia coli was performed according to ISO.
16649-2 (2001) included plating on Tryptone Bile X-glucoronide agar (TBX agar) followed by incubation at 44oC for 24h. Suspected colonies, which showed Greenish-blue colonies were enumerated to obtain coliforms count /gram.
v. Detection of salmonellae was performed according to ISO 6579 [19]
The prepared sample was incubated in buffered peptone water broth at 37°C ± 1°C for 18 ± 2 hours, then transferred to Rappaport Vassilidis broth (RV broth) and incubated at 43°C\ 24hr. One ml of enriched sample was plated on selective XLD agar and Brilliant Green agar, and incubated at 37°C\24h, plates were examined for suspected Salmonella colonies which then isolated for confirmation. The suspected purified salmonella colony was cultured on three biochemical media represented by (TSI agar, Urea agar, and L-Lysine decarboxylation medium) and incubated at 37°C\24hrs.
vi. Enumeration
Staphylococcus aureus was performed by plating 0.1 ml on Baird Parker agar. Suspected colonies were purified and subjected for further biochemical identification following ISO 6888- 1 [20] vii. Detection of Enterotoxins producing.
S. aureus isolates by Reversed Passive Latex agglutination kit (SET-RPLA) test was performed on 24 purified S. aureus isolates according to Igarashi et al. [21].
viii. Detection and enumeration
viable C. perfringens was performed by inoculating one ml of the previously prepared serial dilution on Tryptose sulfite cycloserine agar (TSC agar), followed by anaerobic incubation at 37oc for 20-22h. Suspected colonies were purified and subjected for identification on Lactose sulfite (LS) broth inoculation, which appeared as black ppt and gas formation according to ISO 7937 [22].
ix. Statistical Analysis
The obtained data was statistically treated by one-way ANOVA using SPSS software for Windows (Version 16). Duncan’s post hoc analysis was used to analyze the data, with a p-value of 0.05 being regarded statistically significant [23].
Results
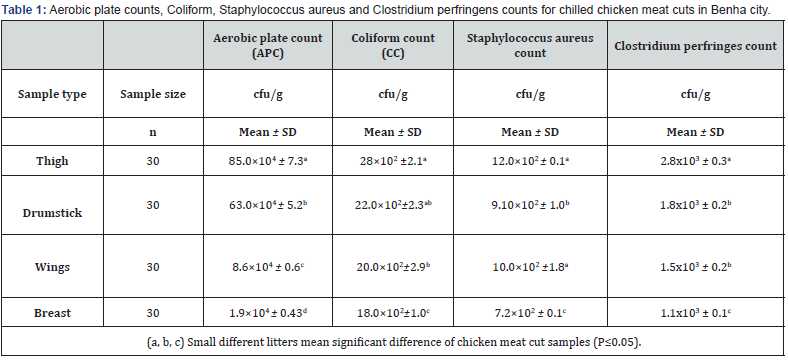
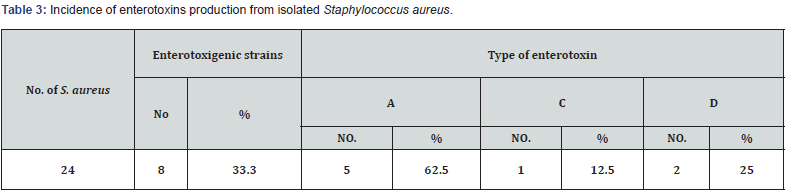
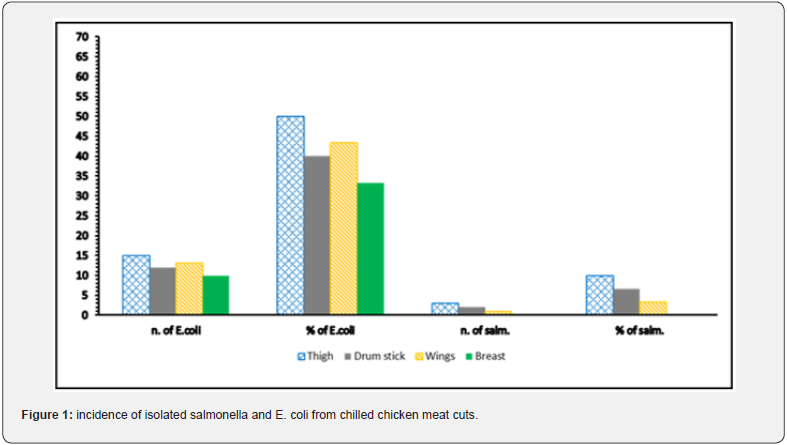
Table 1 showed that the APC, coliform count and C. perfringens count (CFU/g) was significantly (P ≤ 0.05) higher in the thigh samples than in the drumstick, wing, and breast samples, in that order. In terms of S. aureus count (CFU/g), there was no statistically significant difference (P > 0.05) between the thigh and wing samples, but there was (P ≤ 0.05) between the drumstick and breast samples. According to EOS, 2019, the breast samples had acceptable microbiological quality (66.6%, 76.6%, 90%, and 90% for APC, CC, Staphylococcus aureus, and Clostridium perfringens counts, respectively) when compared to the other chicken meat cuts (Table 2). Figure 1 depicts that thigh samples had the highest incidence (50%) of isolated E. coli, while breast samples had the lowest incidence (33 %). While, while a high rate of salmonella was found in 3 samples of thigh (10%) but failed to be detected in breast samples. In addition, Table 3 shows that out of the 24 isolated S. aureus strains, 8 (33.3%) showed positive affinity to produce enterotoxins, with 5 (62.5%) being positive for SEA, 1 (12.5%) being positive for SEC, and 2 (25%) being positive for SED.

Discussion
Chicken meat may be loaded with different foodborne bacteria through all the processing point’s starts with slaughtering and end to the cooking and serving steps [24]. Therefore, continuous microbiological assessment of the retailed poultry meats is recommended. Referring to the recorded results of APC (CFU/g), nearly similar results were reported by Hassanin et al. [25]. (6.13x104 CFU/g in breast samples, while it is considered lower than the current obtained results for drumstick and thigh (7.47x104, 6.51x104); Shaltout et al. [26] (5.9x105 and 7.1x105 in breast and thigh samples, respectively). While higher results were recorded by Wahbah [27] (5.5x106 and 6.8x106 for breast and thigh samples, respectively), and Hassanin et al [24]. (8.16x105, 7.85x105, 6.76x105 and 5.58x105 in wings, drumsticks, thigh and breast samples, respectively). On the other hand, lower counts were reported by Atia [28] (9.28x103 and 2.91x104 in breast and thigh samples, respectively), and Hosny et al. [29] (2x104 and 6x103 in drumstick and wing samples, respectively).
Detection of coliform bacteria in meat products usually indicates the environmental sanitation level around food processing area, or become as a sign of water pollution, personal hygiene and cross contamination may be [30]. Referring to the currently obtained results of coliform count (CFU/g), they were in line with the recorded results by Shaltout et al. [31] (37.3x102 (wing), 21.6x102 (breast) and 27.7x102 (thigh), and Hassanin et al. [24] (2.66×103, 2.12×103, 2.01×103 and 1.84×103 for wing, drumstick, thigh and breast, respectively); while, they were higher than those recorded by (3x102 (wing) and 1x102 (drumsticks)).
Contamination of chicken carcass with E. coli indicates unhygienic environment and possible fecal contamination during slaughtering, manual evisceration, and handling as this bacteria is naturally inhabitant in warm blooded animal gut and in intestine of human [32]. The current prevalence is higher than those recorded by Hassanin et al [24]. (8% (breast), 8% (thigh), 16% (wing) and 18% in (drumsticks), while lower than those recorded by Afify [33] (12% in breast and 18% in thigh samples). Salmonella is the second most common foodborne pathogen associated with zoonotic enteric human infection, which can occur as a result of cross-contamination with internal organs during evisceration or contamination during scalding or deboning [34] The current prevalence of Salmonella species in the examined samples is higher than those recorded by Shaltout et al. [35] (8% of thigh samples), but higher prevalence was reported in the recorded results of Atia [5] (8% and 20% of breast and thigh samples, respectively), and Elsisy [36] (20 and 25% of breast and thigh samples, respectively).
The presence of S. aureus in meat and meat products is indicative of poor hygienic practices, which are primarily the result of improper personal hygiene and a contaminated environment caused by knives, workers’ hands, or inadequately cleaned equipment [37]. The present results of Staphylococcus aureus count (CFU/g) are less than the recorded results of Shaltout et al. [38] (2.5 x103 in thigh, 2.4x103 in breast and 2.17x103 in wing), but the current prevalence came higher than those of Shaltout et al. [34] (10 and 4% of breast and thigh samples, respectively), and Mohamed et al. [39] (4.11x103 and 2.53x103 for thigh and breast samples, respectively); while came in line with those recorded by Mohamed et al. [39] (34.3% of the examined chicken cut samples, where its S. aureus enterotoxigenicity classification by SETRPLA test revealed detection of SEA, SEB and SEC, and Hassanin et al [25] (1.9×102, 2.2x102 and 2.6x102 for breast, thigh and drumsticks, respectively).
Clostridium perfringens (C. perfringens) is commonly found in soils, dust, foods (especially raw meat), human intestinal tracts (10%-30% of adults), and domestic animals (40 percent -80 percent in poultry). Under adverse conditions, C. perfringens can produce spores that are highly resistant to environmental stresses. Infection is typically acquired at schools and camps, or from food caterers or restaurants where large quantities of food are prepared and kept warm for extended periods of time [40]. Therefore, the presence of this bacterium is primarily regarded as fecal contamination. The present prevalence of C. perfringens was lower than the recorded results of Zakaria [41] (25% and 35% of breast and thigh, respectively), and Nabil (2018) (40 and 52% of the examined breast and thigh, respectively); while was nearly similar to Afshari et al. [42] who detected C. perfringens in 15.5% of the examined chicken meat samples. Moreover, lower results were recorded by Thangamani and Subramanian [43] who detected C. perfringens in 3.81% of the examined chicken meat samples. Variations in results among authors may be attributable to differences in sample origin, hygienic practices, personal hygiene, and sample processing status [44-47].
Conclusion
The results indicate that thighs had the highest levels of contamination, followed by drumsticks, wings, and breasts, in that order. This study indicates that fresh chicken meat cuts can harbor a variety of food-poisoning bacteria, resulting in substandard quality and public health risks.
- Ruban S W, Thiyageeswaran M, Sharadha R (2010) Isolation and identification of Salmonella spp. from retail chicken meat by polymerase chain reaction: research. International Journal of Microbiological Research. 1(3): 106-109.
- Saad S M, Edris AM, Shaltout F A, Edris Shimaa (2012) Isolation and identification of salmonellae and E. coli from meat and poultry cuts by using a multiplex PCR.
- Mohammed A Hassan, Shaltout F A (2004) Comparative Study on Storage Stability of Beef, Chicken meat, and Fish at Chilling Temperature. Alexandria journal of veterinary Science 20(21): 21-30.
- Bhaisare D B, Thyagarajan D, Richard Churchill R, Punniamurthy N (2014) Bacterial pathogens in chicken meat review. International Journal of Life Sciences Research 2(3): 1-7.
- Odeyemi O A, Oluwarada Alegbeleye O, Strateva M, Stratev D (2020) Understanding spoilage microbial community and spoilage mechanisms in foods of animal origin. Comprehensive Review of Food Science and Food Safety. 19(2): 311-331.
- Kim J H, Yim D G (2016) Assessment of the microbial level for livestock products in retail meat shops implementing HACCP system. Korean Journal of Food Science of Animal Resource 36(5): 594-600.
- Yulistiani R, Praseptiangga D, Supyani S (2019) Occurrences of Salmonella spp. and Escherichia coli in chicken meat, intestinal contents, and rinse water at slaughtering place from traditional market Surabaya, Indonesia. IOP Conference Series: Materials Science and Engineering 633: 012007.
- Tum S (2015) Policy brief reducing microbial contamination of meat at slaughterhouses in Cambodia. Safety net 9-11.
- Adeyanju G T, Ishola O (2014) Salmonella and Escherichia coli contamination of poultry meat from a processing plant and retail markets in Ibadan, Oyo state, Nigeria. Springer plus 3(139): 2-9.
- Shah M K, Aziz S A, Zakaria Z, Lin L C, Goni M D (2018) A review on pathogenic Escherichia coli in Malaysia. Advances in Animal and Veterinary Sciences 6(2): 95-107.
- Xu Z, Li L, Alam M J, Yamasaki S, Shi L (2008) First confirmation of integron-bearing methicillin-resistant Staphylococcus aureus, Current Microbiology Journal 57: 264-268.
- Shijia W, Nou D, Huajie G, Liling H, Hua Y (2016) A review of the methods for detection of Staphylococcus aureus Enterotoxins, Toxins. 8(7): 176.
- Ohtani K, Shimiz T (2016) Regulation of toxin production in C. perfringens. Toxin 8(7): 207.
- Ameme D K, Alomatu H, Antobre-Boateng A, Zakaria A, Addai L, Fianko K, Janneh B, Afari E A, Nyarko K M, Sackey S O, Wurapa F (2016) Outbreak of foodborne gastroenteritis in a senior high school in south- eastern Ghana: A retrospective cohort study. BMC Public Health 13(16): 564.
- Labbe R G, Juneja V K (2017): Clostridium perfringens. In: Foodborne Diseases. Academic Press 235-242.
- ISO "International Organization for Standardization (2017) International Organization for Standardization. No.6579-1. Microbiology of the food chain Horizontal method for the detection, enumeration and serotyping of Salmonella - Part1: Detection of Salmonella spp.
- ISO "International Organization of Standardization" (2013) International Organization for Standardization No.4833-1. Microbiology of the food chain - Horizontal method for the enumeration of microorganisms - Part 1: Colony count at 30 °C by the pour plate technique.
- ISO International Organization of Standardization (2006) International Organization for Standardization. No.4832. Microbiology of food and animal feeding stuffs-horizontal method for the enumeration of coliforms: colony count technique.
- ISO International Organization for Standardization (2017) International Organization for Standardization. No.6887-1. Microbiology of the food chain - Preparation of test samples, initial suspension and decimal dilutions for microbiological examination - Part 1: General rules for the preparation of the initial suspension and decimal dilutions.
- ISO International Organization for Standardization (2003) International Organization for Standardization No. 6888-1:1999 + A1:2003. Microbiology of food and animal feeding stuffs - Horizontal method for the enumeration of coagulase-positive staphylococci (Staphylococcus aureus and other species) Part 1: Technique using Baird-Parker agar medium
- Igarashi H, Fujikawa H, Shingaki M, Bergdoll M S (1986) Latex agglutination test for staphylococcus toxic shock syndrome toxin 1. Journal of Clinical Microbiology 23: 509-512.
- ISO International Organization of Standardization (2004): International Organization for Standardization No.7937. Microbiology of food and animal feeding stuffs - Horizontal method for the enumeration of Clostridium Perferingens- Colony-count technique.
- R Torrie J (1980) Principles and practices of statistics. McGraw Book Coy Inc. New York, 113-114.
- Lianou A, Panagou E Z, Nychas G (2017) Meat safety. In: Foodborne pathogens and other biological issues. Lawrie´s Meat Science. P:521–552.
- Hassanin F, Shaltout F, Salem A, Maarouf A, Naguib R (2017) Studies on bacteriological profile of chicken meat cuts in Kaliobia governorate. Benha Veterinary Medical Journal 33(2): 402-409.
- Hassanin F, Shaltout F, Maarouf A, El-Sisy S F, Ahmed Y (2020) Bacteriological profile of frozen chicken meat cuts at Qalubiya governorate markets. Benha Veterinary Medical Journal 39(2): 1-5.
- Wahbah S F (2019) Prevalence of salmonella in some chicken meat products. Thesis, Master of veterinary medicine (meat Hygiene), Egypt. Benha Veterinary Medical Journal, 36(2): 33-39.
- Atia G A (2018) Bacteriological and chemical criteria of chicken carcasses. Thesis (Meat Hygiene), Faculty Veterinary Medical Benha University Egypt. Benha Veternary Medicaljournal 16-26.
- Hosny A, Ismail T H, Saleh N, Ahmed N (2022) Bacteriological profile and safety of chicken broiler meat cuts. Journal of Advanced Veterinary Research 12(4): 399-403.
- Feng P, Weagent S D, Grant M A (2002) Bacteriological analytical manual. (8th Edn), Silver Spring, Berlin 1998.
- Shaltout F, Nasief M Z, Lotfy L, Gamil B (2019) Microbiological status of chicken cuts and its products. Benha Veterinary Medical Journal. 37(1): 57-63.
- P, McGill K, Monahan C, Collins J D (2014) The effect of sampling time on the levels of microorganisms recorded from broiler carcass in commercial slaughter planet. Food Microbial 21: 59.
- Afify E, A Shaltout, Zakaria I M (2020) Aerobic plate count of contaminants and molecular characterization of Escherichia coli in raw chicken meat in Ismailia, Egypt. Journal of Veterinary Healthcare 2(2): 23-30.
- Zishiri O T, Mkhize N, Mukaratirwa S (2016) Prevalence of virulence and antimicrobial resistance genes in Salmonella spp. isolated from commercial chickens and human clinical isolates from South Africa and Brazil. Onderstepoort Journal of Veterinary Research. 83(1): 1067.
- Shaltout F, Zakaria I, Afify E (2020) Detection of E. coli O157 and Salmonella species in some raw chicken meat cuts in Ismailia province, Egypt. Benha Veterinary Medical Journal 39(1): 101-104.
- Elsisy S (2019) Enterotoxigenic bacteria as potential hazards threaten the safety of some chilled meat, poultry, and fish under the Egyptian marketing conditions. MVSc Thesis (Meat Hygiene), Faculty of Veterinary Medical Benha University Egypt.
- Perry M, Lewis H, Thomas D R (2018) Need for improved public health protection of young people wanting body piercing: Evidence from a look-back exercise at a piercing and tattooing premises with poor hygiene practices, Wales (UK) 2015. Epidemiol. Infection 146(9): 1177-1183.
- Shaltout F, Zakaria I, Afify A E (2019) Bacteriological profile of some raw chicken meat cuts in Ismailia city, Egypt. Benha Veterinary Medical Journal 39(1): 11-15.
- Mohamed A, Karmi M, Maky A (2021) Incidence of toxigenic genes of Staphylococcus aureus isolated from chicken meat. Aswan University Journal of Environmental Studies (AUJES), 2(3): 162-167.
- Mokhtari F A, Doosti A (2015) Investigation of antibiotic resistance and frequency of Clostridium difficile tcdA and tcdB genes in feces of calves in Chaharmahal Va Bakhtiari province. Journal of Shahrekord University of Medical Sciences. 17: 35-42.
- Zakaria I M (2005) Anaerobic bacteria in chicken meat products. M V Sc Thesis Faculty of Veterinary Medical journal, Zagazig University, Benha branch.
- A Afshari, A Jamshidi, Razmyar J, Rad M (2015) Genotyping of Clostridium perfringens isolated from broiler meat in northeastern of Iran. Veterinary Res. Forum 6(4): 279-284.
- Thangamani A, Subramanian S (2012) Prevalence of Clostridium perfringens in the chicken meat rendered at retail outlets of Namakkal, Tamilnadu. Journal of Advanced Veterinary Research. 2: 157-159.
- ISO "International Organization for Standardization (2020): International Organization for Standardization. No. 7932:2004/AMD 1: Microbiology of food and animal feeding stuffs — Horizontal method for the enumeration of presumptive Bacillus cereus — Colony-count technique at 30 degrees C — Amendment 1: Inclusion of optional tests.
- ISO "International Organization of Standardization" (2018): International Organization for Standardization. No. 16649, part 2. Microbiology of food and animal feeding stuffs – Horizontal method for the enumeration of glucuronidase-positive Escherichia coli.
- Nabil M (2018) Prevalence of anaerobic bacteria in some raw and ready to cook chicken meat products with special reference to Clostridium perfringens. MVSc Thesis (Meat Hygiene), Faculty of Veteirnary Medical Benha University Egypt.
- Shaltout F A, Zakaria I M, Nabil M E (2017) Detection and typing of Clostridium perfringens in some retail chicken meat products. Benha Veterinary Medical Journal 33(2): 283-291.






























