Prevalence and Drug Resistance Status of Enterococcus in Tertiary Care Hospital
Mesbah Uddin Ahmed1*, Afsana Mahbub2 and Shah Zahurul Haque Asna1
1Masters in Microbiology, Bangladesh University of Health Sciences, Bangladesh
2Assistant Professor & Lab Consultant, ZH Sikder Women’s Medical College Hospital, Bangladesh
Submission:November 25, 2020;Published: December 07, 2020
*Corresponding author:Mesbah Uddin Ahmed, Masters in Microbiology, Bangladesh University of Health Sciences, Bangladesh
How to cite this article: Mesbah U A, Afsana M, Shah Z H A. Prevalence and Drug Resistance Status of Enterococcus in Tertiary Care Hospital. Adv Biotech & Micro. 2020; 16(1): 555929.DOI:10.19080/AIBM.2020.15.555929
Abstract
Introduction: Enterococci are common components of the microfloral community of human and other animals and are commonly found in soil, on plants, and in water. It is increasingly causing human infections and is being isolated from various clinical samples.
Methods:This was a cross sectional study was conducted to determine the status and drug resistance pattern of enterococcus in a selected tertiary care hospital. Both descriptive and inferential statistics were used.
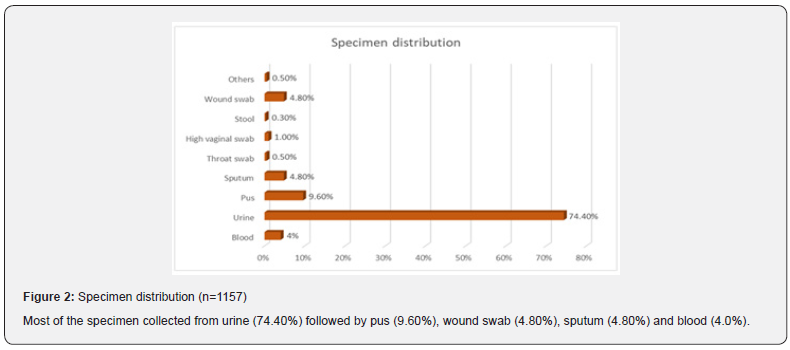
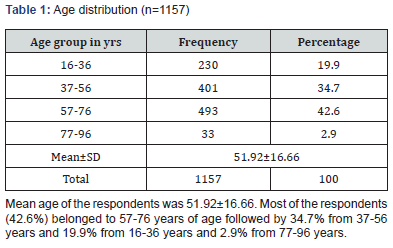
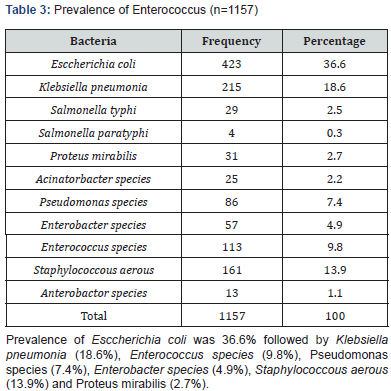
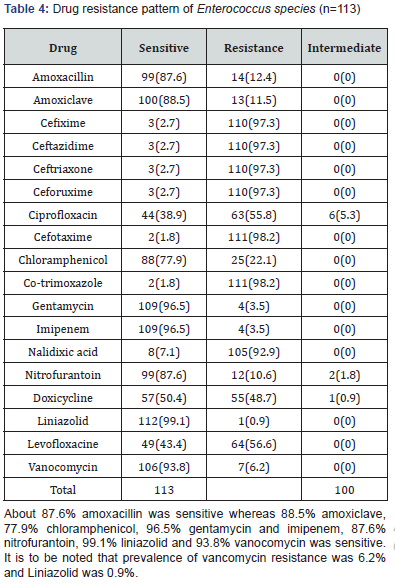
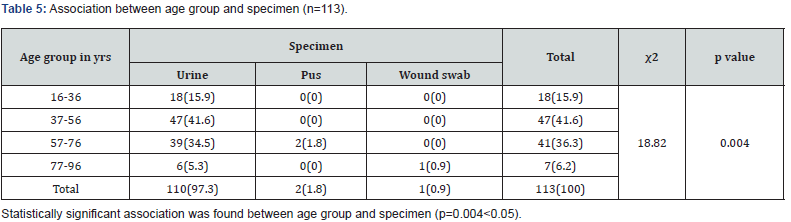
Results: Mean age of the respondents was 51.92±16.66. Mean monthly family income of the respondents was 28331.46±15704.84 BDT. Most of the specimen collected from urine (74.40%) followed by pus (9.60%), wound swab (4.80%), sputum (4.80%) and blood (4.0%). Two-third of the specimen (68.80%) showed significant presence of pus cell. Prevalence of Esccherichia coli was 36.6% followed by Klebsiella pneumonia (18.6%), Enterococcus species (9.8%), Pseudomonas species (7.4%), Enterobacter species (4.9%), Staphylococcous aerous (13.9%) and Proteus mirabilis (2.7%). About 87.6% amoxacillin was sensitive whereas 88.5% amoxiclave, 77.9% chloramphenicol, 96.5% gentamycin and imipenem, 87.6% nitrofurantoin, 99.1% liniazolid and 93.8% vanocomycin was sensitive. Prevalence of Enterococcus species in urine, pus and wound swab was 97.30%, 1.80% and 0.90%. Statistically significant association was found between age group and specimen (p=0.004<0.05).
Keywords:Prevalence of enterococcus; Drug resistance; Status of enterococcus; Tertiary care hospital
Introduction
Enterococci are members of the healthy human intestinal flora, but are also leading causes of highly antibiotic resistant, hospital-acquired infection [1]. There is growing evidence that these bacteria frequently possess several specific traits that enable them to survive in the hospital environment, colonize patients, and cause infections such as bacteraemia, peritonitis, endocarditis and urinary tract, surgical site infection, and device-related infections [2]. Nosocomial or health care-associated infections account for a high morbidity and mortality rate among hospitalized patients [3]. Therapeutic spectrum of enterococci is limited since the organisms are genetically resistant to Cephalosporins and Cotrimoxazole. They also have a tremendous capacity to acquire resistance to penicillins, high concentration of aminoglycoside & vancomycin [3]. Enterococci with High Level Resistance to Aminoglycosides (HLAR), beta lactamase production & glycopeptide resistance including vancomycin resistance are posing a great therapeutic challenge for clinicians as well as health care institutions [4]. Antimicrobial resistance results in increased morbidity, mortality and costs of treatment. Preventing the emergence and dissemination of resistant organisms is critical for control of hospital infections. Appropriate antimicrobial stewardship that includes optimal selection, dose and duration of treatment, as well as control of antibiotic use, will prevent or slow the emergence of resistance among microorganisms [5]. Having an awareness of antimicrobial resistance patterns, particularly in hospitals, is crucial for the selection of appropriate antibiotic therapy to improve treatment outcomes, reduce morbidity and mortality, shorten the hospitalization period and consequently reduce the cost of care. Hence, this study was designed to identify the magnitude of Enterococcal infections and their antibiotic susceptibility pattern in a tertiary care hospital in Dhaka, Bangladesh.
Methodology
Specimen: Blood, Urine & Pus.
Blood culture: Blood culture was done by Bectec Automated Blood Culture System. This system gives signal when growth appears in the media. Subculture was done on MacConkey and blood agar media from the media giving signal for growth.
Pus Culture: Pus culture was done in MacConkey and Blood, Bectec Automated Blood Culture System.
Urine culture: Mid-stream urine was collected and cultured on Blood agar and MacConkey agar and Bectec Automated Blood Culture System All the inoculated media are incubated overnight at 37°C and suspected colonies was picked up and subjected to identification by biochemical tests.
Biochemical test: Bile-esculin test is based on the ability of certain bacteria, notably the group D streptococci and Enterococcus species, to hydrolyze esculin in the presence of bile (4% bile salts or 40% bile).
Antibiotic susceptibility testing The test was done as per modified Kirby-Bauer disc-diffusion test. Briefly, a small inoculum of each pure bacterial isolate was emulsified in 3 ml of sterile normal saline in Bijou bottles, and the density was compared with a barium chloride standard (0.5 McFarland). A sterile cotton swab was dipped into the standardized suspension of bacterial cultures and used to evenly inoculate Mueller-Hinton agar plates.
Study Design: It was hospital based cross sectional study.
Study Period: One year
Study Area: This study was conducted at BIHS Hospital.
Study Population: The study was conducted among all patients in BIHS hospital for treatment purpose
Sample size: Sample size was calculated by using following formula 2

Here,
Z=Standard normal deviate=1.96 corresponding to 95% of CI, p= is the prevalence rate, taken as 50%, i,e, 0.5 (as no study found)
q= 1-p (or, proportion of persons not suffering from the disease) =0.5
d = the acceptable standard error and
n = the required sample size
Therefore, the required sample size, n = {(1.96)2 x 0.5 x 0.5}/0.052 = 384 but I took 1157 to increase statistical power.
Sampling technique: Non-probability convenient sampling technique was applied.
Data collection procedure: Appropriate data was collected by using a pre-designed data sheet. All relevant information was collected from history sheet and investigation papers. Data analysis: Statistical Package for Social Sciences (SPSS 22.0) version was used for classification, presentation and analysis of data.
Ethical consideration
Prior to commencement of study the respective authority approved the research protocol. Proper permission was taken from the department and institution concerned for the study. All the patients included in this study was informed about the nature, risk and benefit of the study. No data was collected without permission of the patient. Participation in this research was fully voluntary. The respondents were remained entirely free to withdraw their participation at any stage or any time of the study. Informed written consent was taken from each patient. Confidentiality was assured and anonymity was maintained. No participant was identified in any report or publication under the study.
Results & Discussion
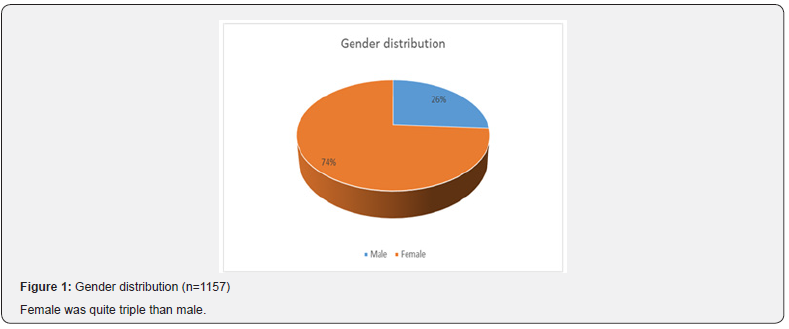
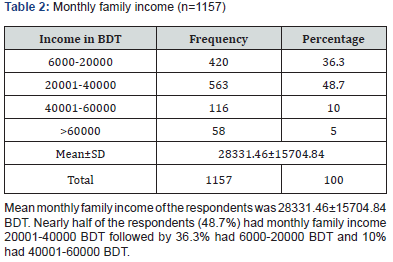
Enterococci are commensal bacteria inhabiting the intestines of both humans and animals, which are the major conditionally pathogenic bacteria that cause hospital-acquired infections. Recently, frequent inappropriate use of antimicrobial agents, increase in invasive therapy, and wide use of immunosuppressants has resulted in a growing rise in the number of clinical infections caused by Enterococcus spp., notably Enterococcus faecium [6]. In addition, the emergence of High-Level Aminoglycoside-Resistant (HLAR) enterococci and Vancomycin-Resistant Enterococci (VRE) causes great difficulties in clinical anti-infective therapy [7-9] (Table 1& Figure1). Most of the specimen collected from urine (74.40%) followed by pus (9.60%), wound swab (4.80%), sputum (4.80%) and blood (4.0%). Two-third of the specimen (68.80%) showed significant presence of pus cell. Prevalence of Esccherichia coli was 36.6% followed by Klebsiella pneumonia (18.6%), Enterococcus species (9.8%), Pseudomonas species (7.4%), Enterobacter species (4.9%), Staphylococcous aerous (13.9%) and Proteus mirabilis (2.7%). About 87.6% amoxacillin was sensitive whereas 88.5% amoxiclave, 77.9% chloramphenicol, 96.5% gentamycin and imipenem, 87.6% nitrofurantoin, 99.1% liniazolid and 93.8% vanocomycin was sensitive. Prevalence of Enterococcus species in urine, pus and wound swab was 97.30%, 1.80% and 0.90%. Statistically significant association was found between age group and specimen. Due to the spread of enterococcal antimicrobial resistance [10,11], the tracing of the infectious sources is of great significance for the control of enterococcal infections and its spreading. Among the 289 enterococcal strainss isolated from a tertiary-care pediatric hospital in Mexico City during an 18-month period, E. faecalis and E. faecium comprised 81.2% of the total isolates, and antimicrobial resistance in Enterococcus spp. was found to be common [12] (Table 2 & Figure 2). Of the 415 enterococcal isolates obtained from clinical samples between January 1999 and 31 December 2001 in the Mubarak Al- Kabeer, Amiri, Adan, Ibn Sina and Maternity hospitals in Kuwait, E. faecalis (85.3%) and E. faecium (7.7%) accounted for 93% of the samples [13]. In China, E. faecium and E. faecalis were also found to be predominant in the enterococci isolated from clinical specimens [14-16]. Similar to these findings, the current study showed that E. faecium (58.7%) and E. faecalis (33%) were predominant in the 1157 clinical isolates of Enterococcus species isolated from our hospital. However, the present study involved a large sample size, compared the antimicrobial resistance in enterococcal strains isolated from different departments of the hospital, and investigated the efflux mechanism of resistance in enterococci, which is rarely reported previously (Table 3 & Figure 3). Enterococcus species are found to be intrinsically resistant to cephalosporins and aminoglycosides. In the current study, a significantly higher prevalence of resistance to penicillin, ampicillin, rifampicin, ciprofloxacin, levofloxacin, fosfomycin, erythromycin and furadantin was detected in E. faecium than in E. faecalis, while a greater prevalence of resistance to chloramphenicol, quinupristin/dalfopristin, minocycline and tetracycline was found in E. faecalis than in E. faecium. In addition, a low prevalence of resistance to linezolid, vancomycin and teicoplanin was detected in both E. faecium and E. faecalis. Limitations of studies are very common in social work. Monthly income of the patients was collected based on response of the study subjects so there might be some discrepancy at concrete (Tables 4,5 & Figures 4,5).
Conclusion
Most of the specimen collected from urine followed by pus. Two-third of the specimen showed significant presence of pus cell. Prevalence of Esccherichia coli was 36.6%. About 87.6% amoxacillin was sensitive whereas 88.5% amoxiclave, 77.9% chloramphenicol, 96.5% gentamycin and imipenem, 87.6% nitrofurantoin, 99.1% liniazolid and 93.8% vanocomycin was sensitive. Prevalence of Enterococcus species in urine was 97.30%. Statistically significant association was found between age group and specimen.
References
- Shankar N, Baghdayan AS, Gilmore MS (2002) Modulation of Virulence within a Pathogenicity Island in Vancomycin-Resistant Enterococcus faecalis. Nature417 (6890):746-750.
- Sava G, Heikens E, Huebner J (2010) Pathogenesis and Immunity in Enterococcal Infections. Clinical Microbiology and Infection16(6): 533-540.
- Mohanty S, Dhawan B, Gadepalli RS, Lodha R, Kapil A (2006) Case Report of Vancomycin Resistant Enterococcus Faecium VanA Phenotype: First Documented Isolation in India. The Southeast Asian Journal of Tropical Medicine and Public Health 37(2):335-337.
- Oberoi L(2010) Multidrug Resistant Enterococci in a Rural Tertiary Care Hospital-A Cause of Concern. JK Science 12:157-158.
- Shlaes DM,Gerding D N,John Jr JF, Craig WA, D L Bornstein, et al. (1997) Society for Healthcare Epidemiology of America and Infectious Diseases Society of America joint committee on the prevention of antimicrobial resistance: Guidelines for the prevention of antimicrobial resistance in hospitals. Infection Control and Hospital Epidemiology 25(3): 584-599.
- Sood S, Malhotra M, Das BK, Kapil A (2008) Enterococcal infections & antimicrobial resistance. Indian J Med Res128(2):111-121.
- Bonten MJ, Willems R, Weinstein RA (2001) Vancomycin-resistant enterococci: Why are they here, and where do they come from? Lancet Infect Dis1(5):314-325.
- Adhikari L (2010) High-level aminoglycoside resistance and reduced susceptibility to vancomycin in nosocomial enterococci. J Glob Infect Dis2(3):231-235.
- Cetinkaya Y, Falk P, Mayhall CG (2000) Vancomycin-resistant enterococci. Clin MicrobiolRev13(4):686-707.
- Shay DK, Goldmann DA, Jarvis WR (1995) Reducing the spread of antimicrobial-resistant microorganisms. Control of vancomycin-resistant enterococci. Pediatr ClinNorth Am42:703-716.
- Klare I, Konstabel C, Badstübner D, Werner G, Witte W (2003) Occurrence and spread of antibiotic resistances in Enterococcus faecium. Int J Food Microbiol88(2-3):269-290.
- Miranda G, Lee L, Kelly C, Solórzano F, Leaños B, Muñoz O, Patterson JE (2001) Antimicrobial resistance from enterococci in a pediatric hospital. Plasmids in Enterococcus faecalis isolates with high-level gentamicin and streptomycin resistance. Arch Med Res32(2):159-163.
- Udo EE, Al-Sweih N, Phillips OA, Chugh TD (2003) Species prevalence and antibacterial resistance of enterococci isolated in Kuwait hospitals. J. Med. Microbiol52(2):163-168.
- Ling JM, Char TS, Cheng AF (2002) Distribution of enterococci in Hong Kong. J Infect45(4):257-262.
- Lu JF, Qian L, Huo JF, Shao KY (2011) Clinical distribution and drug sensitivity analysis of 302 strains of Med. Pharm. J ChinPLA23:42-43.
- Guo ZS, Zhang LH, Lin SS (2012) Analysis of the isolation and variance of resistance to antibiotics of Enterococcus in blood specimens. Lab Med Clin9: 537-539.






























