Prevalence, Antimicrobial Susceptibility Pattern, And Associated Factors of Urinary Tract Infections Among Diabetic Patients at Dongla State, Sudan
Husham M Taha Aloob1*, Samia S Mohamed Ismail2, Abdelhakam H Ali3
1Department of Microbiology and Parasitology, Faculty of Medical Laboratory Sciences, Dongla University, Sudan
2Department of Microbiology, Faculty of Medicine, Dongla University, Sudan
3Department of molecular bacteriology, faculty of medical laboratory sciences, university of al Butana, Sudan
Submission:January 25, 2021;Published: February 09, 2021
*Corresponding author: Husham M Taha Aloob, Department of Microbiology and Parasitology, Faculty of Medical Laboratory Sciences, Dongla University, Sudan
How to cite this article: Husham M T A, Samia S M I, Abdelhakam H A. Prevalence, Antimicrobial Susceptibility Pattern, And Associated Factors of 0010 Urinary Tract Infections Among Diabetic Patients at Dongla State, Sudan. Adv Biotech & Micro. 2021; 16(1): 555930.DOI:10.19080/AIBM.2021.15.555930
Abstract
Urinary tract infections are the most common bacterial infections in humans. UTI causes considerable morbidity in diabetic patients and if complicated, can cause severe renal damage and life threatening infections. The present study was aimed to determine the Prevalence, Antimicrobial Susceptibility Pattern, and Associated Factors of Urinary Tract Infections among Diabetic Patients. A cross-sectional study was conducted during the period from August 2019 to April 2020. Data were collected using structured questionnaire. Clean-catch, midstream urine samples were collected and cultured for UTI diagnosis and antimicrobial susceptibility. significant bacteriuria was defined as a positive urine culture (≥105 colony-forming units). The predominant isolates were Escherichia coli (60%), K. pneumoniae (15%), P. aeruginosa (8%), E. feacalis (6.5%), P. mirabilis (1%), S, aureus (2.5%) and C. albicans (7%). Antimicrobial Susceptibility Pattern of Gram-negative bacteria were showed sensitive to colistin (99%), meropenem (95%), and gentamycin (48%), but were resistant to cefpodoxime (99%) and ciprofloxacin (65%). Gram-positive bacteria isolates showed a higher level of sensitivity to colistin (100%), meropenem (80%), followed by ciprofloxacin, ceftriaxone and gentamycin (60%). Isolation of uropathogenic bacteria and antimicrobial susceptibility testing are crucial for the treatment of UTI in diabetic patients. Resistance rates among common uropathogens continue to evolve and appear to be increasing too many commonly used antimicrobial agents and a continued surveillance of resistance rates among uropathogens is needed to controlling this global problem.
Keywords: Urinary tract infection, Diabetes, Bacteriuria, Sudan
Introduction
Urinary tract infections are the most common bacterial infections in humans, both as community-acquired and healthcare-associated infections. It is the most common nonsurgical nosocomial infection in postoperative patients and the second most common healthcare associated infection [1,2]. Proliferation of bacteria in the urinary tract is the cause of urinary tract infection. The clinical manifestations of UTI depend on the portion of the urinary tract involved, the etiologic organism(s), the severity of the infection and the patient’s ability to mount an immune response to it [3]. Signs and symptoms may include fever, chills, dysuria, urinary urgency, frequency and cloudy or malodorous urine. Infections are almost always ascending in origin and caused by bacteria in the periurethral flora and the distal urethra. These bacteria inhabit the distal gastrointestinal tract and colonize the perineal area. E. coli usually causes a child’s first infection [4] but other gram-negative bacilli and Enterococci may also cause infection. Staphylococcal infections, especially those due to Staphylococcus saprophyticus are common causes of urinary tract infection among female adolescents. Diabetes Mellitus (DM) is a group of metabolic disorder characterized by hyperglycemia resulting from defects in insulin secretion, insulin action, or both [5]. has long been considered to be a predisposing factor for Urinary Tract Infection (UTI) because of sugar in urine, which serves as media for growth of bacteria [6,7]. The colonized urinary tract can also accelerate the prolonged release of bacteria with an increased risk of complications of the urinary system, ranging from dysuria (pain or burning sensation during urination) to the organ damage and sometimes even death [8,9]. Risk factors for UTI among patients with and without DM have been identified e.g. obesity, female sex, and prostate syndrome in men [10,11]. Furthermore, glycosuria, low immunity, and bladder dysfunction, which are associated with DM, are considered particular risk factors for UTI [12,13]. Escherichia coli is the most commonly isolated organism in both diabetic and nondiabetic patients [14,15]. Antimicrobial resistance is emerging as an important public health problem in both the hospitals and the community. Untreatable infections are being recognized more frequently and, as important bacterial pathogens become increasingly resistant, the lack of new or alternative antimicrobial agents makes serious outbreaks a possibility, increased health care costs resulting from treatment failures, and longer hospital stays [16,17]. Frequently encountered MDR bacteria, methicillin resistant S. aureus, cephalosporins, and extended spectrum betalactamase producing Enterobacteriaceae, ceftazidime resistant P. aeruginosa, Imipenem-resistant A. baumannii and vancomycinresistant Enterococci are commonly encountered in the hospital environment [18,19].
Materials and Methods
Study design
A Cross-sectional study was carried to detect Clinical Epidemiology and Antibiogram of UTI among diabetes mellitus patients in Dongla State, Sudan. The study include patients clinically diagnosed by having one or more of the following symptoms: dysuria, frequency, urgency, suprapubic discomfort or flank pain. Non-diabetic and pregnancy were excluded from the study. A total of 200 bacteriuria isolated form Diabetic patients (80 males and 120 females) with age group ranged from 10 to 80 years old. All patients were informed of the purpose of the study and their consent or that of their care provider was obtained before urine samples were collected. during the period from August 2019 to April 2020.
Data collection
A structured questionnaire and referring to the patient clinical sheet were being used; demographic data and other Data (clinical symptoms, previous antibiotic, duration of antibiotic used). verbal consent was obtained from each patient enrolled in this study.
Sample Collection and Processing
Each diabetic patient was instructed how to collect a cleancatch midstream urine specimen. Accordingly, about 5 to 10 ml of urine specimen were collected in a labeled, leak proof, and sterile containers. The specimens were stored at 4°C and transported under aseptic technique.
Isolation and Identification of organisms using Biochemical tests and selective medium
A loop full of urine was inoculated on Cysteine Lactose Electrolyte Deficient (CLED) agar, MacConkey, and Blood agar plates (Oxoid, Ltd., Basingstoke, Hampshire, England) by using a sterile calibrated wire loop with a volume of 0.001ml after the specimen was mixed. The plates were incubated aerobically at 35-37oc for 24 hours and the outcome was judged as significant/ non-significant growth, or contaminated (discarded). Urine culture plates showing ≥105 Colony Forming Units (CFU)/ml of single bacterial species were considered as significant bacteriuria [20]. Gram reaction of the organisms, microscopic appearance and colony characteristics were the presumptive identification criteria. Indole production, citrate utilization, H2S production, gas production, urea hydrolysis, lysine decarboxylation, lactose fermentation and motility were used for further identification of gram negative bacteria. Coagulase, catalase, and mannitol fermentation test were used for further identification of gram positive bacteria [21].
Antimicrobial Susceptibility Testing
Antimicrobial sensitivity testing of all isolates was performed on diagnostic sensitivity test plates according to the Kirby- Bauer method following the definition of the Committee of Clinical Laboratory International Standards. Bacterial inoculums were prepared by suspending the freshly grown bacteria in 5mL sterile saline. A sterile cotton swab was used to streak the surface of Mueller Hinton agar plates. Filter paper disks containing a designated concentration of the antimicrobial drugs were obtained from Hi-Media Laboratories in the following concentrations: Amikacin (30μg), Gentamycin (10μg), Cefotaxime (30μg), ceftriaxone (30μg), Meropenem (10μg) ciprofloxacin (5μg), ofloxacin (5μg), colistin (10μg), Cefepime (30μg). The diameters of zone of inhibition were interpreted according to CLSI standards. Media and disks were tested for quality control with standard strains.
Data analysis
Collected data were analyzed by using Statistical Package for Social Science (SPSS) program 20.
Results
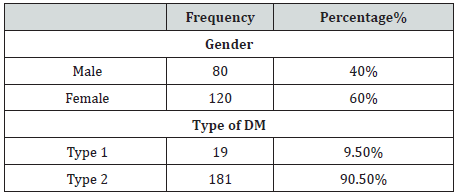
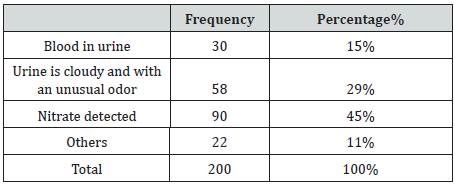
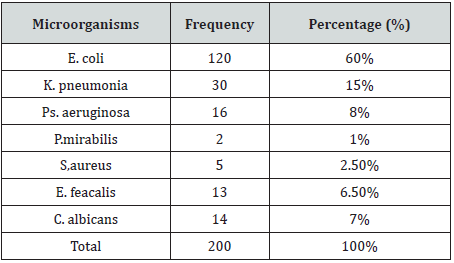
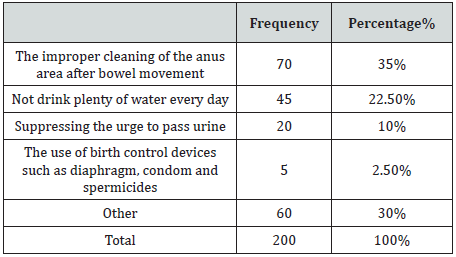
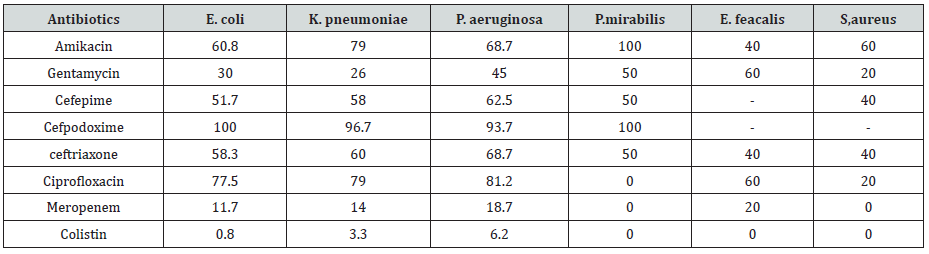
A total of 200 bacteriuria isolated form Diabetic patients (80 males and 120 females, 9.5% had type 1 and 90.5 % had type II DM) with age group ranged from 10 to 80 years old (Table 1). collected from patient’s attendant a different hospital in Dongla State, Sudan. All the clinical isolates were given numbers and then purified by streaking plates containing the appropriate selective and differential culture media and then identified on the basis of the results of microscopically staining reaction (Gram stain), culture characteristic and biochemical tests. Most of urine appearance showed abnormality and cloudy and unusual odor (Table 2). The most frequent isolated were 120 E. coli (60%), 30 K. pneumoniae (15%), 16 P. aeruginosa (8%), 13 E. feacalis (6.5%), 2 P. mirabilis (1%), 5 S, aureus (2.5%) and 14 C. albicans (7%) (Table 3). The percentage of UTI was higher among patient improper cleaning of the anus area after bowel movement (67%) (Table 4). The bacteriuria isolate strains showed differences in susceptibility and resistance patterns to the antimicrobials tested. All most of the isolated were shown to be resistant to Cefpodoxime. The most efficient antibiotics were Colistin and imipenem (Table 5).
Discussion
Urinary tract infection is the commonest bacterial infectious disease with a high rate of morbidity and financial cost. The risk of developing infection in diabetes is higher due to abnormalities in the host defense and high glucose in urine [22]. In the present study all urine sample revealed significant growth for species of bacteria and fungi were isolated. the most commonly isolated agents from urinary tract infections vary, almost all of them are caused by single microorganism type. In this study the most frequently isolated microorganism was E. coli with a rate of (60%), followed by K. pneumoniae (15%), and this result is agreement with [23-27] The higher incidence of E. coli could be attributed to the fact that they are commensals of the bowels and that infections are mostly by fecal contamination due to poor hygiene and the presence of unique structure, which promote colonization of the host epithelial cells within the urinary tract and prevent bacteria from urinary washing [28]. P. aeruginosa, C. albicans, E. feacalis are the next predominant uropathogens isolated [26,29]. In our study the frequency of UTI was higher among duration of DM greater than 4 years this is in agreement with a study done in Saudi Arabia [30], Gondar [31] and Iran [32]. Duration of diabetes had been described as risk factor for complicated UTI, probably because of concurrent neuropathy [30]. Antimicrobial Susceptibility Pattern of Gram-negative bacteria were showed sensitive to colistin (99%), meropenem (95%), and gentamycin (48%), but were resistant to cefpodoxime (99%) and ciprofloxacin (65%). Gram-negative isolates, E. coli K. pneumoniae and P. aeruginosa were howed higher sensitivity to colistin (99.2%, 96.7%, 93,2), meropenem (88.3%,86%, 81.3) and gentamycin (70%,74%,55%), while were resistant to cefpodoxime (100%,96.7%,93.7%). respectively. Proteus spp. were showed higher sensitivity to colistin, meropenem and ciprofloxacin (100%). But showed higher resistant to cefpodoxime and amikacin (100%). Grampositive bacteria isolates showed a higher level of sensitivity to colistin (100%), meropenem (80%), followed by ciprofloxacin, ceftriaxone and gentamycin (60%), This is similar with the report of different studies [26-34]. Multi drug resistance were observed in 90% of the isolated bacterial uropathogens. Reasons for such alarming MDR might be inappropriate and incorrect administration of antimicrobial agents as empirical treatment and lack of appropriate infection control strategies, which can cause a shift to increase prevalence of resistant organism in the community.
Conclusion
The overall prevalence of significant UTI in diabetic patients and non-diabetic patients the most frequently observed organisms were E. coli followed by K. pneumoniae. Multi drug resistance were observed in 90% of the isolated. All Gram-negative and Gram-positive bacteria were showed sensitive to colistin and meropenem.
References
- Benedetta Allegranzi, Sepideh Bagheri Nejad, Christophe Combescure, Wilco Graafmans, Homa Attar, et al. (2011) Burden of endemic healthcare-associated infection in developing countries: systematic review and meta-analysis. The Lancet. 377(9761): 228-241.
- Gastmeier P, Kampf G, Wischnewski N, Hauer T, Schulgen G, et al. (1998) Prevalence of nosocomial infections in representative German hospitals. J Hosp Infect. 38(1): 37-49.
- Foxman B, Brown P (2003) Epidemiology of urinary tract infections: transmission and risk factors, incidence, and costs. Infectious disease clinics of North America. 17(2): 227-241.
- Brkic K (2010) An overview of traffic sign detection methods. Department of Electronics, Microelectronics, Computer and Intelligent Systems. Faculty of Electrical Engineering and Computing Unska, 3,10000.
- American Diabetes Association (2008) Diagnosis and classification of diabetes mellitus. Diabetes Care, 21(1): S62-S67.
- Ribera R, Pascual R, Orozco D, Perez B, Pedrera V, et al. (2006) Incidence and risk factors associated with urinary tract infection in diabetic patients with and without asymptomatic bacteriuria, European Journal of Clinical Microbiology and Infectious Diseases 25(6): 389-399.
- ChitaT, TimarB, Muntean D (2016) Urinary tract infections in Romanian patients with diabetes: prevalence, etiology, and risk factors. Therapeutics and Clinical Risk Management 13: 1–7.
- Mishra N, Tripathi M, Jain M, Mishra R, Patel S, et al. (2016) Bacteriological study of urinary tract infection in diabetic patients. World Journal of Pharmaceutical Sciences. 5(4): 1247–1253.
- Borj M, Taghizadehborojeni S, Shokati A (2017) Urinary tract infection among diabetic patients with regard to the risk factors, causative organisms and their antimicrobial susceptibility profiles at Firoozgar Hospital, Tehran, Iran. International Journal of Life Science and Pharma Research 7(3): L38–L47.
- Al-Rubeaan KA, Moharram O, Al-Naqeb D, Hassan A, Rafiullah MR (2013) Prevalence of urinary tract infection and risk factors among Saudi patients with diabetes. World J Urol. 31(3): 573–578.
- Ribera MC, Pascual R, Orozco D, Pérez Barba C, Pedrera V, Gil V (2006) Incidence and risk factors associated with urinary tract infection in diabetic patients with and without asymptomatic bacteriuria. Eur J Clin Microbiol Infect Dis. 25(6): 389–393.
- Funfstuck R, Nicolle LE, Hanefeld M, Naber KG (2012) Urinary tract infection in patients with diabetes mellitus. Clin Nephrol. 77(1): 40–48.
- Nicolle LE (2005) Urinary tract infection in diabetes. Curr Opin Infect Dis. 18(1): 49–53.
- Ghenghesh KS, Elkateb E, Berbash N, Abdel Nada R, Ahmed SF, et al. (2009) Uropathogens from diabetic patients in Libya: virulence factors and phylogenetic groups of Escherichia coli isolates. J Med Microbiol. 58(8): 1006–1014.
- Hamdan HZ, Ziad AH, Ali SK, Adam I (2011) Epidemiology of urinary tract infections and antibiotics sensitivity among pregnant women at Khartoum North Hospital. Ann Clin Microbiol Antimicrob. 18(10): 2.
- Muhammad UK, Isa MA, Aliyu ZM (2013) Distribution of potential nosocomial pathogens isolated from environments of four selected hospital in Sokoto, North Western Nigeria. J Microbiol Biotechnol Res. 3:139–143.
- Davane M, Suryawanshi N, Pichare A, Nagoba B (2014) Pseudomonas aeruginosa from hospital environment. J Microbiol Infect Dis. 4: 42–43.
- Centers for Disease Control and Prevention (2003) Healthcare infection control advisory committee. Guidelines for environmental infection control in healthcare facilities. Atlanta.
- Lee TB, Baker OG, Lee JT, Scheckler WE (1998) Recommended practices for surveillance. Am J Infect Control. 26(3): 277–288.
- Pezzlo M (2014) Laboratory Diagnosis of Urinary Tract Infections: Guidelines, Challenges, and Innovations. CMN. 36(12): 87–93.
- Cheesbrough M (2006) District Laboratory Practice in Tropical Countries. Cambridge University Press, New York.
- Senthamarai S, Sivasankari S, Anitha C (2016) A study on clinical presentation, bacterial profile and its antibiotic sensitivity pattern in urinary tract infections among diabetic patients attending tertiary care hospital, Tamilnadu. Int J App Res.2(3): 157–159.
- Amin M, Mehdinejad M, Pourdangchi Z (2011) Study of bacteria isolated from urinary tract infections and determination of their susceptibility to antibiotics. Jundishapur J Microbiol 2: 118-123.
- Beyene G, Tsegaye W (2011) Bacterial Uropathogens in Urinary Tract Infection and Antibiotic Susceptibility Pattern in Jimma University Specialized Hospital, Southwest Ethiopia. Ethiop J Health Sci 21(2): 141-146.
- Borj M, Taghizadehborojeni S, Shokati A et al., (2017) Urinary tractinfectionamongdiabeticpatientswithregardtotherisk factors, causative organisms and their antimicrobial susceptibility profiles at Firoozgar Hospital, Tehran, Iran, International Journal of Life Science and Pharma Research, 7(3): L38–L47.
- Ampaire L, Butoto A, Orikiriza P, Muhwezi O, Papazafiropoulou A (2015) Bacterial and drug susceptibility profiles of urinary tract infection in diabetes mellitus patients at Mbarara Regional Referral Hospital, Uganda,” British Microbiology Research Journal, 9(44): 1–5.
- Lyamuya E, Moyo S, Komba E, Haule M (2011) Prevalence, antimicrobial resistance and associated risk factors for bacteriuria in diabetic women in Dar Es Salaam, Tanzania. African Journal of Microbiology Research, 5(6): 683–689.
- Mulvey M (2002) Adhesion and entry of uropathogenic Escherichia coli. Cell Microbiology 4(5): 257–271.
- De Francesco MA, Ravizzola G, Peroni L, Negrini R, Manca N (2007) Etiology of uropathogens and antimicrobial resistance of common uropathogens in Brescia Italy. Med Sci Monit. 6: BR136–BR144.
- Badri AM, Mohamed SG (2017) Clinical Epidemiology and Antibiogram of UTI Patients Attended Different Hospital in Khartoum, Sudan. Clin Microbiol 6: 301.
- Al-Rubeaan K, Moharram O, Al-Naqeb D (2013) Prevalence of urinary tract infection and risk factors among Saudi patients with diabetes. World J Urol. 31(3): 573–578.
- Yismaw G, Asrat D, Woldeamanuel Y (2012) Urinary Tract Infection: Bacterial etiologies, drugresistance profile and associated risk factors in diabetic patients attending Gondar University Hospital, Gondar, Ethiopia European Journal of Experimental Biology. 2(4): 889–898.
- Raoofi A, Ghavami M, Shahhamzeh M (2016) The Impact of Demographic Factors and Blood Sugar Control on the Incidence of Urinary Tract Infections in Khorramabad in 2013. Iran Red Crescent Med J. 18(5): 1–6.
- Al-Qaseer A, Abdul-wahab B, Abbas O (2014) Bacteriological finding of urinary tract infection in diabetic patients. Int J Adv Res. 2(10): 274–279.






























