Green Synthesis and Characterization of Nano- Iron by Fusaium Oxysporum
Aldesuquy HS*, Eldeghidy AM, Abdel-Fattah GM and Ashraf Elsayed
Department of Botany, Faculty of Science, Mansoura University, Egypt
Submission:February 18, 2021; Published: August 30, 2021
*Corresponding author: Aldesuquy HS, Department of Botany, Faculty of Science, Mansoura University, 35516 Mansoura, Egypt
How to cite this article: Aldesuquy HS*, Eldeghidy AM, Abdel-Fattah GM, Ashraf Elsayed. Green Synthesis and Characterization of Nano- Iron by Fusaium Oxysporum. Adv Biotech & Micro. 2021; 16(4): 555942 DOI:10.19080/AIBM.2021.16.555942
Abstract
Recently, nano-science is one of the most important fields. Production of nanoparticles by using microbes is a new area of interest. Therefore, this study aimed to synthesize nano-iron using Fusaium Oxysporum by cell-free water extract method followed by confirmation and characterization of nano-iron by UV-visible spectrophotometry and electron microscopy. Reduction of iron ions to iron atoms was visualized through the change in the color of the mixture from pink to dark brown color with a peak at 226 nm corresponding to the Surface Plasmonic Resonance (SPR) of nano-iron by UV-Vis Spectroscopy. Transmission Electron Microscopy (TEM) showed spherical shaped nano-iron with a size range of 2.5 to 20 nm. The formation of a typical crystalline structure of iron atoms was confirmed by Selected Area Electron Diffraction (SAED). In conclusion, Fusaium Oxysporum is an excellent bio-factory replacing the conventional chemical and physical techniques.
Keywords: Biosynthesis; Fusaium Oxysporum; Nano-iron; Nanotechnology
Introduction
Nanotechnology is defined as the study of particles which are less than 100 nm in size. Nanoparticles (NPs) either formed through transfer of macro-sized material (bulk material) to nano-sized particles by two methods; top-down method or bottom-up methods [1]. The features of nanomaterials over their macro-scaled size exhibiting high quality in most of valuable applications due to their new physical and chemical characters [2]. In addition to that, their unique physical and chemical properties, they reported in frequent optical, photo electrochemical and electronic properties [3]. The issues of the physiochemical methods used for the production of nanomaterials as: short time stability and safety problems can be resolved by the use of other biosynthetic procedures as the use of microorganisms. Either extracellular or intracellular inorganic substances can be produced by several organisms [4].
Magnetite nanomaterials are models of nanoparticles produced by unicellular bacteria [5]. Using fungi in the biosynthesis of nanomaterials was discovered where fungi can produce a large amount of enzymes inclusive in nanoparticles biosynthesis. One of the requirements for the advancing of nanotechnology is the development of new synthetic protocols for synthesizing of nanoparticles with different sizes, shapes and chemical composition. Green nanotechnology uses biological systems as microorganisms including bacteria, fungi, yeast and plant extract in synthesis [6]. Several physical and chemical methods including mechanical milling [7], sodium borohydride reduction [8,9], solvo-thermal method Basavaraja et al. [16], and carbo-thermal synthesis [10,11] have been employed for preparation of the nano-iron.
Iron nanoparticles which synthesized by these methods rapidly agglomerate to form clusters due to inter- particle Vander Waals and magnetic forces and may further undergo rapid oxidation in the presence of oxidants thereby limiting their reactivity [12,13]. Furthermore, Nano-iron could be biologically synthesized using microorganisms such as Pleurotus sp[14], and Sargassum muticum or by plant extract such as an aqueous extract of Passiflora tripartite mollissima fruit [15]. The potential of iron nanoparticles (NPs) for environmental remediation have been indicated in many recent studies. Nano-scale materials such as nanoadsorbents, nanocatalysts, nanofiltration, and nanobiocides such as metal and metal oxide nanoparticles are currently being employed for remediation of water and wastewater pollutants. Among these metallic nanoparticles, nano-iron (FeNPs) has promising advantages that can combat environmental pollution [15]. This work was undertaken to synthesize and characterize nano-iron particles using Fusarium oxysporum.
Materials and Methods
Culturing of Fusarium oxysporum
Fusarium oxysporum was donated from Dr. Ashraf Elsayed, Botany Department, Faculty of Science, Mansoura University, and tested for the synthesis of nano-iron. Fusarium oxysporum was cultured on PDA medium (potato extract 200 ml, glucose 20g, agar 20 g in 1L distilled H2O) and incubated at 25 °C for five days till sufficient fungal growth. The pure fungus was maintained at 4°C in slant for further use [1].
Biosynthesis of Nano-iron By Fusarium oxysporuum:
Fusarium biomass was obtained by growing 3-5 fungal agar discs in broth MGYP medium (malt extract 3 g, glucose 10 g, yeast extract 3 g, peptone 5 gm in 1 L distilled H2O). Broth medium was sterilized in an autoclave at 121±1◦C for 15 minutes. After incubation time at 28◦C, 180 rpm, the fungal biomass was separated from medium components by filtration, then extensively washed three times by sterile distilled water for removing any residue. To each 100 ml sterile deionized water, 20 g fresh fungal biomass immersed and incubated at the same previous conditions (at 28◦C, 180 rpm) for 24 hours. After that, the cell-free water extract was obtained by filtration through Whatman filter paper No 1. To each 100 ml cell free water extract, 1mM carefully weighed FeCl3 was added. The mixture was incubated at 28◦C, 180 rpm in dark condition to prevent photo-oxidation of ferrous ions. Control sample composed of cell-free water extract free from iron ions.
Characterization of Fe-NPs:
i. Ultra violet–visible spectral study
The initiatory detection of nano-iron was estimated by optical observations or observing the color change. These samples were later submitted to visual measurements executed by UV-visible scan spectrophotometer (Uni cam UV-VIS). Spectral scan is one of the most important techniques used to verify the formation of nanoparticles that providing its surface Plasmon resonance [16].
ii. Transmission electron microscopy
TEM (JEOL JEM-2100, U.S.A) was used to determine the size and shape of nanoparticles.
The TEM characterized by its high magnification power, which reaches to million and half million than the real size [17]. The analysis was done by drop-coating of nano-colloidal solution into carbon coated grid (Type G 200, 3.05 μ diameter, TAAP, U.S.A).
iii. Selected Area Diffraction Pattern (SADP) Analysis
Selected Area Diffraction Pattern (SADP) is a crystallographic experimental technique that can be performed inside a transmission electron microscope [15]. In this analysis a very thin sectioned specimennearly 100 nm and a high energy electron volt (100-400 k.ev) were required. Electrons will act as wave not particle when interacting with analyzed material. Atoms in the substrate will act as a grating making diffraction of falling electrons, so the diffraction pattern appears as a bright spot. SADP is preferred in the analysis of crystalline structure [18].
Results
Biosynthesis of Nano-iron by Fusarium oxysporum
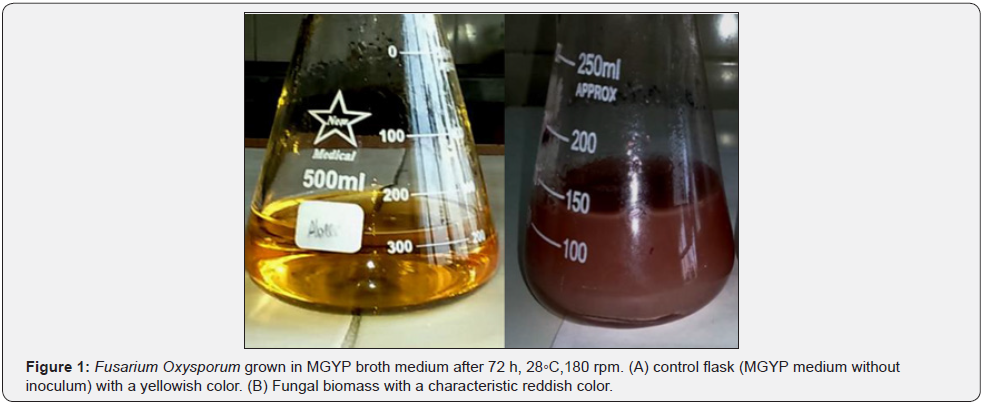
Fusarium oxysporum was tested for the production of nano-iron, this fungus was grown on PDA medium showing a characteristic pinkish white color on aerial hyphae after 7 days at 25◦C.
After 3 days, the biomass of Fusarium oxysporum on MGYP medium at 28◦C, 180 rpm obviously appeared as a reddish color (Figure 1). After incubation of the cell-free water extract and FeCl3, iron nanoparticles synthesized. The visible change in color from pink to brown color was recognized as an indicator for nanoiron formation. This may happen due to a gradual reduction of iron ions to zero state iron atoms (Figure 2).

Optical properties of synthesized nano-iron
i. U.V-visible spectral analysis
Nano-iron particles produced by Fusarium oxysporum were scanned by U.V-VIS spectra ranging from 200 to 400 nm, a characteristic peak at 226 nm was recorded. This peak is considered as an indication for the presence of the biosynthesized nano-iron (Figure 3).

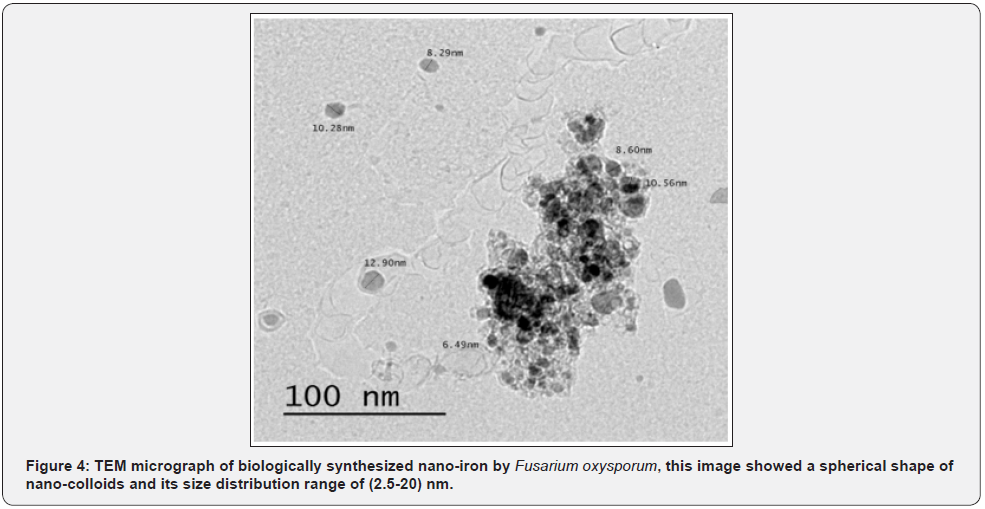
ii. Morphology of nano-iron by TEM
By using TEM microscopy, the morphology and size of nanoiron particles could be detected. The size of nano-iron ranged from 2.5 to 20 nm, and the shape was mostly spherical (Figure 4).
iii. Selected area diffraction pattern (SADP) analyses
After analysis of one drop from nano-colloidal solution by SADP, the diffraction pattern of nano-iron particles seemed as lighted spots on the dark field, this pattern reflected the crystalline structure of nano-iron and its diffraction rings (Figure 5).

Discussion
Nanoparticles are defined as small objects with a size range of 10-100 nm [19]. The biological method has been developed to obtain a size-controlled nanoparticles, inexpensive, not toxic so it is considered as an eco-friendly technique [19]. The result presented here showed that, nano-iron was produced by the tested Fusarium oxysporum through the reduction of aqueous ferrous (III) FeCl3 solution of 1mM concentration in the dark conditions. The formation of nano-materials was assured firstly by the visual appearance of brown color followed by UV-visible spectrophotometry recording 226 nm absorbance peak. These results were very close with some previous studies in which iron oxide nanoparticles recorded one peak at 222 nm [20] and another iron nanoparticles formed by Pleurotus sp. Recorded two peaks at 226 and 276 nm [14]. In contrast, two absorption peaks of nanoiron biosynthesized by Sargassum muticum aqueous extract were introduced at wave lengths 402 nm and 415 nm [15]. This shifts in peaks of nanomaterials may be related to the size of particles.
From TEM micrograph, the biosynthesized nano-iron was mostly spherical shaped with mean size 2.5-20 nm. Similar results obtained from nano-iron biosynthesized by reduction of ferric chloride solution with a green method using brown seaweed (Sargassum muticum) aqueous extract contains sulfated polysaccharides as the reducing agent recording 18±4 nm size with cubic shapes [15]. Also, iron oxide nanoparticles biologically synthesized using an aqueous extract of Passiflora tripartite mollissima fruit showed a mean particle size of spherical 22.3±3 nm [21-23].
Conclusion
Summering up, the obtained data from this study showed that Fusarium oxysporum could be used as a good source of reducing agents for synthesis of nano-iron particles.
References
- Katan T, Shlevin E, Katan J (1997) Sporulation of Fusarium sp. lycopersici on surfaces of tomato plants and aerial dissemination of inoculum. Phytopathology 87(7): 712-719.
- Feynman R (2018) There’s plenty of room at the bottom. Feynman and computation p. 63-76.
- Pető G, G.L Molnár, Z Pászti, O Geszti, A Beck, et al. (2002) Electronic structure of gold nanoparticles deposited on SiOx/Si (100). Materials Science and Engineering: C 19(1-2): p. 95-99.
- Mann S (1993) Molecular tectonics in biomineralization and biomimetic materials chemistry. Nature 365(6446): 499-505.
- Lovely DR, John F Stolz, Gordon L Nord Jr, Elizabeth JP Phillips (1987) Anaerobic production of magnetite by a dissimilatory iron-reducing microorganism. Nature 330(6145): 252-254.
- Sintubin L, Willy Verstraete, Nico Boon (2012) Biologically produced nanosilver: current state and future perspectives. Biotechnology and Bioengineering, 109(10): 2422-2436.
- Karimi E (2014) A novel method for fabrication of Fe catalyst used for the synthesis of carbon nanotubes. Bulletin of Materials Science 37(5): 1031-1038.
- Cai H, Xiao An, Jun Cui, Jingchao Li, Shihui Wen, et al. (2013) Facile hydrothermal synthesis and surface functionalization of polyethy eneimine- coated iron oxide nanoparticles for biomedical applications. ACS applied materials and interfaces 5(5): 1722-1731.
- Woo H, Junboum Park, Seockheon Lee, Seunghak Lee (2014) Effects of washing solution and drying condition on reactivity of nano-scale zero valent irons (nZVIs) synthesized by borohydride reduction. Chemosphere 97: 146-152.
- Makarov V, A J Love, O V Sinitsyna, S S Makarova, I V Yaminsky(2014) Green nanotechnologies: synthesis of metal nanoparticles using plants. ActaNaturae (англоязычнаяверсия), 6(1): :35-44.
- Shen Y, (2015) Carbothermal synthesis of metal-functionalized nanostructures for energy and environmental applications. Journal of Materials Chemistry A 3(25): 13114-13188.
- Huang G, Mianmian Wang, Yongyou Hu, Sihao Lv, Changfang Li (2017) Synthesis, characterization, and debromination reactivity of cellulose-stabilized Pd/Fe nanoparticles for 2, 2', 4, 4'-tretrabromodiphenyl ether. PloS one 12(3): e0174589.
- Stankic S, Sneha Suman, Francia Haque, Jasmina Vidic (2016) Pure and multi metal oxide nanoparticles: synthesis, antibacterial and cytotoxic properties. Journal of Nanobiotechnology 14(1): 73.
- Mazumdar H, Haloi N (2011) A study on biosynthesis of iron nanoparticles by Pleurotus sp. J Microbiol-Biotechnol Res 1(3): 39-49.
- Mahdavi M (2013) Green biosynthesis and characterization of magnetic iron oxide (Fe3O4) Nano-particles using seaweed (Sargassum muticum) aqueous extract. Molecules, 18(5): p. 5954-5964.
- Basavaraja S (2011) Solvothermal synthesis and characterization of acicular α-Fe2O3 Bulletin of Materials Science 34(7): 1313-1317.
- Saif S, Tahir A, Chen Y (2016) Green synthesis of iron nanoparticles and their environmental applications and implications. Nanomaterials 6(11): 209.
- El‐Temsah YS, Joner EJ (2012) Impact of Fe and Ag nanoparticles on seed germination and differences in bioavailability during exposure in aqueous suspension and soil environmental toxicology 27(1): 42-49.
- Wang Z (2000) Transmission electron microscopy of shape-controlled nanocrystals and their assemblies. ACS Publications.
- Ruud C, Barrett CS, Russell PA, Clark RL (1969) Selected area electron diffraction and energy dispersive X-ray analysis for the identification of asbestos fibres, a comparison. Micron 7(2): 115-132.
- Mansoori GA, Soelaiman TF (2005) Nanotechnology—an introduction for the standards community. Journal of ASTM International 2(6): 1-22.
- Kumar B, Kumari Smita, Luis Cumbal, Alexis Debut (2014) Biogenic synthesis of iron oxide nanoparticles for 2-arylbenzimidazole fabrication. Journal of Saudi Chemical Society 18(4): 364-369.
- Pal S (2014) Antimicrobial activity of iron oxide nanoparticles.






























