Anticancer Activity Exerted by Novel Bioactive Protein from Bacillus Endophyticus Strain DR92 Isolated from Cow Urine
Sheetal Pardeshi1,2, Raghavendra Ramappa1*, Aravinda Subrayashastry1, Shankarling Mavinamar3, Kiranmayee4 and Mary Shobha Rani Inala4
1Talent Development Centre, Indian Institute of Science, Challakere Campus at Kudapura, India
2Department of Microbiology, Modern College of Arts, Science and Commerce, Shivajinagar, India
3Department of PG studies in Botany, Karnataka State Akkamahadevi Wmen’s University, Vijayapura, India
4Department of Cell Biology and Molecular Genetics, Sri Devaraj Urs Academy of Higher Education and Research, India
Submission:February 18, 2021; Published: September 13, 2021
*Corresponding author: Raghavendra Ramappa, Talent Development centre, Indian institute of Science Challakere Campus at Kudapura, Challakere-577536 Chitradurga District, Karnataka, India
How to cite this article: Sheetal P, Raghavendra R, Aravinda S, Shankarling M, Kiranmayee, et al . Anticancer Activity Exerted by Novel Bioactive Protein from Bacillus Endophyticus Strain DR92 Isolated from Cow Urine. Adv Biotech & Micro. 2021; 16(4): 555943 DOI:10.19080/AIBM.2021.16.555943
Abstract
Breast cancer is a complex and most frequently diagnosed cancer among other types. Increasing prevalence necessitates search for new anticancer substances and medicines. Among them, bacterial proteins are a promising group of bioactive proteins and potential anticancer drugs. In this study, we attempted to isolate bacteria from cow urine. The useful bacterium was identified as Bacillus endophyticus strain DR92 by 16S rRNA gene sequence comparison. Low molecular weight proteins have been extracted, purified from B. endophyticus, confirmed and characterized by SDS-PAGE and MALDI-TOF MS. The observed molecular weights were around 8531 and 8731 daltons. The purified protein was screened for anti-proliferative activity by MTT assay against breast cancer cell line Zr-75-1 which showed 13% viability. To conclude from the study, the purified protein from B. endophyticus could be a promising anti-proliferative drug for cancer. Further work has to be done to find out the mode of action.
Keywords: Anticancer peptide; Cow urine; 16S rRNA; MALDI-TOF/TOF; SDS-PAGE; Bacillus endophyticus
Introduction
Global scenario with respect to occurrence of cancer in individuals of all ages is alarming and mortality due to the same is increasing rapidly. cancer occurrence in an individual depends on many factors such as diet, lack of exercise, exposure to carcinogenic pollutants through inhalation, ingestion [1,2]. oncogenic pathogen infections [3] and most importantly genetic predisposition [4-6]. Among all, breast cancer is the leading type of cancer occurring in females causing around 25% morbidity and 15% mortality worldwide. Statistical analysis of data for the past 30 years of Indian population shows that cancer occurrence has increased from 0.6 million in 1991 to 1.4 million in 2015, of which 21% cases are of breast cancer [7].
The present treatment of breast cancer uses a combination of chemotherapy, hormonal therapy, immunoglobulin administration and radiation. Unfortunately, 100% removal of cancer cells may not be possible in all cases using these treatments and thus recurrence is inevitable in such patients. Many reports from different parts of the world are coming up describing alarming development of acquired resistance in secondary cancer cells towards anticancer agents used in treatment of primary cancer in the patient with incidence of recurrence [8-10]. Due to developing resistance among cancer cells for existing anticancer drugs, there is imperative need of bio-prospecting possible resources for new anticancer candidates.
Microorganisms have been employed in numerous processes such as alcohol fermentation and cheese production since ancient times but with the advent of technologies in study of microorganisms especially with respect to the primary and secondary metabolites they produce, their applicability for medicinal purposes has increased. Microorganisms have been reported for production of metabolites with myriad of bioactivities and are of choice for large scale production of such bioactive compounds due to ease in cultivation, maintenance, genetic manipulations and downstream processing. [11] have described anticancer potential of azurin protein and peptide p28 isolated from Pseudomonas aeruginosa, whereas Bacillus thuringiensis extracellular polysaccharide has been reported to show anticancer activity [12]. Use of Brevibacillus choshinensis transformant as bacterial cancer therapy to deliver anticancer proteins in the body efficiently is also reported by [13]. Thus, bacteria as a potential resource for anticancer peptides have been validated through various reports. In present study, we aimed at isolation of microorganisms with potential to produce anticancer proteins that may serve as drug candidates for future drug development.
As per ancient Indian literature, cow urine is described to have multiple medicinal properties and is recommended to be used to treat various ailments including cancer [14]. Reports coming from different parts of India and some other countries have illustrated scientific proof of the same [15,16]. However, bioactive potential of microflora of cow urine has been less explored and thus was undertaken in present study.
Materials and Methods
Sources of sample
Cow urine sample were collected inasterile container from Kudapura, Challakere taluk, Chitradurga district. Karnataka. India. Sample was brought to laboratory and used immediately for isolation of bacteria.
Chemicals used
All the chemicals and reagents used for experiments were of analytical grade, obtained from Sigma Chemical Company (St. Louis, Mo., USA).
Isolation of bacteria from cow urine
One ml of cow urine sample was serially diluted0.1 ml of each aliquot was spread on Nutrient agar with pH 7.2 ± 0.2. Plates were incubated at 370C for 24 hr. The colony showing iridescence was selected and purified on Nutrient agar plate. Culture was maintained and preserved on the same media slant at 40C until further use [17].
Identification of Bacteria
Preliminary identification of isolate was carried out by morphological studies which included Gram staining, motility, endospore staining and cultural characteristics on agar plates such as colony morphology and pigmentation. Biochemical characterization of isolate was performed for catalase, oxidase, carbohydrate fermentation, H2S production, starch hydrolysis, urease production and nitrate reduction [17].
16S rRNA gene sequencing and phylogeny
Genomic DNA was isolated according to the manufacturer’s protocol given in DNA extraction kit (Chromgene, Bangalore, India). Universal primers 27F and 1492R were used to amplify 10 to 12 ng of DNA template by PCR. The amplified 1.5 kb product was purified by QI quickgel extraction kit (Qiagen, Germany) and directly sequenced on ABI (Applied Biosystem, Sigma, Mumbai) automated sequencer as recommended by manufacturer. 16S rRNA gene sequence was aligned with submitted sequences available in the NCBI database using clustalX software. Phylogeny was established using maximum likelihood method.
Purification of protein from Bacteria
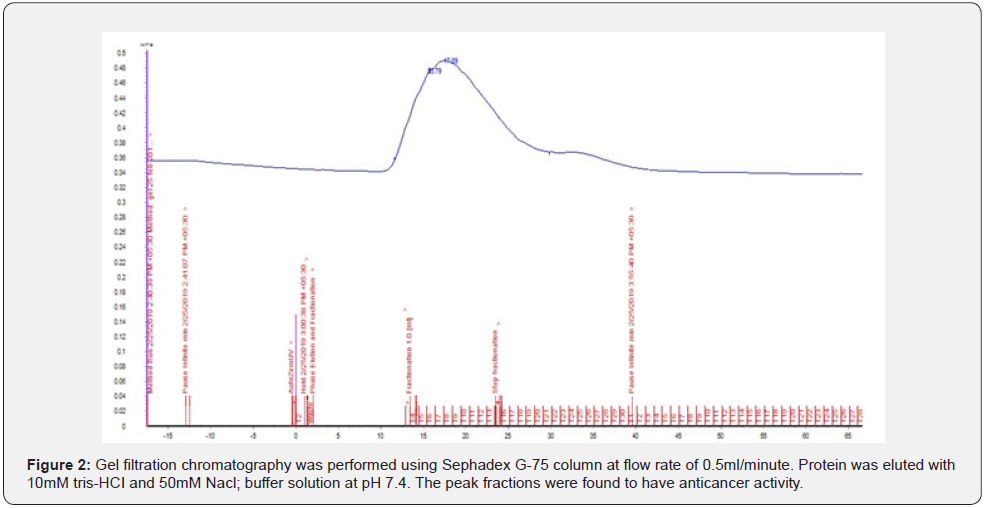
The pure culture of bacterium was inoculated into enrichment medium containing peptone 5; sodium chloride 5; yeast extract 1.5; starch 2% followed by incubation at 37°C under shaking conditions (150 rpm) for 2 days at initial pH 7.4. Sample was centrifuged at 10,000 rpm for 20 min at 40 C and the supernatant was collected and subjected to Ammonium sulphate precipitation. Initial 20% and final 65% precipitation was collected and dialyzed using cut off 3kDa membrane with 10mM -Tris -HCl, 50mM NaCl, and 20mM Imidazole. Dialysate was concentrated using 3kDa cut off centricons; protein was subjected to further purification using FPLC (AkTA START) Sephadex G-75 column (Sigma chemicals Co. St. Louis, MO, USA) matrix and the flow rate of eluate as maintained at 0.5ml /minute using 10mM Tris-HCl; 50mM NaCl. Each 2ml fraction was collected and optical density (OD) was recorded at a wavelength of 280 nm. Purified protein peak fractions were collected and concentrated using 3kDa cut offcentricons which were further characterized using SDS-PAGE and MALDI-TOF.
Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis
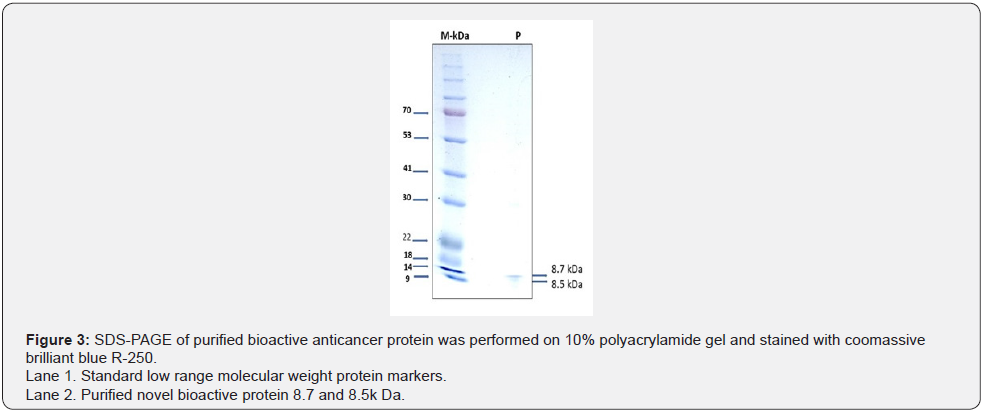
The purified protein was subjected to SDS-PAGE for molecular mass determination according to the procedure of Laemmli and Favre 1973. By using 10 % separating gel, 4 % stacking gel under non reducing conditions. The molecular mass of the purified protein was estimated by comparison of its electrophoretic mobility with those of molecular mass marker proteins (GE Healthcare, Bangalore, India). The gel was stained with Coomassie brilliant blue R-250 and de-stained with solution containing 15 % methanol and 5 % acetic acid.
Determination of Molecular Mass by Gel Filtration
Gel-filtration chromatography was performed using a Sephadex G-75 column (GE Healthcare, Bangalore, India) with a conventional FPLC system equilibrated with 10 mM Tris–HCl buffer containing 50 mM NaCl (pH 7.5) at a flow rate of 0.5 mL/ min and monitored at 280 nm.
MALDI-TOF-MS Analysis
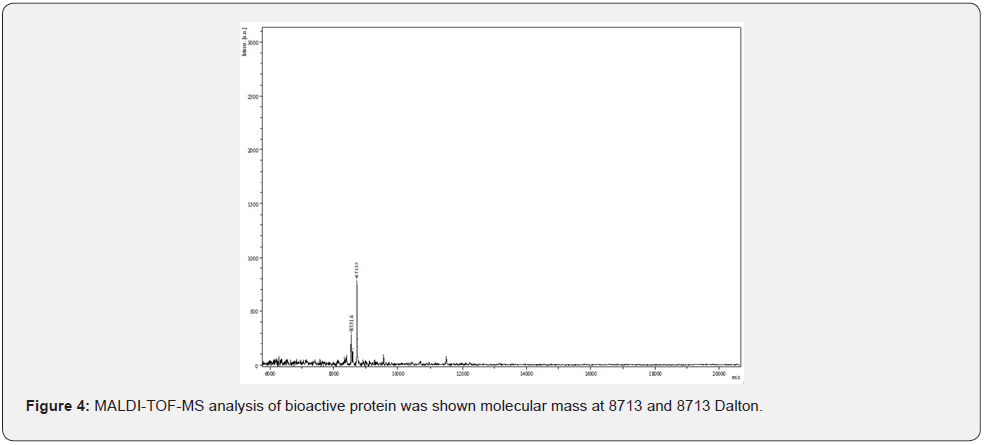
Matrix-assisted laser desorption ionization time-of-flightmass spectrometer (MALDI-TOF-MS) was used to determine the molecular weight of the protein. Analysis was performed using a Reflex IV MALDI-TOF-MS system (Bruker Daltonics, Bremen, Germany). The purified protein 0.5 μl was spotted on an Anchor chip target plate with 0.5 μl of freshly prepared matrix solution2,5- Dihydroxybenzoic acid. The above mixture was loaded on the Anchor chip target plate and the sample was allowed to dry at room temperature for 10 min. The dried target plate was inserted into the mass spectrometer and analysis was performed. The mass spectrum was acquired on a MALDI-TOF/TOF mass spectrometer (Autoflex II; Bruker Daltonics) equipped with a pulsed nitrogen laser (337 nm) in positive reflection mode.
Estimation of protein
Protein estimation was performed using the standard procedure (Ly et al 1951) with bovine Serum Albumin (BSA) as standard protein.
Preparation of Cell culture
The obtained breast cancer cell lines (Zr 75-1) from National Centre for Cell Science (NCCS) Pune were sub-culturedcryopreserved in liquid nitrogen / - 800Cdeep freezer until use. Prior to the analysis, the cells were thawed and were grown for required cell density by using RPMI 1640 along with 10% foetal bovine serum. All the cultures were incubated at 37oC in an atmosphere of 5% CO2 and 95 % air.
Cytotoxic activity of protein sample on breast cancer cell lines
The cytotoxic activity of protein sample was measured by using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT). Cells of 200 μl (1 x 10 5 cells/ml density) were seeded in a 96 well plates overnight. The Concentrated purified protein stock of 1mg/ml solution was made and 100 μg/ml was added to the plate containing cell lines and incubated for 24h. Rinsed the cells with 1X PBS and incubated with 20 μl of 5 mg/ ml MTT at 370C for 3-4 h. After incubation; dissolved the dark blue crystals of formazan were dissolved with 100 μl of DMSO and continued incubation for about 10 minutes. The level of reduced MTT was determined by measuring the absorbance at 570 nm. Cell viability was calculated by using the formula given below.
Results
Characterization, Identification and phylogeny of the isolate
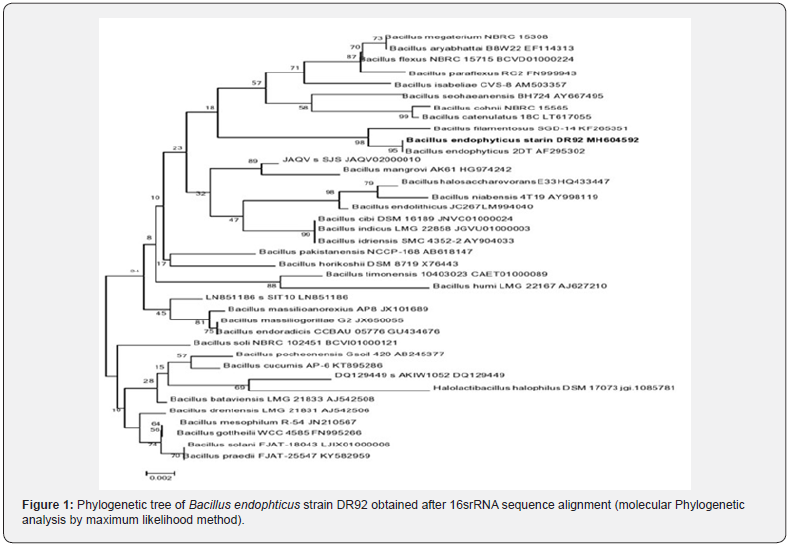
The isolate was Gram positive, rod shaped, approximately 3μm in length showing flat circular colonies with iridescence, round margin and mucoid consistency. The biochemical feature of the isolate is described in table 1. The partial 16S rRNA gene sequence of 445bp length was deposited in NCBI with the accession number MH604592. Based on the results of the phenotypic and genotypic analyses, we concluded that the isolate belongs to Bacillus endophyticus (Figure 1).

Protein purification
Protein purification was performed by using Sephadex G-75 desalting column by using system FPLC (Akta start). Protein peak fractions were collected (Figure 2), concentrated to 1mg /ml using 3kDa cut-off centricons. The resultant protein was used for further characterization.
Characterization of anticancer protein
The molecular weight of purified novel bioactive protein was determined by comparison with Rf values of standards in 10% SDS-PAGE. The observed protein band was corresponding to the molecular weight of 8 kDa (Figure 3). Further the protein was characterized using MALDI-TOF-MS (Figure 4).
Cytotoxic activity of protein sample on breast cancer cell lines
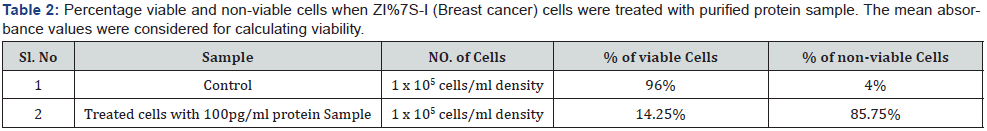
The results obtained demonstrated that the purified protein from cow urine has a significant anticancer potential on top ranked cancer i.e; breast cancer cell line (Zr-75-1). After calculating the viability, the test protein exhibited 14.25 % viability and 85.75 % dead or non-viability. Below given table 2 describes the percentage viable and non - viable cells up on treatment.
Discussion
Biochemical composition of cow urine has been studied to a great extent and is reported to contain essential elements, enzymes and hormones that can exert health benefits to humans Satyapal Singh, 2019, Joshi and Adhikari, 2019. Different researches have also validated the therapeutic potential of cow urine as antioxidant, anticarcinogenic, antidiabetic and antimicrobial agent [18-21] Recently, patents have been granted for use of cow urine distillate along with antibiotic quinolone or fluoroquinolone and antifungal agents such as azoles, clotrimazolesmystatin or amphotericin which enhance antimicrobial effect of these agents [22], as well as, for the use of bioactive fraction from cow urine distillate as a bioavailability facilitator and pharmaceutically acceptable additives for anti-infective and anticancer agents [23,24]. It will be interesting to know if any of these bioactive compounds are metabolites of microflora of cow urine imparting medicinal properties to cow urine which was one of the objectives of this study. Discovery of new anticancer agents and especially of anticancer peptides is of significance due to rising resistance of cancer cells to chemotherapeutic agents [25-28]. In coherence with this, in present study, we report novel bioactive proteins from Bacillus endophyticus strain DR92 isolated from cow urine against breast cancer cell lines (Zr 75-1). Previously, Bacillus endophyticus JUPR15 isolated from environmental samples has been presented to produce protease inhibitors exhibiting promising activity and to induce apoptosis in HeLa, HepG2 and MCF-7 cancer cell lines [29,30]. which is in consistency with our results. Apart from anticancer activities, metabolites from genus Bacillus are described to display variety of bioactivities [31]. Bacteria such as Streptomyces and other genera of actinobacteria are reported to be able to produce anticancer metabolites [32]. Production of anticancer agents has also been attributed to endophytic fungi for which comprehensive review can be found elsewhere [33-35] have reported potential of <3kDa peptide fraction from Spirullina platensis to significantly reduce viability of Human colon adenocarcinoma cell line (SW480).
Anticaner agents such asgenistein, lycopene, resveratrol (currently under clinical trials) and taxene paclitaxel (approved for human use in 1992) from plants have also been reported [36], but mass production of such compounds is a problem due to their plant origin. Peptides from hydrolysates of food derived proteins such as milk, eggs, oyster, soybean, peas, Spirulina are reported to have anticancer and immunomodulatory properties [37]. but production of these peptides requires hydrolysis of proteins using commercial proteases, fermentation with bacterial strains and gastrointestinal digestion which increases the cost of process and makes purification protocols laborious. The present study findings about the functionality of the protein are significant and bacterial protein production is easy in large scale. Anticancer peptides have been known to act directly by binding or indirectly to kill cancer cells Hilchie, et al. [38] have published a broad review on mechanisms of anticancer peptides. Some reports elaborate on use of nano-carriers and liposomes for delivery of anticancer peptides to increase their half-life in experimental animals [38]. A detailed study will be needed in order to elucidate exact mode of action of the novel protein reported here.
Bacterial proteins or peptides already used in various cancer treatments include actinomycin D from Actinomycesantibioticus, bleomycin from Streptomyces verticillus, doxorubicin from Streptomyces peucetius var. caesius, mitomycin C from Streptomyces caespitosus and diphtheria toxin, while p28, arginine deiminase ADI are in clinical trials. Therapeutic peptides that are GnRH agonists such as buserelin, gonadorelin, leuprolide, nafarelin, goserelin, triptorelin, and histrelin, cetrorelix, abarelix, degarelix are in clinical use for treatment of breast and prostate cancer [27]. This signifies our work of presenting more options for the established successful treatment protocols of cancer.
Conclusion
Cancer prevalence is increasing alarmingly and multi drug resistance in cancer cells is of utmost concern. Present study signifies the potential of tiny life forms of holding a tremendous potential to produce bioactive compounds that can be used in a variety of applications including cancer treatment. In this study, we report purification of novel anticancer protein from bacteria isolated from cow urine. The bacterial isolate was found to be Gram positive short rod and was characterized using various biochemical tests. The isolate was identified to be Bacillus endophyticus by 16S rRNA sequencing (Accession number MH604592). Protein fraction from isolate was obtained by ammonium sulphate precipitation and was purified by SDS PAGE and FPLC. Further we report that the novel bioactive protein thus purified is having molecular weight of 8kDa using MALDI TOF MS analysis and exhibits anticancer activity against breast cancer cell line Zr 75-1 which showed only 13% cell viability after treatment with novel protein. The findings of present study are significant in the wake of increasing anticancer drug resistance where alternatives to current therapies are needed. Studies like this can help identify potential anticancer candidates for future use. Moreover, ease of handling, mass production, purity and safety of bioactive compounds makes this approach striking and economic.
Acknowledgement
Authors are grateful to Prof M S Hegde Convener Talent development centre; Indian institute of science, Challakere, Chitradurga district, Karnataka state, India, for his support in present work. We are greatly thankful to Sri Devaraj Urs Academy of Higher Education and Research centre Kolar district, Karnataka state, India, for their aid in conducting anti-proliferative studies.
References
- Wang Y, Yue S, Zheng B, Hao Z, Chen J (2018) A general method for evaluating the effects of air pollutants on lung cancer prevalence. Journal of the Air & Waste Management Association 68(12): 1366-1377.
- Gaskin J, Coyle D, Whyte J, Krewksi D (2018) Global estimate of lung cancer mortality attributable to residential radon. Environmental Health Perspectives, 126(5): 057009.
- Shrestha AD, Neupane D, Vedsted P, Kallestrup P (2018) Cervical cancer prevalence, incidence and mortality in low- and middle-income countries: a systematic review. Asian Pac J Cancer Prev 19(2): 319-324.
- Engel C, Rhiem K, Hahnen E, Loibl, S, Weber KE, et al. (2018) Prevalence of pathogenic BRCA1/2 germline mutations among 802 women with unilateral triple-negative breast cancer without family cancer history. BMC Cancer 18(1): 265.
- Singh J, Thota N, Singh S, Padhi S, Mohan P, et al. (2018) Screening of over 1000 Indian patients with breast and/or ovarian cancer with a multi‑gene panel: prevalence of BRCA1/2 and non‑BRCA mutations. Breast Cancer Research and Treatment.
- AravindKumar M, Singh V, Shaik MN, Shanker U, Lakshmi Narasu (2017) Microarray-based SNP genotyping to identify genetic risk factors of triple-negative breast cancer (TNBC) in South Indian population. Mol Cell Biochem 442(1-2): 1-10.
- Mathew A, George PS, Jagathnath Krishna KM,Vasudevan D, James FV (2019) Transition of cancer in populations in India.Cancer Epidemiology 58: 111-120.
- Paulson, KG, Voillet V, McAfee MS, Hunter DS, Wagener FD, et al. (2018) Acquired cancer resistance to combination immunotherapy from transcriptional loss of class I HLA.Nature communications, 9(1): 3868
- Nikolaou M, Pavlopoulou A, Georgakilas AG, Kyrodimos E (2018) The challenge of drug resistance in cancer treatment: a current overview. Clinical and Experimental Metastasis. 35(4): 309-318.
- Robey RW, Pluchino KM, Hall MD, Fojo AT, Bates SE, et al. (2018) Revisiting the role of efflux pumps in multidrug-resistant cancer. Nat Rev Cancer 18(7): 452-464.
- Garizo AR, Bernardes N, Chakrabarty AM, Fialho AM, (2019) Chapter 9: The anticancer potential of the bacterial protein Azurin and its derived peptide p28. Chakrabarty AM., FialhoAM. (ed). Microbial infections and cancer therapy CRC Press.
- Sathishkumar R, Ananthan GS, Lakshmana Senthil, Moovendhan M, Jeganathan A (2018) Structural characterization and anticancer activity of extracellular polysaccharides from ascidian symbiotic bacterium Bacillus thuringiensis. Carbohydrate Polymers 190: 113-120.
- Mukai H, Takahashi M, Watanabe Y (2018) Potential usefulness of Brevibacillus for bacterial cancer therapy: intratumoral provision of tumor necrosis factor-alpha and anticancer effects. Cancer gene ther 25(3-4): 47-57.
- Ashwinikumar A Raut, Ashok DB, (2018) Vaidya. Panchgavya and cow products: A trail for the holy grail. Journal of Ayurveda and Integrative Medicine 9: 64-66.
- Meena M, Patel P, Saini S, Gurjar T, Gogoi, et al. (2019) Go mutra (Cow urine) and its uses: An overview. Journal of Entomology and Zoology Studie 7(3): 1218-1222.
- Suk KH, Subash CB, Gopinath, Anbu P, Lakshmi priya T (2018) Cellulose nanoparticles encapsulated cow urine for effective inhibition of pathogens. Powder Technology.
- Holt JG, Krieg NR, Sneath PHA, Staley JT, Williams (1994) Bergey’s ST Manual of determinative Bacteriology (9th edition). Williams and Wilkins.
- Satyapal Singh (2019) Biochemical appraisal of Gomutra (Cow urine). Journal of Pharmacognosy and Phytochemistry 8(3): 4089-4092.
- Joshi DR, Adhikari N (2019) Benefit of cow urine, milk, ghee, curd, and dung versus cow meat. Acta Scientific Pharmaceutical Sciences 3(8):169-175.
- Randhawa GR, Sharma R (2015) Chemotherapeutic potential of cow urine: A review. J Inter cult Ethno pharmacol 4(2): 180-186.
- Dhama K, Chauhan RS, Singhal L (2005) Anti-cancer activity of cow urine: current status and future directions. International Journal of Cow Science 1(2): 1-25.
- Khanuja (2002) Pharmaceutical composition containing cow urine distillate and an antibiotic. Patent no. US 6,410,059 B1. United states patent and trademark office.
- Khanuja (2005) Use of bioactive fraction from cow urine distillate (GO-MUTRA) as a bioenhancer of antiinfective, anticancer agents and nutrients. Patent no. US 6,896,907 B2. United states.
- Randhawa, (2010) GR Letters to the editor: Cow urine distillate as bioenhancer. Journal of Ayurveda and Integrative Medicine 1(4).
- Qin Y, Qin ZD, Chen J, Cai CG, Li L, et al. (2019) XF, From antimicrobial to anticancer peptides: the transformation of peptides. Recent Patents on Anti-Cancer Drug Discovery 14(1): 71.
- Kurrikoff K, Aphkhazava D, Langel U (2019) The future of peptides in cancer treatment. Current Opinion in Pharmacology, 47: 27-32.
- O Brien-Simpson NM, Hoffmann R, Chia CSB, Wade JD (2018) Editorial: Antimicrobial and anticancer peptides. Front. Chem. 6: 13.
- Venkatachalam P, Nadumane VK (2018) Overexpression of p53 and Bax mediating apoptosis in cancer cell lines induced by a bioactive compound from Bacillus endophyticus JUPR15, Process Biochemistry,
- Venkatachalam P, Nadumane VK (2019) Purification and characterization of a protease inhibitor with anticancer potential from Bacillus endophyticus JUPR15. Current cancer therapy reviews 15(1): 74-82.
- Sansinenea E, Vaca J, Rojas Ne, Vázquez C (2019) A wide spectrum of antibacterial activity of secondary metabolites from Bacillus amyloliquefaciens ELI149. Bio sci. J, Uberlândia 36(1): 235-244.
- Chen C, Ye Y, Wang R, Zhang Y, Wu C, et al. (2018) Streptomyces nigra sp. nov.is a novel actinobacterium isolated from mangrove soil and exerts a potent antitumor activity in vitro. Front Microbiol 9: 1587.
- Sharma N, Sharma V, Abrol V, Panghal A, Jaglan S (2019) Chapter 6: An update on bioactive natural products from endophytic fungi of medicinal plants. Pharmaceuticals from Microbes, Environmental Chemistry for a Sustainable World 28, Arora et al. D (eds.), Springer Nature Switzerland AG.
- Vasundhara M, Reddy MS, Kumar A (2019) Chapter 18: Secondary metabolites from endophytic fungi and their biological activities. New and future developments in microbial biotechnology and bioengineering.
- Sadeghi S, Jalili H, Ranaei, Siadat SO, Sedighi M (2018) Anticancer and antibacterial properties in peptide fractions from hydrolyzedSpirulina protein. J Agr Sci Tech 20: 673-683.
- Buyel JF (2018) Plants as sources of natural and recombinant anti-cancer agents. Biotechnology Advances 36: 506-520.
- Chalamaiah M, Yu W, Wu J (2017) Immunomodulatory and anticancer protein hydrolysates (peptides) from food proteins: A review. Food Chemistry 245: 205-222.
- Hilchie AL, Hoskin DW, Power, Coombs MR (2019) Chapter 9: Anticancer activities of natural and synthetic peptides. Matsuzaki K (ed.), Antimicrobial Peptides, Advances in Experimental Medicine and Biology 1117. Springer Nature Singapore Pte Ltd.
- Karpinski TM, Adamczak A (2018) Anticancer activity of bacterial proteins and peptides. Pharmaceutics 10(2): 54.






























