Assessment of Salicylic Acid Protective Role Against Botrytis Cinerea Induced Damage in Tomato, In Various Storage Periods
Ehab A Salem1* and Yasser M Abd El Shafea2
1 National Center for Radiation Research and Technology (NCRRT), Atomic Energy Authority, Egypt
2Regional Center for Food and Feed (RCFF), Agricultural Research Center (ARC), Egypt
Submission: February 07, 2020; Published: June 17, 2020
*Corresponding author:Ehab A Salem, National Center for Radiation Research and Technology (NCRRT), Atomic Energy Authority, Nasr City, Egypt
How to cite this article: Ehab A S, Yasser M Abd El S. Assessment of Salicylic Acid Protective Role Against Botrytis Cinerea Induced Damage in Tomato, In Various Storage Periods. Adv Biotech & Micro. 2020; 15(5): 555923.DOI: 10.19080/AIBM.2020.15.555923
Abstract
Post-harvest pathogenic fungi are one of the most important causes of fruits decay. screenings were done in four districts produced tomato in Sharkia governorate and found that Botrytis cinerea was the most Prevailing fungi which the main causal agent of tomato grey mold disease. This study was conducted to evaluate the antifungal effect of different concentrations salicylic acid. Salicylic acid significantly reduced the linear growth of Botrytis cinerea in vitro especially high concentrations (6 g/l and 0.8g/l) in contact and fumigation methods respectively. The severity of artificially infected tomato increase gradually with increasing of storage periods (1, 2 and 3 weeks) while treatment with salicylic acid especially in high concentrations in either contact and fumigation lead to significant decrease of the severity of infection throughout all storage periods. Fumigation method was found to be more effective than contact method throughout both linear Botrytis cinerea growth and in vivo. The quality characteristics of tomato (soluble solids, acidity and vitamin C) was markedly affected in artificially inoculated tomato fruits comparing with the non-inoculated during different storage periods. This study has demonstrated that the salicylic acid could be considered as a potent and promising antifungal and preservative which is safe to environment.
Keywords: Salicylic acid; Antifungal activity; Botrytis cinereal; Tomato
Introduction
Tomatoes (Solanum lycopersicon L.) are one of the major vegetable components of the human diet worldwide. The fleshy fruits, however, are susceptible to decay and loss of quality once they have been harvested. Gray mold rot, caused by Botrytis cinerea, is the major postharvest disease of tomato fruits [1]. Infestation is stimulated by high humidity, particularly if free moisture is present on the plant surface and low temperatures [2]. A number of alternative strategies to the use of synthetic, chemical fungicides have been evaluated for their ability to prevent postharvest decay and extend the shelf-life of tomato fruits, including the application of chemical treatments that induce host resistance [3-5]. Although the synthetic fungicides are effective, their continued or repeated application has disrupted biological control by natural enemies and led to disease outbreaks, widespread development of resistance to various types of fungicides [6-10], toxicity to non-target organisms and environmental problems [11]. Therefore, there is a need for development of alternative strategies to control gray mold of tomato that are safe, effective, economical, and compatible with commercial handling practices. Because of their well-known antimicrobial activity, these phytochemicals have been extensively reported to be a rich source of eco-friendly antifungals for food safety applications [12]. Phenolics are compounds possessing one or more aromatic rings with one or more hydroxyl groups. Antifungal effects of these compounds against phytopathogenic fungi have also been reported. Mechanisms underlying antifungal properties of phenolic compounds concern their ability to suppress cellular functionality by affecting membrane integrity. the use of phenolic compounds is of interest, not only due to the important roles of these compounds in plant defenses against pathogens, but also because they have proven to be beneficial to human health exhibiting antioxidant and anti-carcinogenic effects. Salicylic acid (SA), a phenolic acid with an aromatic ring linked to a hydroxyl group or functional derivatives. This molecule is an endogenous plant hormone involved in plant growth development and cell signaling [13] and involved in the biosynthesis of defensive compounds in plants and exhibited antimicrobial effects against various fungi that cause rot. SA is thought to be a good alternative to control diseases, many studies have evaluated its antifungal activity against several postharvest pathogens, including P. expansum, Botrytis cinereal, Fusarium oxysporum in tomatoes and Rhizopus stolonifera. In addition, SA can help to preserve the physical and chemical characteristics of fruits by preventing, for example, the weight loss of stored strawberries [14] the total soluble solids content, and titratable acidity of grapes. Thus, this study aimed to assess the potential effect of SA to protect, improve the quality characteristics and increase shelf life of tomato fruits against damage induced by Botrytis cinerea. Such approach is assumed to be crucial for the development of efficient alternative strategies to replace the use of fungicides to control gray mold in tomato fruits.
Materials and Methods
Tomato fruits
Collected from local markets in Sharkia Governorate were classified into two groups, healthy and decayed fruits. Decayed fruits were examined after 3 days storage at 15 °C. The developing fungal colonies were picked up and transferred to PDA plates for microscopic examination. The fungi isolated were purified and identified. The purified cultures were maintained on Potato- Dextrose Agar medium (PDA) containing Potato extract, 230 ml; glucose, 20 g and water 770 ml. Potato extract was prepared by adding 100 gm potatoes (peeled and sliced in a mincer) to 300 ml tap water, it was left overnight at 4°C and filtered through cloth(9). The fungus was identified according to Raper and Thom in Mycological lab. In Fungal Disease Research Department, Plant Pathology Research Institute, Agricultural Research Center, Giza, Egypt. The media used for identification was Czapek´s- Dox agar medium.
Salicylic acid: were obtained from El-Gomhoria medical company, Cairo, Egypt.
Fungal species: Botrytis cinerea was obtained from Fungal Disease Research Department, Plant Pathology Research Institute, Agricultural Research Center, Giza, Egypt.
Pathogenicity test
Healthy tomato fruits were carefully washed rinsed with sterile water then kept to dry at room temperature, surface disinfested in 70% ethyl alcohol for one minute. By the aid of a small scratch wounds were made in each fruit and inoculated by fungus using equal mycelial disks (3 mm in diameter), taken from 7 days old PDA cultures and set one disk in each scratch. Check treatments were similarly carried out using fungal free medium. Three replicates each containing ten fruits were used for each treatment. The inoculated fruits for each treatment were put in sterilized carton box with 5 kg capacity and kept at room temperature for 7 days. The resulted rot was calculated using the decay index and severity of infection according to Chastanger and Ogawa (1979) based on visual inspection of each fruit infection. Infected fruits were placed in one of five categories:

Salicylic acid in vitro treatment (Contact)
Different concentrations 0, 0.25, 0.5, 1, 2, 4 and6g/L of pure salicylic acid were added individually to Erlenmeyer flasks (250 ml capacity) each contain 100 ml sterilized PDA medium. The prepared media were poured in sterilized Petri-dishes. After solidification, the dishes were inoculated singly at the center with equal discs (3 mm diameter) of fungal growth, taken from 10 days old culture grown on PDA medium incubated at 20oC. The linear growth of fungi tested was measured when the control plates reached full growth. Three replicates were used for each treatment.
Salicylic acid in vitro fumigation treatment
Discs (3 mm diameter) of 10 days old cultures of Botrytis cinerea were fumigated with salicylic acid vapor at different concentrations 0.2, 0.4, 0.6, 0.8 and 1.0 g/l for 30 min in container with enclosed current of air. Fumigated discs were transferred to Petri-dishes containing PDA medium. Unfumigated fungi discs used as control. The plates were incubated at 20 °C, linear growth of fungi was measured when control plates reached full growth and three discs were used as replicates for each treatment.
Salicylic acid treatment in vivo
Healthy tomato fruits divided into two lots, the first was sterilized by 70% ethanol for one minute and left to dry at room temperature. Standard amount of inoculum discs (3mm diameter) of Botrytis cinerea were introduced through very small scratch in the middle surface of fruit. The second set of fruits left without inoculation; two methods were used to control tomato fruit rots.
Dipping treatment: Previously inoculated and noninoculated tomato fruits were dipped in 0, 2, 4 and 6 g/l of salicylic acid solutions for 3 min and air dried for 2 h in laminar flow hood, tomato fruits were packed in plastic net bags, then put in perforated sterilized carton box and stored for 1, 2 and 3 weeks in cold storage room at 13oC and 90-95 relative humidity for each treatment three replicates were used, each replicate containing 10 fruits. The results were recorded as severity of infection
Fumigation treatment: Inoculated and non-inoculated fruits were fumigated with salicylic acid vapor 0.2, 0.4, 0.6, 0.8 and 1.0 g/L in air closed glass container for 30 min. Fumigated-inoculated fruits were air dried in laminar flow hood for 2h, then packed in plastic bags, then put in perforated sterilized carton box and stored for 1, 2 and 3 weeks at 13 °C and 90-95% relative humidity, for each treatment three replicates were used, each containing 10 fruits, the results were recorded as severity of infection.
Chemical properties of fruit:
Total soluble solids (T.S.S %)
Using hand refractometer according to (AOAC 1990)
Acidity
Titratable acidity was expressed as percentage of citric acid (g citric acid / 100 mL juice) according to (AOAC1990).
L-Ascorbic acid (mg/100g fresh weight)
It was determined as mg/100g fresh weight (AOAC 1980).
Statistical analysis:
Statistical analysis of the obtained data was done using the least significant difference test (LSD) at the 5% level of probability as outlined by Snedecor and Cochran 23. Using the Duncan test institute program used a computer in the statistical analysis.
Result and Discussion
Data presented in Table 1 showed that the frequency of occurrence of isolated fungi Botrytis cinerea, Mucor. Sp., Alternaria alternate, Fusarium solani, Rhizopus stolonider, Aspergillus niger which causing fruit rots of tomato from four districts in Sharkia governorate. Botrytis cinerea was found that the most common fungi in all four regions.
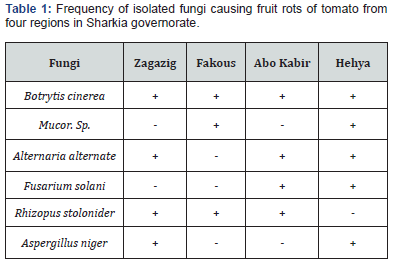
In vitro contact effect of salicylic acid on linear growth of Botrytis cinerea:
Effect of different concentrations of salicylic acid 0, 0.25, 0.5, 1, 2, 4 and 6 g/L added to the media in contact on linear growth of A. alternata represented in Table (2). The linear growths of Botrytis cinerea decreased as the concentration of salicylic acid increase and at concentrations 4 and 6 g/l of salicylic acid completely inhibit the mycelial growth of Botrytis cinerea.
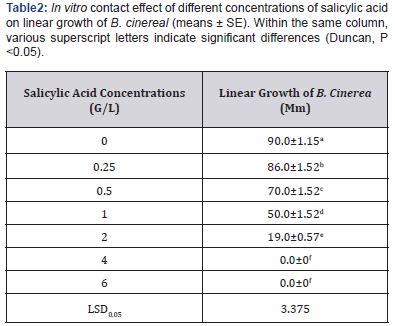
In vitro fumigation, effect of salicylic acid on linear growth of Botrytis cinerea:
Results in (Table 3) show that as salicylic acid concentrations increased linear growth of Botrytis cinerea decreased. Salicylic acid concentration at 0.8 and 1.0g/l inhibit the linear growth of fungus. Also, our results showed that from Minimum Inhibitory Concentration (MIC) of salicylic acid in contact and in fumigation the fumigation methods is more effective than contact due to the direct effect of salicylic acid vapors on Botrytis cinerea.
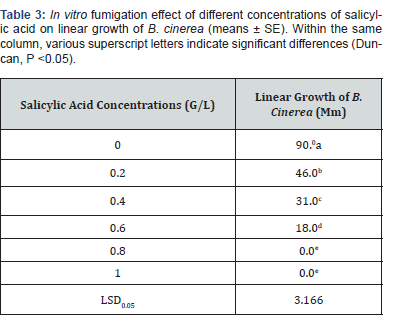
In vivo contact effect of acetic acid on postharvest tomato rots caused by Botrytis cinerea
Inoculated tomato fruits were dipped in 2, 4 and 6 g/l concentration of salicylic acid for 3 min and air dried for 2 h they kept in plastic net bags as previously showed severity of tomato rots infection during different storage periods 1, 2 and 3 weeks was calculated in (Table 4). As salicylic acid concentrations increased severity of infection decreased and we found that 6 g/l from salicylic acid concentration was the most effective concentration throughout the storage periods.
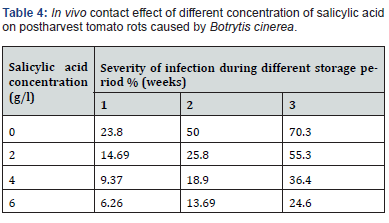
In vivo effect of salicylic acid fumigation on postharvest tomato rots caused by Botrytis cinerea:
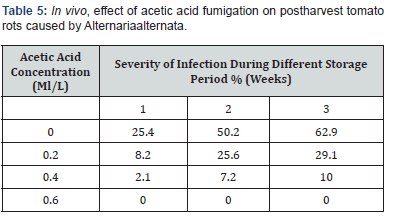
As previously described, different salicylic acid vapors (0.2, 0.4 and 0.6 g/l) were used to fumigate inoculated tomato fruits in air closed container for 30 min. After packing in plastic bags, the severity of infection calculated at different storage periods (1, 2 and 3 weeks). The obtained results in (Table 5) showed that 0.6g/l of salicylic acid concentration completely inhibit postharvest tomato rots caused by Botrytis cinereal for 3 weeks of storage. Also, as in in vitro the fumigation methods is more effective than dipping methods due to the direct effect of salicylic acid vapors on Botrytis cinereal the causing agent of tomato rots.
Effect of inoculation with Botrytis cinerea on tomato fruits quality characteristics.
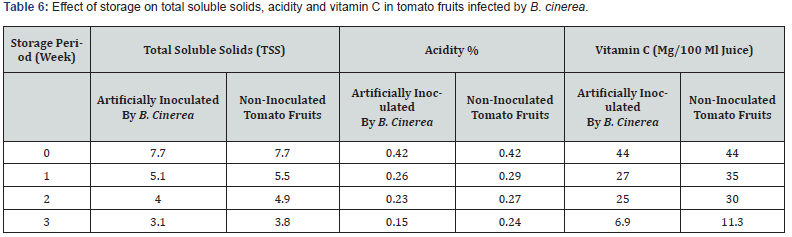
In the results obtained in Table 6 it was obviously that there a negative marked change in the quality characteristics of tomato fruits either in total soluble solids, acidity % and vitamin C content during all storage periods.
Discussion
The present efforts indicated that different concentrations of salicylic acid wither liquid or as vapor were significantly reduced the growth of B. cinerea. Also, submersed tomato fruits in different concentrations of salicylic acid solution significantly reduced the severity of B. cinerea infection. These results are in harmony with those of [15]. The in hibitory effect of salicylic acid not related to pH alone but carbon chain length and inherent susceptibility of the microorganisms were also important. Also, the undissociated part from the acid was primarily responsible for its antimicrobial activity where it can penetrate the microbial cell and exert its toxic effect [16] and mentioned that the mechanism of salicylic acid effect on inhibiting microorganisms is apparently due to its effect on the cell membrane through the interfering with transport of metabolites and maintenance of membrane potential. Also, Shehata (2006) reported that all the tested salicylic acid concentrations, i.e. 5, 10, 15, 20 and 25%, when applied for 1 h at 13 °C, significantly reduced the percentage of infected areas in fruits compared with the control. Salicylic acid vapors with concentrations 0.8 and1.0g/l is more effective than its solution 4.0 and 6.0 g/l in inhibiting mycelial growth and controlling postharvest disease of tomato fruits by the tested fungi. Increasing penetration ability of salicylic acid than that in the liquid state through the fungal cell might be attributed to the vapor state. One of the most important results obtained during this work is that salicylic acid treatments either as liquid or vapor state at any concentration prevent completely natural infection of healthy non-inoculated tomato fruits (check treatments) during the time of experiment (3 weeks). Data also in the harmony of those obtained [17]. Many other researchers recommended salicylic acid treatment for controlling postharvest decay of fruits [18-21].In conclusion the present data clearly showed that salicylic acid which has long been used been safely used long as food additive, can completely preventdecay of tomato fruits when dipped in 6 g/l acetic acid solution or exposed to its vapor at 0.6g/l. This procedure can be easily applied and inexpensively top reserve tomato fruits for long periods without any side effects, in refrigerators at home, market, and storage and exportation level.
References
- Abd El Kareem F (2001) Fumigation of table grapes with acetic acid vapours for controlling grey mould decay. Egypt J Phytopathol29: 89-98.
- Agrios GN (1997) Plant Pathology, 4th Academic Press, San Diego, CA.
- Barkai Golan R (2001) Postharvest Diseases of Fruits and Vegetables: Development and Control, Elsevier Science B.V., Amesterdam, 418 pp.
- Chastanger GA, JM Ogawa (1979) A fungicide wax treatment to suppress Botrytis cinerea and protect fresh market tomatoes. Phytopathology 69: 59-63.
- Davidson PM, Juneja VK (1990): Antimicrobial agents. 83-137 in AL Branen, PM Davidson and S. Salminen (eds) Food Additives Marcel Dekker Inc., New York, USA.
- Doores S (1990) pH control agents and acidulants. In: Branen AL, Davidson PM, Salminen S (Eds) Food Additives, Marcel Dekker Inc., New York, USA, pp. 477-510.
- Doores S (1993) Organic acids. In: Davidson PM, Branen AL (Eds) Antimicrobials in Foods (2nd Edn), Marcel Dekker, Inc, New York, USA, pp. 95-136.
- Gill A, Holley R (2003) Nature of Echerchia coli and Lactobacillus saeiinhubution by eugenol, Cinnamaldehyde and sodium lactate. Int. Assoc. Food Prot., Annual Meet.
- Giovanni A, Marinna U (2001) Fungitoxic activity of 12 essential oils against four postharvest citrus pathogens: Chemical analysis of Thymus capitatus oil and its effect in sub atmospheric pressure conditions. J Food Prot 64 (7): 1025-1029.
- Holly RA, Patel D (2005) Improvement in shelf-life and safety of perishable foods by plant essential oils and smoke antimicrobials. Food Microbiol 22(4): 273-292.
- Isman MB (2000) Plant essential oils for pest and disease management. Crop Protection19(8-10): 603-608.
- Peter Sholberg (2009) Control of postharvest decay by fumigation with acetic acid or plant volatile compounds. Fresh Produce 3 (Special Issue 1) 80-86© Global Science Books.
- Plaza P, Sanbruno A, Usall J, Lamarca N, Torres R, et al. (2004) Integration of curing treatments with degreening to control the main postharvest diseases of Clementine mandarins. Postharvest Biology and Technology 34(1): 29-37.
- Plotto A, Roberts D, Roberts G (2003) Evaluation of plant essential oils as natural postharvest disease control of tomato (Lycopersicon esculentum). Acta Horticulture 628: 737-745.
- Seaton WH (1993) Acetic acid. In: Agreda VH, Zoeller JR (Eds) Acetic Acid and its Derivatives, Marcel Dekker. Inc., New York, NY, USA, pp. 73-100.
- Sholberg PL, Gaunce AP (1995) Fumigation of fruit with acetic acid to prevent postharvest decay. Hort Sci 30: 1271-1275.
- Sholberg PL, Shephard T, Moyls AL (2003) Monitoring acetic acid vapor concentrations during fumigation of fruit for control of postharvest decay. Canadian Biosystems Engineering 45: 313-317.
- Slininger J, Burkhead D, Schisler A (2004) Antifungal and sprout regulatory bioactivities of phenylacetic acid, indole-3-acetic acid, and tyrosol isolated from the potato dry rot suppressive bacterium Enterobacter cloacae S11: T: 07. J Ind Microbiol Biotechnol 31(11): 517-524.
- Soliman KM, Badeaa RL (2002) Effect of oil extracted from some medicinal plants on different mycotoxigenic fungi. Food Chem. Toxicol 40(11): 1669-1675.
- Unnikrishnan V, Nath S (2002) Hazardous chemicals in foods. India J Dairy Biosci 11: 155-158.
- Yousefi VO (2000) Agrochemical in South Africa. African Newsletter, Johannesburg, South Africa.






























