Phytoplankton structure and cyanobacteria specific composition of Foum-Gleita Lake, Mauritania
Ahmed Sidi Sadegh1,2*, Zeinebou Sidoumou1 and Juan Luis Gomez Pinchetti3
1Unit of Marine Eco-biology, Environment, Health and Nutrition (EBIOMESN), Department of biology, University of Nouakchott Al-Aasriya (UNA), Africa
2Laboratory of Biology and Ecology of Aquatic Organisms (LEBOA), Mauritanian Institute of Oceanographic and fisheries Research (IMROP), Africa
3Spanish Bank of Algae (BEA) and Institute of Oceanography and Global Change (IOCAG), University of Las Palmas de Gran Canaria, Spain
Submission:July 27, 2021; Published: August 23, 2021
*Corresponding author: Ahmed Sidi Sadegh, Unit of EBIOMESN, Department of biology, University of Nouakchott Al-Aasriya and LEBOA, Mauritanian Institute of Oceanographic and fisheries Research, Mauritania, Africa
How to cite this article: Sadegh S A, Sidoumou Z, Gomez-Pinchetti JL. Phytoplankton structure and cyanobacteria specific composition of Foum-Gleita Lake, Mauritania. Adv Biotech & Micro. 2021; 16(4): 555941 DOI:10.19080/AIBM.2021.16.555941
Abstract
In order to study the structure and the relative abundance of phytoplankton and the specific composition of cyanobacteria in the Foum-Gleita Lake, the surface waters of this Lake were subject of a regular monthly sampling extending over 12 months, from September 2017 to August 2018. Analysis of the phytoplankton by inverted microscopy method identified 28 taxa distributed over six phyla (Chlorophyta, Cyanobacteria, Bacillariophyta, Cryptophyta, Miozoa and Euglenozoa). However, analysis of the Cyanobacteria by same method showed that this phylum is specifically composed of eight genera (Microcystis, Gloeocapsa, Chroococcus, Dolichospermum, Oscillatoria, Planktothrix, Lyngbya, and Arthrospira), five of them are recognized as potentially toxic (Microcystis, Dolichospermum, Oscillatoria, Planktothrix and Lyngbya) and are mainly dominated by the species Microcystis aeruginosa and Dolichospermum flos-aquae during the hot season (May to September).
Keywords: Phytoplankton; Cyanobacteria; Abondance; Lake; Foum-Gleita; Mauritania
Introduction
The surface waters are often subject to eutrophication, mainly due to the various sources of pollution and to the natural process of soil erosion providing various elements, which can be at the origin of the deterioration of quality of these waters [1]. This growing phenomenon is manifested by massive proliferation of phytoplankton, in particular the blooms of cyanobacteria which have very wide geographic and ecological distributions [2]. In Mauritania, the state of the lakes is very little studied while they receive an increasing volume of waste, in particular nutrients especially phosphorus (P) and nitrogen (N). For a long time, the Foum-Gleita Lake has been subject of significant demographic pressure due to arrival of the nomad’s victims of growing drought in the Sahel. After several years of domestic, agricultural and industrial use of this lake, previous surveys have shown that its waters are evolving towards eutrophication [3]. The objective of this work is to study the structure and the relative abundance of phytoplankton and the specific composition of cyanobacteria in the surface waters of Foum-Gleita Lake (Mauritania).
Materials and Methods
Studied area and sampling
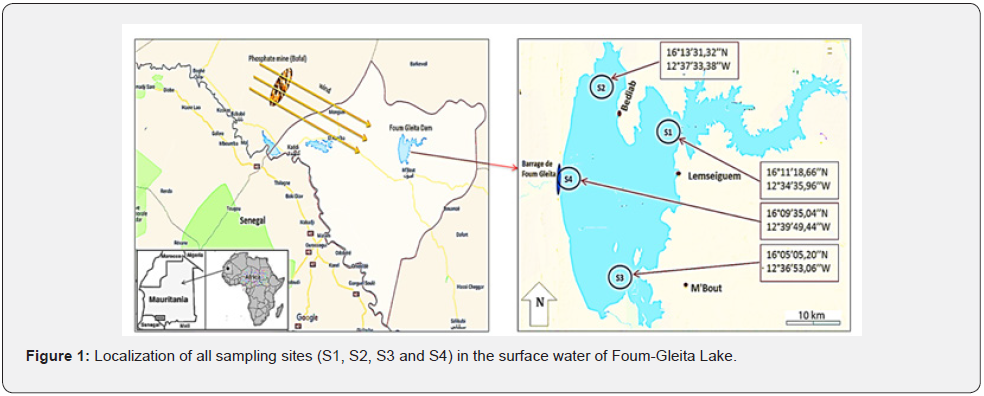
Foum-Gleita Lake is geographically encamped in southern part of Mauritania between latitudes 16°15’ N and 16°03’ N and longitudes 12°39’ W and 12°32’ W (mean altitude: 32 m). Precisely, it is located in the Gorgol region, at the department of M’bout, 120 km east of Kaédi (capital of the region), and 520 km southeast of Nouakchott (capital of the country). Its water body is 160 km2 (normal area) and 50 km2 (minimum area) and has a storage capacity of 1 billion m3 of water. The watershed of studied lake is located between latitudes 15°50’ N and 17°40’ N and longitudes 11°40’ W and 12°35’ W, with a total area of approximately 21,000 km2 and a 180 km long; The watershed crosses soft (clayey) but also hard (rocky) formations. The watershed climate is characterized by a long dry season (8 months), high temperatures (22 to 44°C), low rainfall (180 to 400 mm/year), long sunshine period (290 days/year), permanent winds (3 to 7 m/s) and the presence of a phosphate mine (94,500 ha). Socio-economically, Foum-Gleita Lake plays a key role in the irrigation of cultivated areas (11,200 ha), the supply of drinking water (26,500 inhabitants), the animals drinking (53,300 heads) and in the practice of continental fishing for a production of 800 tonnes/year. Ecologically, this lake plays an important role for birds (nesting and migrating), mammals, reptiles and insects. In this lake, the sampling was carried out between September 2017 and August 2018 in four different sites (S1, S2, S3 and S4) chosen according to their accessibility (Figure 1), at the rate of a monthly sampling per site. The samples were taken from the surface (0.1- 0.5 m). The phytoplankton concentrates were sampled using a plankton net with a 20-μm mesh gap according to the method described in Briand et al. [4]. Upon arrival at the laboratory, the samples are stored at 4°C until analysis.
Phytoplankton analysis
Identification and enumeration of phytoplankton was performed using inverted microscopy following Utermöhl’s method [5]. For each preserved phytoplankton sample, 10 mL aliquots were settled for 24 hours on a gridded chamber before being visually scanned on an inverted microscope (Leitz, Fluovert) at 20x and 40x. The two dominant cyanobacteria taxa have been identified at the species level, while the other minor ones have been identified only at the genus level, using universally accepted taxonomic keys [6-9], and then all have been quantified with a minimum of 100 units counted per sample. The biovolume (mm3/L) of Microcytis sp., Dolichospermum sp., Oscillatoria sp. and Planktothrix sp. was then estimated. Briefly, the mean number and the dimension of cells from 50 Microcystis colonies and 50 Dolichospermum filaments per sample were estimated. For Oscillatoria and Planktothrix, the average length and width of 50 filaments per sample were also estimated. The biovolume of each taxa was calculated by assuming each cell as sphere for Microcystis and Dolichospermum and each filament as a cylinder for Oscillatoria and Planktothrix species. The number of cells per liter for Microcystis and Dolichospermum species and filaments per liter for Oscillatoria and Planktothrix species were then multiplied by the average cell and filament biovolume [10]. Other phytoplankton groups were identified according to the morphological characteristics described in Bourrelly, Reynaud, Laloë, and Olenina [11-15], and then quantified as cell per liter with a minimum of 100 units counted per sample.
Results
Structure and relative abundance of phytoplankton in Foum-Gleita Lake
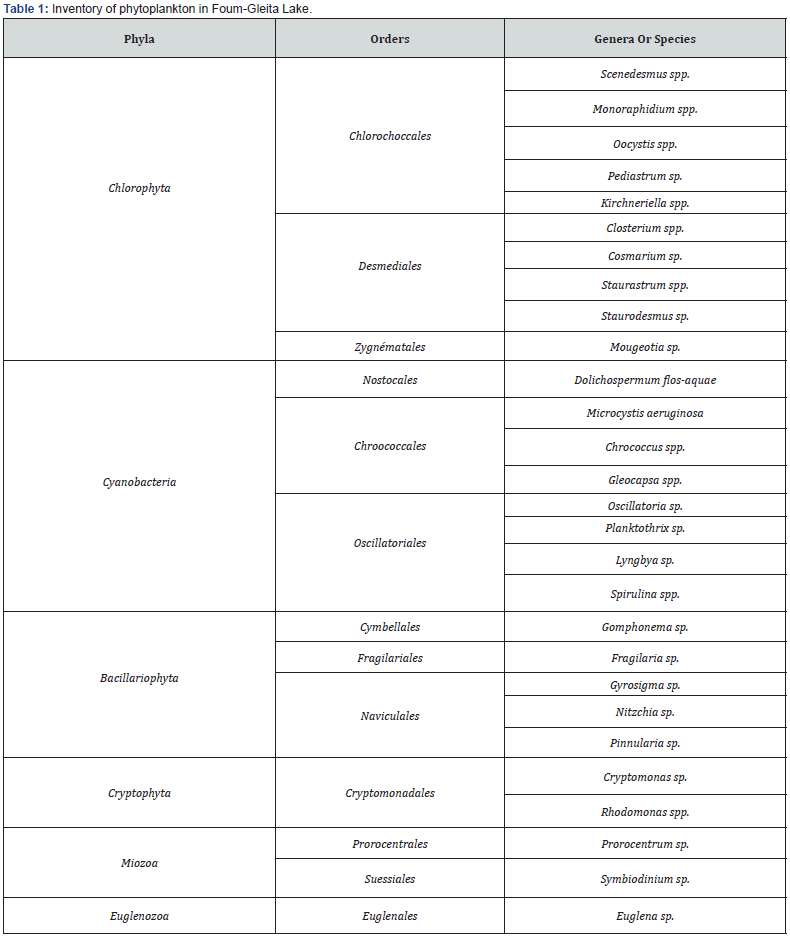
The monthly monitoring of the evolution of phytoplankton in Foum-Gleita Lake, allowed us to identify 28 taxa spread over six phyla among which we cite Chlorophyta, Cyanobacteria, Bacillariophyta, Cryptophyta, Miozoa and Euglenozoa (Table 1). The qualitative analysis of phytoplankton present in the water samples from the four sampling sites made it possible to obtain the specific composition shown in Figure 2. Within this composition, Chlorophyta is the most present phylum. It represents 42 to 50% of the total number of phytoplankton species. This phylum is mainly dominated by Desmidiales, in particular the genera Closterium, Staurodesmus and Staurastum. Site 1 has the highest density of Chlorophyta compared to sites 2, 3 and 4, respectively. Cyanobacteria represents the second phylum (17 to 32%) with higher densities at sites 4 and 3 than at other sites 2 and 1, respectively. Principally, this phylum is represented by the genera Microcystis, Gloeocapsa, Chroococcus, Dolichospermum, Oscillatoria, Planktothrix, Lyngbya, and Arthrospira. Bacillariophyta phylum occupies the third position after Chlorophyta and Cyanobacteria where they represent 11 to 13%. It is essentially dominated by Pennatophycidae, in particular the genera Pinnularia, Fragilaria and Gomphonema, with higher densities on site 2 compared to other respective sites: 1, 3 and 4. Cryptophyta phylum represents the fourth class (7 to 9%).
This phylum is mainly represented by the genera Cryptomonas and Rhodomonas. Miozoa phylum represented by the genera Prorocentrum and Symbiodinium come in fifth position where it represents 5 to 6% of the total number of phytoplankton species. Euglenozoa phylum constitutes only 3 to 5% of the phytoplankton encountered and is dominated by the genus Euglena.
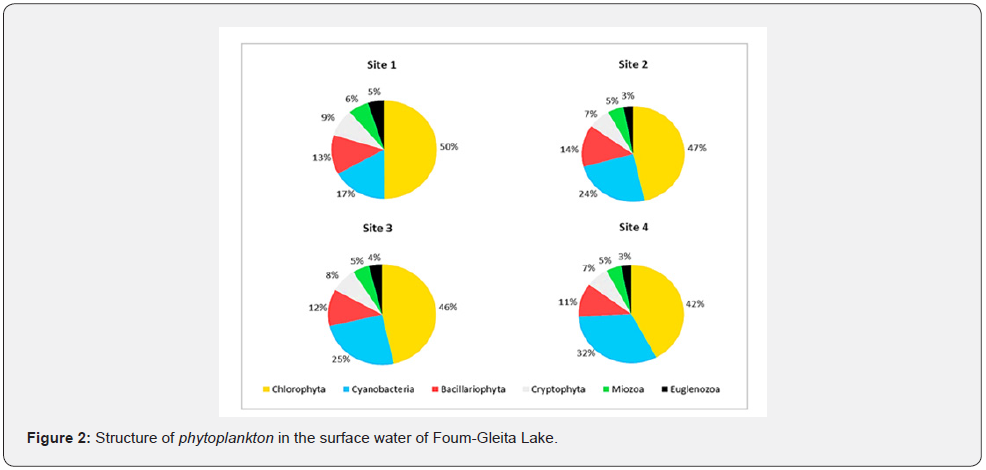
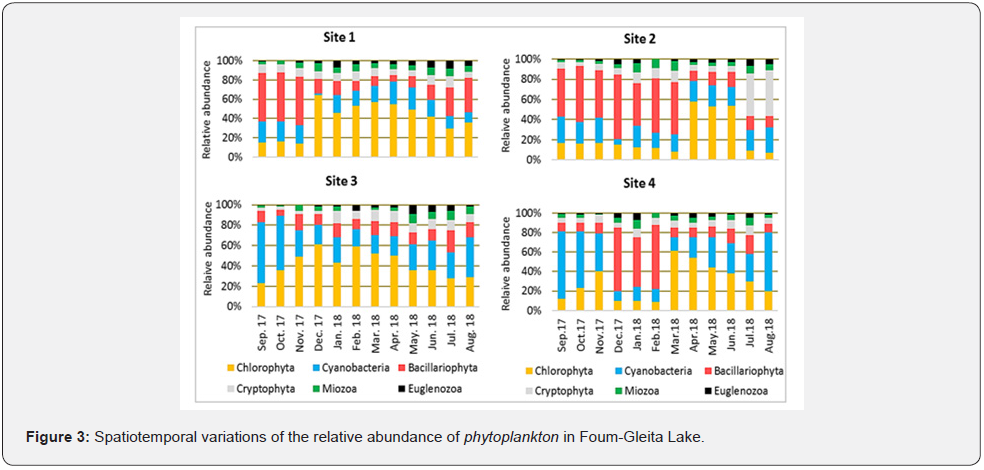
In addition, the analysis of spatiotemporal evolution of relative abundance of the different phytoplankton phyla (Figure 3), revealed the dominance of cyanobacteria on sites 3 and 4 during the hottest months of the study period (August, September and October).
Specific composition of cyanobacteria in Foum-Gleita Lake
The cyanobacteria observed in Foum-Gleita Lake during the period of this study consisted of 8 genera (Figure 4), three having a colonial form (Microcystis, Gloeocapsa and Chroococcus) and five having a filamentous form (Dolichospermum, Oscillatoria, Planktothrix, Lyngbya, and Arthrospira). The two genera, Microcystis and Dolichospermum, which represent respectively 38 and 48% of total number of the cyanobacteria species, however, showed a constant presence in the different sampling sites throughout the study period. They were exclusively represented by the species Microcystis aeruginosa and Dolichospermum flos-aquae, respectively. The two genera Gloeocapsa and Chroococus, which respectively constitute 8.3% and 5.3% of the total number of cyanobacteria, also remained present throughout the study period, but with fairly low average densities. However, the two genera Oscillatoria and Planktothrix, which total only 0.4% of the total number of cyanobacteria, showed an irregular monthly presence. The genus Oscillatoria only appeared in samples from all four sites during the first three months of the study. The genus Planktothrix was only present at sites 2, 3 and 4, with very low irregular densities. The two genera Arthrospira and Lyngbya, which together represent only 0.02% of the total number of cyanobacteria, accidentally appeared only once at site 3, with low densities.
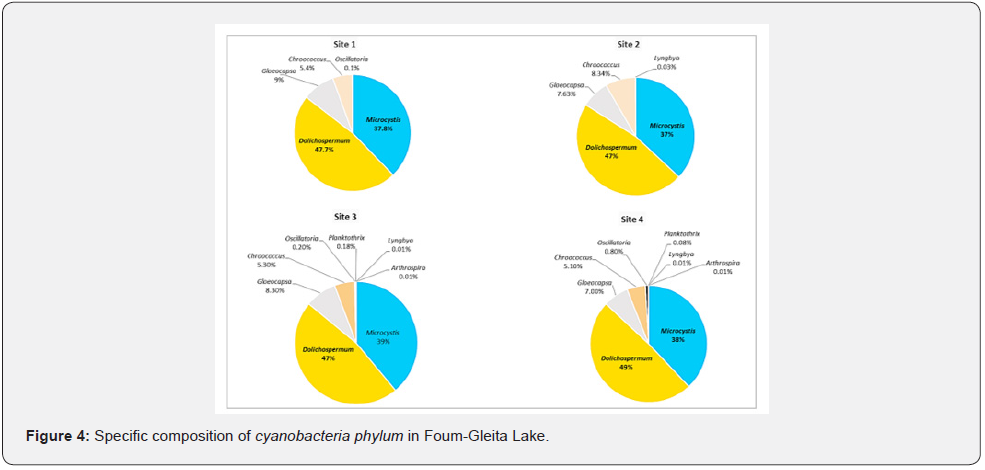
In addition, we note in the Foum-Gleita Lake the massive presence of potentially toxic cyanobacteria detected from monthly samples over an annual cycle. Analysis of their occurrence in this lake (Table 2) showed that the first two genera, Microcystis and Dolichospermum, were ubiquitous, the third, Oscillatoria, and the fourth, Planktothrix, were irregular. While the last one, Lyngbya, was accidental.
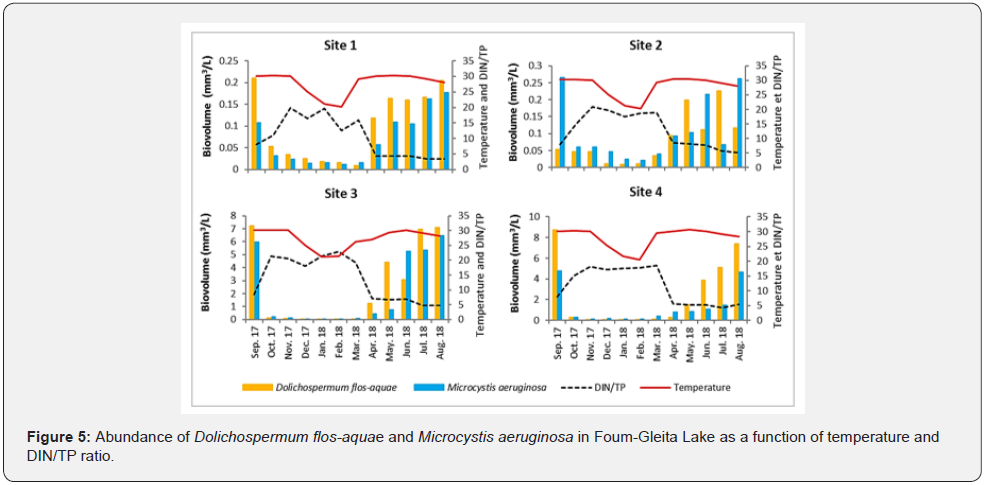
However, the analysis of natural samples during the periods of strong development of these toxic cyanobacteria showed that the main species responsible for the blooms in the prospected lake were Microcystis aeruginosa and Dolichospermum flos-aquae. Its blooms appear in summer to reach their maximum proliferation in September with significant annual variability in biomass. The highest biovolumes of these two dominant species, D. flos-aquae and M. aeruginosa, were observed when the DIN/TP ratio was less than 10 and the water temperature reached 30°C (Figure 5).
Discussion
The structure of phytoplankton and the spatiotemporal variations in phytoplankton densities observed in the Foum- Gleita Lake during the period of this study is a logical response to the seasonal change in the physicochemical conditions of the environment, where the growth of phytoplankton depends on temperature, nutrients and light. Microscopic analysis of the water samples showed that the phytoplankton of Foum-Gleita Lake was mainly composed of a Chlorophyta/Cyanobacteria assemblage, with some genera of Bacillariophyta. Other phyla such as Cryptophyta, Miozoa and Euglenozoa were also present but with much less importance, although local short-term developments can sometimes observed, as for Cryptophyta phylum at site S2 in July-August. Globally, the phytoplankton in Foum-Gleita Lake was quantitatively dominated by Cyanobacteria phylum during the rainy period (July-September) especially in sites S3 and S4 where anthropological pollution was most important with high concentrations of Dissolved Inorganic Nitrogen (6.53 and 8.22 mg/L, respectively) and Total Phosphorus (0.56 and 0.59 mg/L, respectively) combined with elevated water temperature (30.4 and 30.7°C , respectively) [3]. However, when the water temperature begins to drop during the dry season months (November-April), Chlorophyta become the dominant phylum in these sites (S3 and S4) although cyanobacteria are still present as the second phylum with relative abundances of 17-29%. In the other two sites, S1 located at mouth of the Gorgol Noir River and S2, far from any anthropogenic pollution, the phytoplankton community was characterized by a distinct abundance succession of Bacillariophyta/Chlorophyta. However, Cyanobacteria were always present in third position except in the S2 site in July-August where they were present in fourth position after Cryptophyta. These results are in accordance with other studies, which have shown that phytoplankton succession in tropical aquatic ecosystems is characterized by a distinct change between dry and rainy seasons: Chlorophyta/Cyanobacteria in Lake Tanganyika in Tanzania [16], Bacillariophyta/Cyanobacteria in Lake Victoria in Kenya [17] and in Lake Guiers in Senegal [18]. While in temperate regions such as North Africa, phytoplankton communities were dominated by Bacillariophyta phylum in winter and Chlorophyta phylum in spring-summer, succeeded by Cyanobacteria phylum in autumn [19,20].
In other regions such as Europe and America, an analysis of data from 143 lakes showed that cyanobacteria biomass increases sharply with temperature in lakes with high rates of light absorption [21]. In addition, Jankowiak et al. [22] reported that cyanobacteria abundance in Lake Erie (international border between Canada and United States) increased dramatically in response to an increase in nitrogen, with large increases combined at high concentrations of nitrogen and phosphorus, and water temperature. In contrast, Almanza et al. [23] showed that certain genera of cyanobacteria such as Aphanizomenon, Aphanocapsa, Aphanothece and Dolichospermum can form blooms in the freshwater ecosystems of south-central Chile at low temperatures in autumn and winter (10,8- 15.6 °C), which suggests that eutrophication is the main factor in the proliferation of these genera regardless of water temperature.
The cyanobacterial community of Foum-Gleita Lake was mainly composed of Dolichospermum flos-aquae and Microcystis aeruginosa, which were known among the most microcystin-producing (MCs) and bloom-forming species in the world [24]. The growth trend of these two dominant species followed the same pattern at all four sites (S1 to S4); showing low abundance between December and February when the water temperature was a little lower (around 22°C). However, the biovolume of both species begins to increase in parallel with temperatures from March to peak in August and September when the water temperature reaches 30°C. In non-limiting Nitrogen and Phosphorus ecosystems like our lake, high water temperatures can promote the development of cyanobacteria by maximizing their growth rates compared to other phytoplankton phyla [25]. Although the growth trend of Dolichospermum flos-aquae and Microcystis aeruginosa in this lake was similar in the four sampling sites, their biovolume was approximately 30 times higher in sites S3 and S4. This difference in spatial dynamics could be explained by the fact that S3 and S4 were the sites most exposed to anthropogenic pollution.
Conclusion
The present study has contributed to understanding, for the first time, the structure and the seasonal variations of phytoplankton community of the surface waters of Foum-Gleita Lake. It showed that the phytoplankton taxocenosis of this lake is poorly diversified (28 taxa spread over six phyla). It also revealed a period of strongest phytoplankton development extending from May to September. In addition, this study identified 8 taxa of cyanobacteria dominated in summer-autumn by the two toxic species, Microcystis aeruginosa and Dolichospermum flos-aquae. This situation is probably linked to the urbanization, the agricultural activities, the phosphate mines present in the watershed of the lake and the increase in water temperature due to the global change. The massive presence of Microcystis aeruginosa and Dolichospermum flos-aquae, which are potentially toxic, in this lake, clearly demonstrates the need for regular monitoring of cyanobacteria and cyanotoxins in the waters of this lake.
Acknowledgment
J.L. Gomez Pinchetti thank the support of the European Territorial Cooperation Program PCT-MAC 2014-2020 through the project REBECA-CCT (MAC/1.1.B/269).
References
- Huisman J, Codd GA, Paerl HW, Ibelings BW, Verspagen JMH, et al. (2018) Cyanobacterial blooms. Nat Rev Microbiol 16(8): 471-483.
- Preece D, Becerra R, Robinson K, Dandy J (2017) Assessing alexithymia: Psychometric properties and factorial invariance of the 20-item Toronto Alexithymia Scale in nonclinical and psychiatric samples. J Psychopathol Behav Asses 40: 276-287.
- Sadegh AS, Sidoumou Z, Dia M, Pinchetti JLG, Bouaïcha N (2021) Impacts of phosphorus loads on the water quality and the proliferation of harmful cyanobacteria in Foum-Gleita Reservoir (Mauritania). Inter J Limnol 57:1.
- Briand JF, Robillot C, Quiblier-Llobéras C, Humbertd J F, Couté A, et al. (2002) Environmental context of Cylindrospermopsis raciborskii (Cyanobacteria) blooms in a shallow pond in France. Water Res 36(13): 3183-3192.
- Utermöhl H (1958) On the perfection of quantitative phytoplankton method. Int. Ass. Theo. Appl Limnol Comm 9: 1-38.
- Geitler L (1932) Cyanophyceae. Akademische Verlagsgesellshaft, Leipszig.
- Komárek J, Anagnostides K (1999) Cyanoprokaryota, Part 1: Chroococcales, Süsswasserflora von Mitteleuropa, Bd 19/1, Spektrum Akademischer Verlag.
- Komárek J, Anagnostides K (2005) Cyanoprokaryota, Part 2: Oscillatoriales, Süsswasserflora von Mitteleuropa, Bd 19/2, Spektrum Akademischer Verlag.
- Wacklin P, Hoffmann L, Komárek J (2009) Nomenclatural validation of the genetically revised cyanobacterial genus Dolichospermum (Ralfs ex Bornet and Flahault) comb nova Fottea 9: 59-64.
- Hillebrand H, Durselen CD, Kirschtele D, Pollingher U, Zohary T (1999) Biovolume calculation for pelagic and benthic microalgae. J Phycol 35(2): 403-424.
- Bourrelly P (1972) Les algues d’eau douce. Initiation à la systé Tome I: Les algues vertes. Editions N. Boubée & Cie.
- Bourrelly P (1981) Les algues deau douce. Initiation à la systé Tome II: Les algues jaunes et brunes. Chrysophycées, Phéophycées, Xanthophycées et Diatomées. Editions N. Boubée & Cie.
- Bourrelly P (1985) Les algues d’eau douce. Initiation à la systématique. Tome III: Les algues bleues et rouges. Eugléniens, Péridiniens et cryptomonadines. Editions N. Boubée & Cie.
- Reynaud PA, Laloë F (1985) La méthode des suspensions-dilutions adaptée à l'estimation des populations algales dans une riziè Revue d'écologie et de biologie du sol 22: 161-192.
- Olenina I, Hajdu S, Edler L, Andersson A, Wasmund N, et al. (2006) Biovolumes and size-classes of phytoplankton in the Baltic Sea. HELCOM Baltic Sea Environment Proceedings 106.
- Descy JP, Hardy MA, Sténuite S, Pirlot S, Leporcq B, et al. (2005) Phytoplankton pigments and community composition in Lake Tanganyika. Freshwat Biol 50(4): 668-684.
- Kling HJ, Muggidde R, Hecky RE (2001) Recent changes in the phytoplankton community of Lake Victoria in response to eutrophication. In The Great Lakes of the World (GLOW): Food web, Health, and integrity (Eds: Munawar M, Skjoldal H.R). Backhuys, Leiden, 47–65.
- Tian C, Lu X, Pei H, Hu W, Xie J (2012) Seasonal dynamics of phytoplankton and its relationship with the environmental factors in Dongping Lake, China. Environ Monit Asses 185(3): 2627-2645.
- Gellati FZ, Touati H, Tambosco K, Quiblier C, Bensouilah M (2017) Unusual cohabitation and competition between Planktothrix rubescens and Microcystis sp. (Cyanobacteria) in a subtropical reservoir (Hammam Debagh) located in Algeria. PLoS ONE 12(8): e0183540.
- Hammou HA, Latour D, Samoudi S, Mouhri K (2018) Occurrence of the First Toxic Microcystis Bloom in a Recent Moroccan Reservoir. Water Resour J 45: 409-417.
- Kosten S, Huszar VLM, Bécares E, Costa LS, Donk E, et al. (2012) Warmer climates boost cyanobacterial dominance in shallow lakes. Glob Change Biol 18(1): 118-126.
- Jankowiak J, Hattenrath-Lehmann T, Kramer BJ, Ladds M, Gobler CJ (2019) Deciphering the effects of nitrogen, phosphorus, and temperature on cyanobacterial bloom intensification, diversity, and toxicity in western Lake Erie. Limnol Oceanogr 64(3): 1347-1370.
- Almanza V, Pedreros P, Laughinghouse HD IV, Féliz J, Parra O, et al. (2019) Association between trophic stat, watershed use, and blooms of cyanobacteria in south-central Chile. Limnol 75: 30-41.
- Paerl HW (2018) Why does N-limitation persist in the world’s marine waters? Mar Chem 206: 1-6.
- Carey CC, Ibelings BW, Hoffmann EP, Hamilton DP, Brookes JD (2012) Ecophysiological adaptations that favour freshwater cyanobacteria in a changing climate. Water Res 46(5): 1407-1394.






























