Vascularized Bacterial Nanocellulose- Hydroxyapatite Scaffolds for Bone Tissue Engineering
Derce O S Recouvreux1, Gisele V Souza2, Paulo F Dias3, Janice Koepp4, Luismar M Porto4, and Carlos R Rambo5*
1Graduate Program in Engineering and Mechanical Sciences, Federal University of Santa Catarina Brazil
2Department of Chemical and Food Engineering, Federal University of Santa Catarina Brazil
3Department of Cell Biology, Embryology and Genetics, Federal University of Santa CatarinaBrazil
4Biocelltis Biotechnology SA, Florianópolis, Brazil
5Department of Electrical and Electronic Engineering, Federal University of Santa Catarina, Brazil
Submission:April 29, 2021; Published: May 19, 2021
*Corresponding author: Carlos R Rambo, Department of Electrical and Electronic Engineering Federal University of Santa Catarina, 88040-900, Florianópolis, Brazil
How to cite this article: Derce O S R, Gisele V S, Paulo F D, Janice K, Luismar M P, e al. Vascularized Bacterial Nanocellulose-Hydroxyapatite Scaffolds for Bone Tissue Engineering. Adv Biotech & Micro. 2021; 16(2): 555935 DOI:10.19080/AIBM.2021.16.555935
Abstract
In order to tailor biomaterials to facilitate a sufficient supply of oxygen and nutrients to the central regions of large tissue-engineered scaffolds we used dynamically cultures of K. hansenii and nylon templates to produce branching vessel networks inside nanocellulose (BNC) hydrogels. Hydroxyapatite (HAp) was synthesized in situ by a biomimetic method. HAp precipitated and crystallized on cellulose nanofibers. The morphology and distribution of HAp particles on the 3D-BNC nanofibers were analyzed by scanning electron microscopy (SEM) and Field Emission Scanning Electron Microscopy (FESEM), which revealed that HAp was homogeneously distributed on the surface of the cellulose nanofibers. The formed vessels are delimited by a dense, semi-permeable BNC membrane with thickness varying from 0.2 to 2 μm. Human osteoblast cells (HOB) successfully adhered and proliferated in the 3D BNC-HAp hydrogel composite. Due to its natural compatibility, this biomaterial may potentially be implanted directly in tissue-deficient regions or used as cell carrier scaffolds for therapeutic and regenerative bone tissues applications.
Keywords: Vascularized scaffolds; Osteoblast adhesion; EMC-like scaffolds; bacterial nanocellulose
Introduction
A large variety of synthetic and natural approaches have being successfully applied to produce new biocompatible ECM-like materials [1,2]. Bacterial Nanocellulose (BNC), in particular, has attracted great interest for numerous medical and tissue engineering applications due to its unique nanostructure and biocompatibility [3-7]. On the other hand, synthetic hydroxyapatite (HAp), which is similar to bone apatite, has been used as a ubiquitous biomaterial to solve several bone tissue engineering problems, due to its biocompatibility and osteoinductive properties [8,9]. Despite the efforts to develop adequate biocompatible scaffolding materials for tissue engineering, scaffolds vascularization is essential to supply cells with oxygen and nutrients especially in newly implanted grafts where nutrients and oxygen are supplied mainly by diffusion [10-12]. Osteogenesis, e.g., is a vascular-dependent process in which nutrients and oxygen supply is key for implant maintenance [13,14]. The lack of vascularization, and hence oxygen deficiency in scaffolds for bone grafting may lead to failure in implant integration and hinder angiogenesis [13]. Anyway, production of properly vascularized scaffolds remains so far, a challenge. In this work, we developed 3D large scaffolds composed of BNC hydrogels incorporated with hydroxyapatite. In order to tailor biomaterials, which would then facilitate a sufficient supply of oxygen and nutrients to the central regions of large tissue-engineered constructs, we produced branching vessel networks to supply the scaffolds. The biocompatibility of BC-HAp hydrogel composites was assessed using primary human osteoblasts cells.
Material and Methods
Bacterial cellulose production by Komagataeibacter hansenii ATCC 23769 from the Collection of Tropical Culture (CCT) (André Tosello Foundation- Brazil) was carried out in orbital agitator at 30°C, 150 rpm and mannitol was used as carbon source, as described in [7]. Nylon lines (approx.10 cm long) were used as templates to produce branching vessel networks in situ in the scaffolds during bacteria cultivation and BNC growth, according to the procedure reported in [5]. The hydrogels of BNC produced were purified with 0.1M NaOH and used as supports for precipitation and crystallization of HAp by soaking the hydrogels in alternated solutions of CaCl2 and Na2HPO4.
The morphology and distribution of CB-HAp-crystals on the BNC multilayer were evaluated by Field Emission Scanning Electron Microscopy (FESEM, JEOL JSM-6701F) and Scanning Electron Microscopy (SEM, JEOL JSM-6390LV) together with Energy Dispersive X-ray Spectroscopy (EDS) used to determine the Ca:P ratio. For microscopy observations the samples were freeze dried and placed over a stub and coated with gold. Cell culture was performed as follows. One milliliter of medium (DMEM, with 10% fetal bovine serum) containing approximately 1.8×105 primary human osteoblasts (HOB-obtained from the Rio de Janeiro Cell Bank) cells per well were seeded on slices of the scaffolds and maintained in a humidified incubator at 37°C and 5% CO2 for 72 hours. The cell adhesion and proliferation were observed by SEM.
Results and Discussion
Figure 1a shows a synthesized massive cocoon-like vascularized BNC hydrogel. The 3D massive cocoon-like hydrogel with mass of approximately 80 g and 80 cm3 of volume is composed of an outer membrane with higher fiber density than its inner part, where a sparse nanofibrous network that resembles the Extracellular Matrix (ECM) is formed during agitation [7]. After HAp precipitation the BNC hydrogel exhibits an opaque whitish color (Figure 1b). SEM micrographs of a produced vessel are shown in Figures 1c and d. In Fig. 1c, an ellipsoid-shaped vessel with largest diameter of around 3 mm is shown. The vessel’s shape is deformed from the original circular cross sectioned template due to the drying process. The vessel is delimited by a dense but semi-permeable BNC membrane with thickness varying from 0.2 to 2 μm, in which the microstructure and fiber density differs from the inner regions of the hydrogels. This higher nanofiber density was formed because of the higher oxygen supply for the bacteria attached around the nylon line template [5].
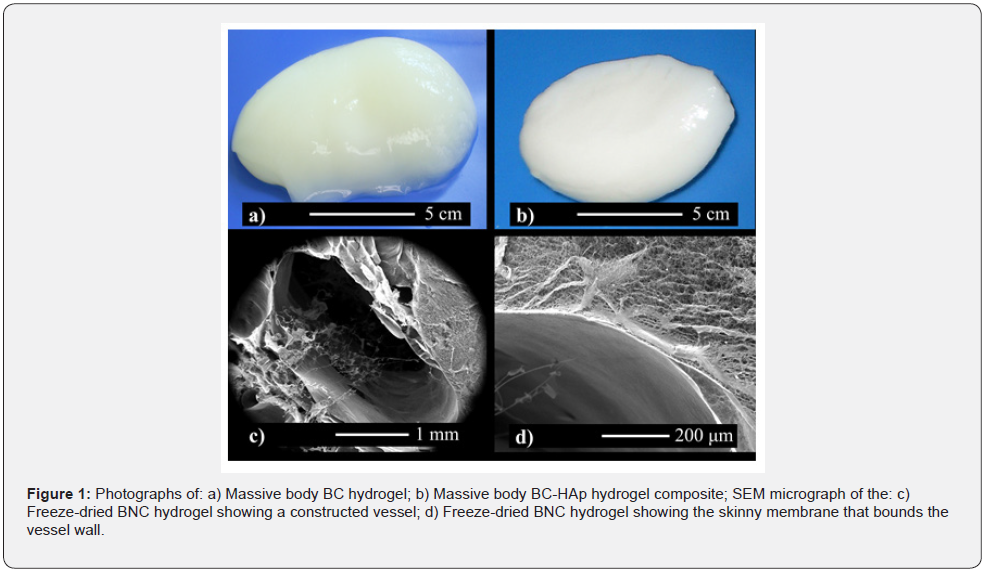
Figure 2 shows the FESEM micrographs (Figures 2 a and b) of a surface of the cross-section view of BNC hydrogel slices before (Figure 2a) and after HAp precipitation (Figure 2b). Figure 2a shows that the membrane is composed of a dense and homogeneous matrix forming an interlaced network of cellulose nanofibers with average diameters less than 100 nm and a high aspect ratio typical of BNC membranes. HAp precipitated and crystallized onto cellulose nanofibers resulting in a relatively rough surface as shown in Figure 2b. The HAp crystals are uniformly distributed on the surface of the cellulose nanofibers. Moreover, cellulose nanofibers are dispersed between hydroxyapatite particles, forming a two-phase system. The hydroxyapatite particles form agglomerates with an apparent homogeneous size distribution ranging from 1 to 2 μm. The EDS spectrum (inset in Figure 2b) indicates formation of a layer rich in Ca and P, whose Ca: P ratio is 1.37. This ratio is smaller than the stoichiometric ratio characteristic of hydroxyapatite (1.67). Hydroxyapatite prepared by precipitation from aqueous solutions usually results in a lower Ca: P ratio, which can vary from 1.33 to 1.67 [15]. The hydroxyapatite-nanocellulose system can provide additional mechanical resistance to the biocomposite.
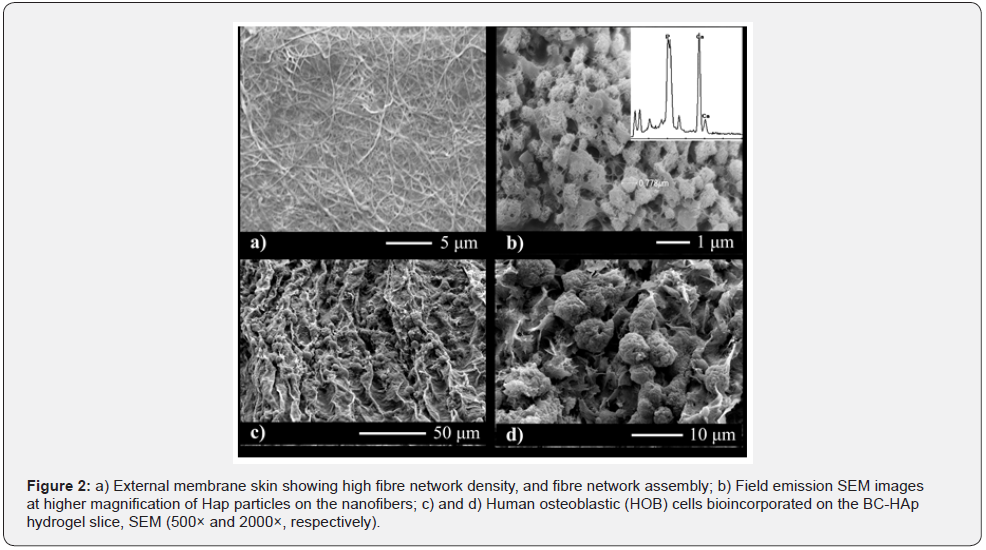
Human Osteoblast Cells (HOB) successfully adhered and proliferated in the 3D BNC-HAp hydrogel composite forming a cell carpet on its surface (Figure 2c). After 72 hours, the cells are already confluent and exhibit a regular active osteoblast morphology on the scaffold’s surface (Figure 2d). Actin filaments of HOB cell indicate a good adhesion on the surface of BNC-HAp scaffold material. Adhered osteoblasts can be then supplied with oxygen due to the formed vessels. Further improvements with other cell types like endothelial cells and fibroblasts, could induce angiogenesis and promote an enhanced integration of the implanted bio-composite.
Conclusion
In this work, we used dynamically cultures of K. hansenii and nylon templates to produce branching vessel networks in nanocellulose large tissue-engineered constructs. A dense, semipermeable BNC membrane delimits the formed vessels. HAp was successfully synthesized by a biomimetic method and precipitated homogeneously on the surface of the cellulose nanofibers. Human osteoblast cells successfully adhered and proliferated in the 3D BC-HAp hydrogel composite. Due to its biocompatibility this biomaterial may potentially be implanted directly in tissue-deficient regions or used as cell-carrier scaffolds for therapeutic and regenerative bone tissue applications.
Acknowledgement
The authors gratefully thank the Central Laboratory of Electronic Microscopy (LCME-UFS) for SEM and FESEM analysis.
Funding Statement
This work was funded by National Council for Scientific and Technological Development (CNPq, Brazil), Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina (FAPESC, Brazil) and Coordination for the Improvement of Higher-Level Personnel (CAPES, Brazil) – Finance Code 001.
References
- Svenja Hinderer, Shannon Lee Layland, Katja Schenke-Layland (2016) ECM and ECM-like materials-Biomaterials for applications in regenerative medicine and cancer therapy. Adv Drug Deliv Rev 97: 260-269.
- Hussey GS, Dziki JL, Badylak SF (2018) Extracellular matrix-based materials for regenerative medicine. Nat Rev Mater 3: 159-173.
- Klemm D, Schumann D, Udhardt U, Marsch S (2001) Prog Polym Sci 26: 1561-1603.
- Czaja WK, Young DJ, Kawecki M, Brown RM (2007) The future prospects of microbial cellulose in biomedical applications. Biomacromolecules 8 (1) 1-12.
- Rambo CR, Recouvreux DOS, Carminatti CA, Pitlovanciv AK, Antonio RV, et al. (2008) Template assisted synthesis of porous nanofibrous cellulose membrane for tissue engineering. Materials Science and Engineering 28 (4): 549-554.
- Andrade FK, Costa R, Domingues L, Soares R, Gama M (2010) Improving bacterial cellulose for blood vessel replacement: Functionalization with a chimeric protein containing a cellulose-binding module and an adhesion peptide. Acta Biomaterialia 6(10): 4034-4041.
- Recouvreux DOS, Rambo CR, Berti FV, Carminatti CA, Antonio RV, et al. (2011) Novel three-dimensional cocoon-like hydrogels for soft tissue regeneration. Material Science Engeneering C 31(2): 151-157.
- Hartgerink JD, Beniash E, Stupp SI Self-assembly and mineralization of peptide-amphiphile nanofibers. Science 294 (5547) 1684-1688.
- E Saiz, L Gremillard, G Menendez, P Miranda, K Gryn, et al. (2007) Preparation of porous hydroxyapatite scaffolds. Materials Science and Engineering: C 27 (3): 546-550.
- Gerber HP, Ferrara N (2000) Angiogenesis and bone growth. Trends Cardiovasc Med 10(5): 223-228.
- H Bramfeld, G Sabra, V Centis, P Vermette (2010) Scaffold Vascularization: A Challenge for Three-Dimensional Tissue Engineering. Curr Med Chem 17(33): 3944-3946.
- Liu X, Jakus AE, Kural M, et al. (2008) Vascularization of Natural and Synthetic Bone Scaffolds. Cell Transplant 27(8): 1269-1280.
- Bishop A (2008) Role of oxygen in wound healing. J Wound Care17(9):399–402.
- Guo S, Dipietro LA (2010) Factors affecting wound healing. J Dent Res 89(3): 219–229.
- Mortier A, Lemaitre J, Rodrique L, Rouxhet PG (1989) Synthesis and thermal behavior of well-crystallized calcium-deficient phosphate apatite. Journal of Solid State Chemistry 78(2): 215-219.






























