Remediation of Crude Oil Contaminated Pit Water Using Pseudomonas Aeruginosa Strains
Kongkana Goswami1, 2, Anisha Sehgal1, Jiumoni Lahkar1, Dipankar Neog1, Binoy Kumar Saikia1, Tobiul Ahmed Hussain1, Anil Kumar Singh1, 2 and Hari Prasanna Deka Boruah1, 2*
1 CSIR-North East Institute of Science & Technology, Jorhat-785006, Assam, India
2 Academy of Scientific and Innovative Research, New Delhi, India
Submission: February 07, 2020; Published: June 17, 2020
*Corresponding author:Hari Prasanna Deka Boruah, CSIR-North East Institute of Science & Technology, Academy of Scientific and Innovative Research, Jorhat-785006, New Delhi, India
How to cite this article: Kongkana G, Anisha S, Jiumoni L, Dipankar N, Binoy K S, et al. Remediation of Crude Oil Contaminated Pit Water Using Pseudomonas Aeruginosa Strains. Adv Biotech & Micro. 2020; 15(5): 555922.DOI: 10.19080/AIBM.2020.15.555922
Abstract
A huge quantity of water contaminated with oil and other drilling materials is stored in large ponds within the oil drilling sites. This contaminated water cannot be released to the surrounding environment without proper treatment. In the present study, we are reporting the treatment of such water and the possible field application through microbial treatment by using two bacterial strains namely Pseudomonas aeruginosa TP16 and Pseudomonas aeruginosa N002. Physical, chemical and biological properties confirmed that the pit water samples contained > 1 % crude oil, high phenolphthalein alkalinity, methyl orange alkalinity, total dissolved solids, and total suspended solids. Bacterial strains TP16 and N002 utilized/converted the crude oil components under control conditions after the treatment process. UV-Vis spectra, GC chromatogram, NMR and FT-IR spectra confirmed the utilization/conversion of the individual oil components including 3-oxo-20-methyl-11-a-hydroxyconaine-1,4-diene,trans-2-phenyl-1,3-dioxolane-4-methyloctadec-9,12,15-trienoate,4,4-dideuterio-6,6-dimethylcyclohexanone,2,5-octadecadiynoic, methyl ester (CAS), benzene-1-methoxy-4-(1-propenyl)-(CAS), phenol-2-methoxy-4-(2-propenyl)-(CAS) and 10-(tetrahydro-pyran-2-Yloxy)-tricyclo-[4.2.1.1.2,5] DEC-7-EN-9-one; as sole C-source. The plant germination bioassay performed for comparison of control and treated wastewater on mustard green (Brassica juncea) was found to be non-toxic. It can be concluded that the microbial treatment followed by chemical clarification is the suitable approach for the treatment of crude oil contaminated large pit water.
Keywords: Bioremediation; Crude oil; Pit-water; Wastewater treatment; Bio-assay
Introduction
Oil drilling is a requisite process in all the oil-recovery fields around the globe. During the oil recovery process, water fractions are utilized as a lubricant and pumped in high pressure inside the earth crust to recover the oil from the deep wells. Following the process, a substantial quantity of water containing a high amount of oil fractions; aromatic hydrocarbon, aliphatic hydrocarbon, organic acid, and organic materials are generated [1-4].But this generated waste-water can’t be released to the surrounding environment without proper treatment and thereby stored in an artificially designed reservoir. Accidental leakage of this oil-contaminated water to the environment creates environmental problems as different oil components can exert adverse effects on the nearby flora and fauna [4-7]. Therefore, the treatment of oil-contaminated wastewater requires attention before disposal. The oil-based industries are mostly located in the remote and hilly areas and their oil production efficiency is not uniform all the time. Therefore, the generated wastewater is allowed to store in the abandoned oil well sites for a long time due to the high cost involved in the treatment process. In contrast to physical and chemical methods, biological treatments are the alternative approach, where microbes are used to treat the contaminated water [8]. Further, the biological treatment can be tailored to the site as remediation of oil-contaminated sites is mostly unique to its origin [9,10]. In this approach, microorganisms such as bacteria, fungi, algae, and protozoa were used which could metabolize the complex contaminants present in the wastewater [11-13]. Compared to other microbes, bacterial remediation has shown high potentiality in terms of accumulation or degradation of hazardous dyes, crude oil, nutrients and hydrocarbons in a short span of time by utilizing mechanisms like exopolysaccharide production, floccs production, and biofilm production. Bacterial interaction with various waste materials triggers the production of exopolysaccharides, biofilms, floccs, and granule that enhance the treatment efficiency by reducing the toxicity, degradation of organic matter and removal of the nutrients present in the contaminated water [14-17].
These convenient aspects of microbes make them suitable for waste treatment in combination with the traditional physicochemical processes. Assam, a state of NE-India produces crude oil since the 18th century under the agencies like Oil and Natural Gas Corporation Limited (ONGCL) and Oil India Limited (OIL). These oil production and recovery sites have many abandoned pit areas having the capacity of carrying 30 kl contaminated pit water. Earlier studies reported the necessity of environment-friendly technology to clean up the crude oil contaminated areas of Assam [18]. Very few reports are available on large scale remediation of oil-contaminated pit water stored near the oil drilled sites. Therefore, it is an urgent need to decontaminate this harmful waste-water generated during the oil drilling. The aim of the present study is to evaluate the remediation efficiency of the oil- contaminated pit water produced by the oilbased industries through the applications of two crude oil utilizing Pseudomonas strains under the controlled conditions citing pit water of KHBB Golaghat, operated under ONGC. Stress was given to study the proposition of the large scale oil-contaminated water reservoir.
Materials and Methods
Sampling
Crude oil contaminated pit water samples were collected from the oil drilled sites of KHBB, Golaghat, Assam (25038 N and 92015 E). This site is operated under Oil and Natural Gas Corporation, Jorhat, India assets [Figure S1].

The bacterial strains used in the current work were previously isolated by our group from oil-contaminated soil sites [19,20]. Further, the bacterial cultures were revived and seed culture was prepared in 50 ml nutrient broth and incubated for 24 h at 30 °C. Finally, 500 μl of the seed culture was transferred to 50 ml mineral media supplemented with 0.1 % (v/v %) crude oil and again incubated for 7 days in shaking incubator at 30 °C and 120 rpm. After 7 days of incubation, strains showing maximum activity in terms of growth, crude oil utilization potential and UVspectrometric analysis were selected for the treatment.
Characterization of the pit water
Crude oil contaminated pit water samples were collected from the oil drilled sites of KHBB, Golaghat, Assam (25038 N and 92015 E) (Figure S2). Physical, chemical and biological characteristics of the pit water collected from the oil drilling sites of KHBB, Golaghat were analyzed as per the methods described in the Indian Standard (IS 30125 2012) to ensure the bio-remediation process. For this, the pH of the water was determined using an automatic glass electrode pH meter (Eutech pH700). Biological Oxygen Demand (BOD) was determined following the Winkler method. The turbidity of the collected water was tested using turbidimeter (ALTISTM instruments). The alkalinity of water was determined using indicator method, while sulfate, magnesium and total suspended solids content was measured gravimetrically. The chloride content was analyzed through the argentometric method while calcium content was determined through EDTA titrimetric method. Finally, the total iron content was measured through 1, 10 phenanthroline method.
Pit water Treatment
For the treatment of the pit water, the collected samples were passed through mesh sieves (0.5-2.5 mm) to remove any large particles. During primary treatment, the collected water is allowed to stand for an hour for settlement of light particles and oil under gravity. The soil containing sludge part settled in the bottom while the water fraction containing the oil was collected for secondary treatment. The collected contaminated water samples were treated with 18 h old enriched cultures of selected efficient bacterial strains. For this, 100 ml of the contaminated water was transferred to 250 ml conical flasks supplemented with 0.01% nutrient solution (yeast extract) except for the carbon source. 1.0 ml of the enriched culture of the selected strains were inoculated and incubated in room temperature at 120 rpm for 48 h. Pit water amended with 0.01 % nutrient solution without bacterial inoculums was served as control and incubated at room temperature for 48 h. After 48 h post incubation, 0.8 % of Poly Aluminum Chloride (PAC) was added and mixed well to settle down the bacterial cells and dissolve solid particles present in the treated pit water. After 1 h of the coagulation process, clarified liquid fractions were separated from the residual sludge. The physical, chemical and biological characteristics of the clarified water, as well as the sludge, were determined as mentioned earlier to verify the efficiency of the bacterial treatment.

Fourier Transform –Infra-Red spectroscopy (FT-IR)
The functional groups present in the contaminated pit water samples before and after treatment was determined using Fourier- Transform infrared spectroscopy (Perkin-Elmer Spectrum 100). For this, a pinch of the dried sample was placed on KBr disc and IR spectra of the samples were obtained in the range of 4000-400 cm-1.
Nuclear Magnetic Resonance (NMR)
For Nuclear Magnetic Resonance analysis, 5ml of the pit water samples were mixed with the deuterated chloroform (CDCl3) to determine the compounds present (Bruker AV500 Avance-III 500 MHz FT-NMR). Top Spin processing and control software (Version 3.2.b.10.) was used to compare the NMR spectra of the treated samples with their respective controls.
Gas Chromatography/Mass Spectroscopy (GC-MS)
The compounds present in the sample pre and post-treatment was further characterized using Gas Chromatography/Mass Spectrometry (Perkin Elmer Clarus 600). The sample preparation and analysis was performed using the Elite 5 MS column as reported in our previous study [21]. Analysis of spectrum was done by comparing the retention time obtained for our samples with the standard, MS-library, and literature.
Scanning electron microscopy
The bacterial interaction with the treated residues and their surface topology was determined via SEM (Sigma HV 05-07). For this, a dried portion of the sample was fixed on a cover-slip by 4 % (w/v) glutaraldehyde and incubated for 24 h. After the fixation step, samples were washed with distilled water and dehydrated using different grades of ethanol (30 %, 50 %, 70 %, 80 %, and 95 %) for 3 min. All the samples were dried in a vacuum and coated with gold particles prior to SEM analysis.
Energy-Dispersive X-ray spectroscopy (EDX)
Energy dispersed X-Ray analysis (EDX) was used to determine the elemental composition of the treated residual samples from the captured SEM images by using Oxford instruments X-MaxN, model no: sigma HV 05-07.
Proximate analysis
Proximate analysis was performed to determine the physical and chemical properties of the treated residual samples using Thermogravimetric Analyzer (Leco TGA701) (ASTM, 2011a,b,c)
Plant germination bio-assay
Seed germination and seedling vigor test was performed on mustard green (Brassica juncea) to investigate the nontoxicity of the treated samples. For this, both the residual and clarified liquid fractions of the treated samples were separated and clarified liquid fractions were neutralized [22]. Seeds of mustard green were surface sterilized using 0.01 % mercuric chloride and 70 % ethanol. Finally, the sterilized seeds were sown in both the clarified and residue of the treated samples and monitored regularly for 7 days. Various growth parameters such as dry biomass, seedlings, and root length were recorded in three replicates to determine the non- toxicity of the treated samples on plant growth.
Statistical analysis
Each sample was studied in triplicates and results were expressed in mean± standard deviation. Comparison of the variance before and after treatment was measured by oneway ANOVA and their significant difference was determined by student’s T-test at p < 0.05.
Result and Discussion
Assam produces >5 million metric tons of crude oil, which are explored under different oil wells. The selected site (KHBB, Golaghat, Assam) have been drilled in 2015 and has started production of oil since 2016. Each oil well produces approximately 30 kl of the waste pit which is stored in artificially designed ponds with an area of 100 m × 50 m and 1-15 m depth. It is essential to remediate these contaminated pit water fractions by the environment-friendly way before proper disposal, since accidental leakage to the nearby low-lying areas cause havoc [23]. In the present study, out of the 40 screened bacterial strains, Pseudomonas aeruginosa TP16 (accession no KC505184) and P. aeruginosa N002 (accession no: JX035794.1) [Fig. S2] was selected to treat the oil contaminated pit water samples on the basis of their growth, crude oil utilization potential, and UVspectrometric analysis. Our previous study revealed that the strain N002 contains eleven types of bio-surfactant producing genes that enhance the crude oil degradation by reducing the surface tension, critical micelle concentration and interfacial tension in the hydrocarbon mixed aqueous solution. Moreover, the genes responsible for hydrocarbon degradation by emulsification are also well equipped in the strain [20]; whereas, the strain TP16 could thrive in crude oil environment (data not published) and has additional plant growth promoting activity. Consortia of these two strains showed enhance crude oil degrading/ utilizing capacity after 12 h of inoculation as compared to its individual capacity.
Characteristic of the pit water before and after the treatment
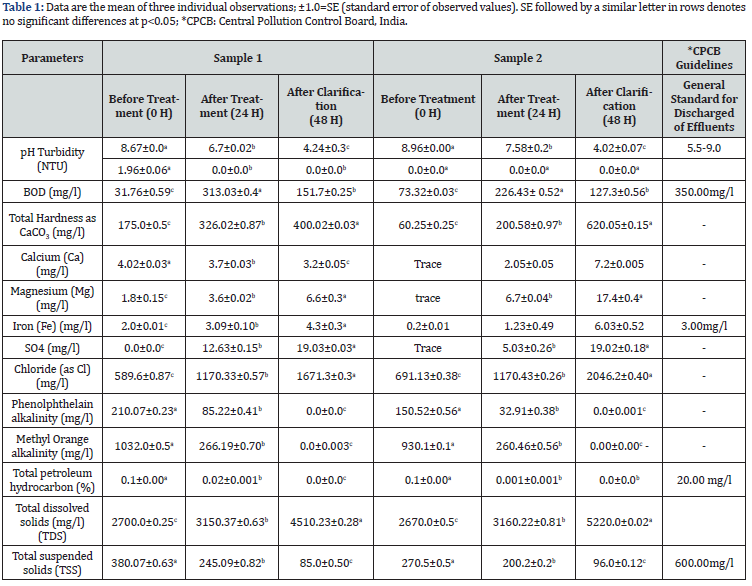
The characteristics features of the oil-contaminated pit water samples collected from the oil drilled site of KHBB; Golaghat, Assam is described in Table 1. The pit water samples were basic in nature (pH 8.67 - 8.96) with 0.1 % oil content. The samples were found to contain dissolved contents, chloride, iron, methyl orange alkalinity, phenolphthalein alkalinity, and total suspended solids, whereas, sample2 also contained calcium, and magnesium in a trace amount. After 24 h of treatment, the concentration of petroleum content was found to be decreased by 80 % and 99%; whereas 74.21% and 72 % methyl orange alkalinity, 59.43 % and 78.13 % phenolphthalein alkalinity, and 35.51 % and 26 % total suspended solids were removed in sample 1 and sample 2 respectively. Mg, Fe, S, total hardness, and dissolved solids were increased substantially than before. The significant changes in the physical and chemical parameters of the pit water after treatment were due to the utilization/conversion/degradation of crude oil and other suspended particles. The enhanced availability of Mg, Fe, S and total dissolve salt was might be due to the release of these materials from the immobilized form and also the addition of coagulating agent PAC [24]. Santhini et al. [25] reported that changes in different parameters of the waste effluents are the indicators of the bacterial utilization process. Hence, the decreased and enhanced components indicated bacterial utilization process. Interestingly, the basic nature of the contaminated water changed to mild acidic nature (4.24-4.04), which could be considered as a sign of bacterial degradation of crude oil to smaller fractions. The lowering of pH during the utilization of oil by microbes is already an established phenomenon [26,27]. Overall, the bacterial treatment in crude oil contaminated pit water leads to diminishing crude oil but subsequently enhanced the inorganic salts and total hardness.
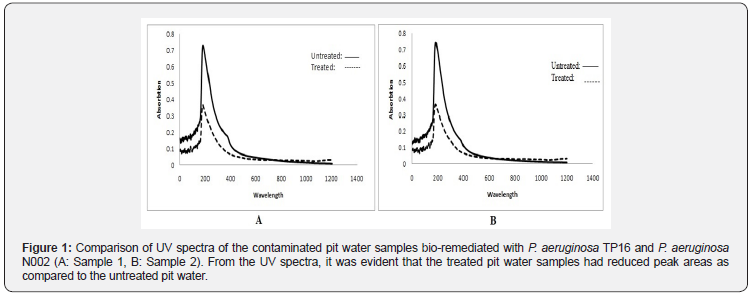
Biological Oxygen Demand (BOD) is considered as one of the parameters to determine the quality of water. BOD of the untreated samples was found to be 31.7 mg/l and 73.3 mg/l. After 24 h of treatment, the recorded BOD values were enhanced by 89.85 % and 67.62 %, indicating utilization of the dissolved oxygen present in the water samples. The changes in the pit water after the treatment was also verified by the absorption spectra recorded at 200-900 nm. (Figure 1) Loss of peak areas in the treated samples is a sign of degradation process, as the non-biological process tends to show the same effect on both the samples and their respective controls [28, 29].
FT-IR
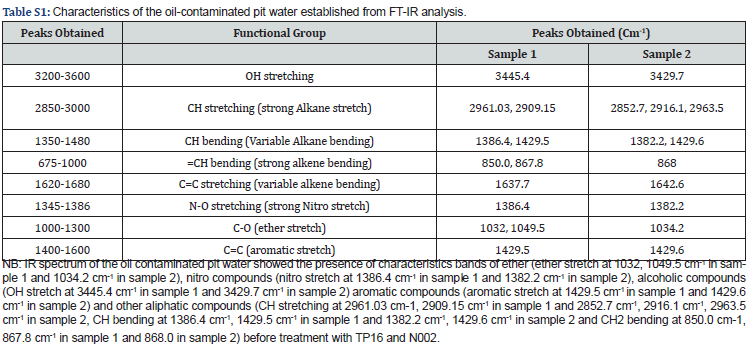
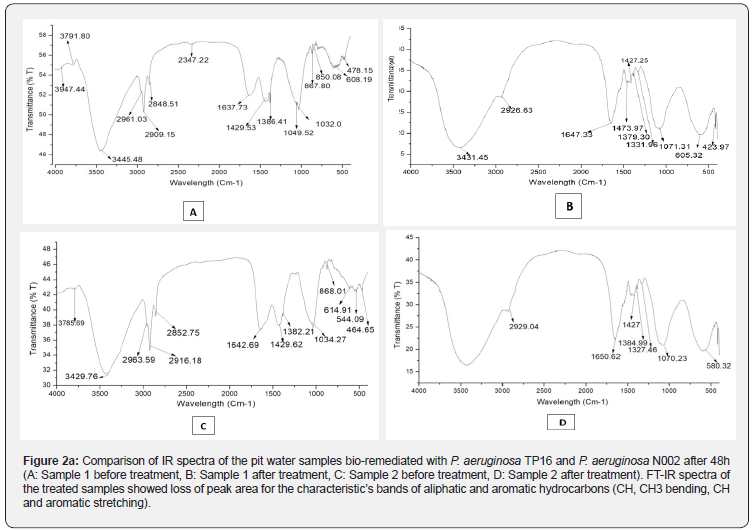
Comparative analysis of the functional groups present in both the treated and untreated samples was studied by FT-IR analysis (Figure 2a). The IR spectra of the untreated sample 1 showed the characteristic bands of CH, CH3 bending at 1386.4 cm-1, 1429.5 cm-1 and 850.0 cm-1, 867.8cm-1, aromatic stretch at 1429.5 cm-1 and C-H stretching at 2961.0 cm-1 and 2909cm-1. While bands of CH, CH3 bending at 2852.7 cm-1, 2916.1 cm-1, 2963.5 cm-1 and 868.0 cm-1, C-H stretching at 2852.7 cm-1, 2916.1 cm-1, 2963.5 cm-1 and aromatic stretch at 1429.6cm1 were found in sample 2 [Table S1]. Loss of peak areas in the treated samples confirmed that both TP16 and N002 utilized oil and other contaminants present in the waste-water.
NMR
Differences in NMR spectra between the control and the treated samples are described in Fig. 2b. In the present study, loss of hydroxyl group was confirmed by a decreased peak area at 4.78 ppm in the treated sample 1, whereas, loss of peak in the range of 4.15-4.20 ppm and 7.24-7.26 ppm in sample 2 confirmed the degradation of hydroxyl and aromatic compounds after the treatment process. However, NMR spectra of both the samples showed a new aromatic peak at 7.25-7.26 ppm after 48 h of the treatment. The loss and formation of a new peak in the NMR spectra supported the degradation and conversion of the pit water components which is comparable to our previous studies on crude oil utilizing hydrocarbon clastic bacteria (mentioned above [19,21])
GC-MS analysis
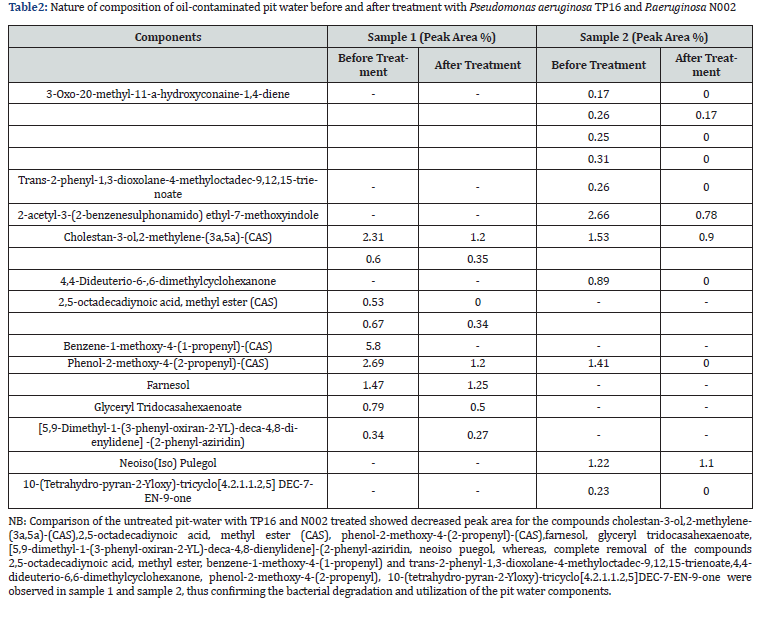
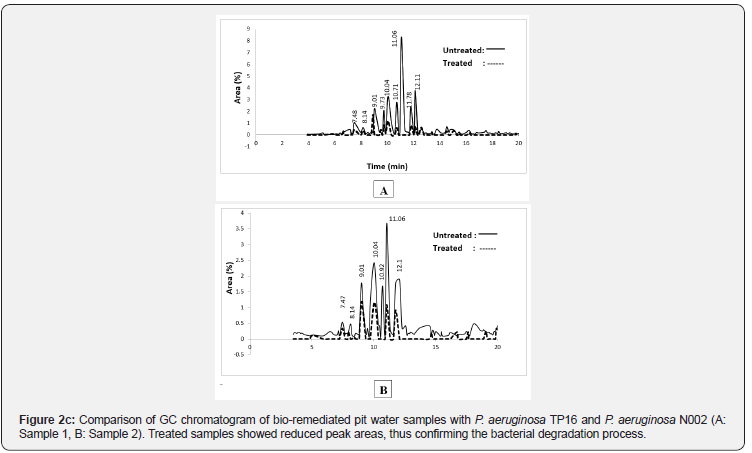
The comparison of GC-MS spectra for the treated and the untreated water samples was done by analyzing the retention time and the MS library (Figure 2b,2c). Various compounds such as hexadecadienoic acid, methyl ester, phenolic compounds, farnesol, and benzene were found in the untreated pit water samples which were removed or reduced to a minimum level in the TP16 and N002 treated water samples (Table 2). The observed changes in the peak areas were at RT 7.48, 8.14, 9.01, 9.73, 10.04, 10.71, 11.06, 11.78, 12.11 and 13.76 min in Sample 1 while 7.47, 8.14, 9.01, 10.04, 10.92, 11.06 and 12.1 min in Sample 2 as compared to their respective controls. Reduction of these compounds to a minimum level and loss of peak areas signified the utilization of the oil and the other components present in the contaminated pit water as carbon source.
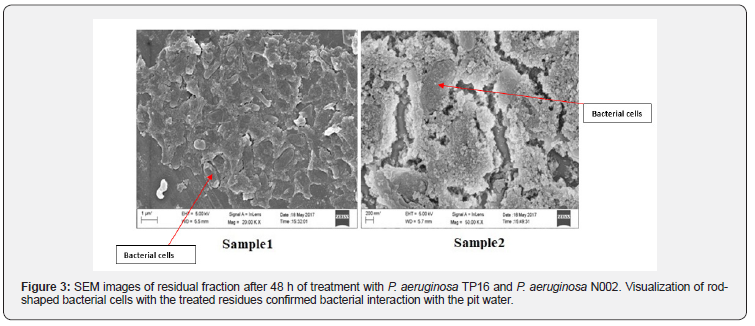
SEM
Surface topography of the bacterial strains and their interaction with the treated residual samples were analyzed via SEM (Figure 3). SEM analysis revealed the presence of rod-shaped bacterial strains TP 16 and N002 in the treated residual samples. Presence of the bacterial strains in the residue confirmed the interaction between the bacterial strains and the oil-contaminated water samples and their ability to adapt in that stress conditions.
EDX
Presence or absence of toxic metals or any other elements in the treated pit water was analyzed by EDX (Table 3). EDX analysis confirmed that the residue contained phosphorus, sulfur, and carbon in Sample 1, whereas, potassium, sulphur, magnesium, zinc, and carbon were found in sample 2 after the treatment with TP16 and N002. Srivastava and Thakur [30] also reported the elemental composition of waste effluents via EDX analysis. In the present findings, the treated residual samples were found to contain the essential elements only. Whereas, the detected amount of zinc in sample 2 is safe for disposal as per CPCB guidelines (Central Pollution Control Board, India). Hence our study supports the safety of the treatment processes.


Proximate analysis
Proximate analysis of the treated residual samples was carried out to evaluate the physical and chemical properties of the treated residual samples via fixed carbon, volatile matter, moisture content and ash content (Table 3). The present study confirmed that the treated residues were dominated by volatile matter (48.59 % and 41.23 %), ash content (28.32 % and 28.12 %), moisture content (17.54 % and 16.54 %), and fixed carbon (5.55 % and 14.02 %) in Sample 1 and Sample 2 respectively. These parameters have already been studied in biological treatment processes to evaluate the physical and chemical properties of the treated samples [31,32]. In the present study, the treated residual samples were dominated by volatile matter, which might be resulting from the degraded or converted oil components present in the treated residue. Detection of moisture and ash content confirmed the presence of water fractions and various metals and/or elements in the treated residue.
Plant germination bio-assay
Safe disposal of the treated samples to the surrounding environment is a deciding factor in every waste-water treatment process. To ensure the non-toxic nature of the treated pit-water, a laboratory scale experiment was performed on mustard green seeds. In this analysis, we studied different parameters such as germination rate, dry biomass, and root length to confirm plant growth and survivability (Table 4). It was found that the treated pit water and its residues showed no inhibition on seed germination, seedling growth, and total dry biomass accumulation.

Conclusion
The bacterial strains TP16 and N002 are two native Pseudomonas aeruginosa strains that can utilize/convert/ degrade crude oil. From the present study, it can be concluded that both TP16 and N002 could efficiently remediate the oil contaminated pit water samples after 48 h of treatment. The efficacy of the treatment process was confirmed by the plant germination bioassay that ensured the non-toxic nature of the treated samples.
Acknowledgement
Authors are thankful to the Director CSIR-NEIST, Jorhat for the support and KHBB, Golaghat, Assam for providing the samples for the analysis. The fellowship to the first author from the CSIR is also acknowledged.
References
- Chand J (1990) Environmental pollution monitoring in oil exploration and exploitation. In RBKN Rao, J Au, B Griffiths (Eds.), Condition Monitoring and Diagnostic Engineering Management. Springer, Dordrecht, pp. 218-224.
- Neff J, Lee K, DeBlois EM (2011) Environment Risks and Advances in Mitigation Technologies. In K Lee and J Neff (Eds.). Produced water: overview of composition, fates, and effects in Produced water. Springer Science+ Business, LLC, New York, USA, pp. 3-54.
- Bakke T, Klungsoyr J, Sanni S (2013) Environmental impacts of produced water and drilling waste discharges from the Norwegian offshore petroleum industry. Mar environ res 92: 154-169.
- Lindsey K (2016) Salting the Earth: The Environmental Impact of Oil and Gas Wastewater Spill. Environ Health Perspectv 124(12): 230-235.
- Aas E, Baussant T, Balk L, Liewenborg B, Andersen OK (2000) PAH metabolites in bile, cytochrome P4501A and DNA adducts as environmental risk parameters for chronic oil exposure: a laboratory experiment with Atlantic cod. Aquat toxicol 51(2): 241-258.
- Incardona JP, Collier TK, Scholz NL (2004) Defects in Cardiac function Precede Morphological abnormalities in Fish embryos exposed to polycyclic aromatic hydrocarbons. Toxicol Appl Pharmacol 196(2): 191- 205.
- Hu G, Zeng G (2013) Recent development in the treatment of oily sludge from petroleum industry. J Haz Mat 261: 470-490.
- Camarillo KM, Stringfellow TW (2018) Biological treatment of oil and gas produced water: a review and meta-analysis. Clean Techn Environ Policy 20 (6): 1127–1146.
- Crawford G, Sandino J (2010) Energy efficiency in wastewater treatment in North America: a compendium of best practices and case studies of novel approaches. Water Environment Research Foundation.
- Cai L, Zhang T (2013) Detecting human bacterial pathogens in wastewater treatment plants by a high throughput shotgun sequencing technique. Environ Sci Technol 47(10): 5433–5441.
- Bundy JG, Paton GI, Campbell CD (2004) Combined microbial community level and single species biosensor responses to monitor recovery of oil polluted soil. Soil Biol Biochem 36 (7): 1149-1159.
- Grijalbo L, Garbisu C, Martin I, Etxebarria J, Manero FJG, et al. (2015) Functional diversity and dynamics of bacterial communities in a membrane bioreactor for the treatment metal-working fluid wastewater. J Water Health 13 (4): 1006-1019.
- Shah MP (2016) Industrial Wastewater Treatment: A Challenging Task in the Industrial Waste Management. Adv Recycling Waste Manag 2(1): 1-11.
- Raszka A, Chorvatova M, Wanner J (2006) The role and significance of extracellular polymers in activated sludge. Part I: literature review. Acta Hydrochim Hydrobiol 34(5): 411–424.
- Moy BYP, Tay JH, Toh SK, Liu Y, Tay STL (2002) High organic loading influences the physical characteristics of aerobic sludge granules. Lett Appl Microbiol 34(6): 407–412.
- Hamza RA, Laorhemen OT, Tay HJ (2016) Advances in biological systems for the treatment of high-strength wastewater. Journal of Water Process Engineering 10: 128-142.
- Andersson S, Dalhammar G, Rajarao GK (2011) Influence of microbial interactions and EPS/polysaccharide composition on nutrient removal activity in biofilms formed by strains found in wastewater treatment systems. Microbiol Res 166(6): 449–457.
- Yenn R, Borah M, Deka Boruah HP, Sharma Roy A, Baruah R, et al. (2014) Phytoremediation of abundant crude oil contaminated drill sites of Assam with the aid of a hydrocarbon- degrading bacterial formulation. Int J Phytoremediation 16(9): 909-925.
- Roy AS, Baruah R, Borah M, Singh AK, Deka Boruah HP, et al. (2014) Bioremediation potential of native hydrocarbon degrading bacterial strains in crude oil contaminated soil under microcosm study. Int Biodet Biodeg 94: 79-89.
- Das D, Baruah R, SarmaRoy A, Singh AK, DekaBoruah HP, et al. (2015) Complete genome sequence analysis of Pseudomonas aeruginosa N002 reveals its genetic adaptation for crude oil degradation. Genomics 105 (3): 182-190.
- Baruah R, Kalita DJ, Saikia BK, Gautam A, Singh AK, et al. (2016) Native hydrocarbonoclastic bacteria and hydrocarbon mineralization processes. International Biodeterioration & Biodegradation 112: 18-30.
- Petruzzelli D, Petrella M, Boghetich G, Calabrese P, Petruzzelli V, et al. (2009) Neutralization of Acidic Wastewater by the Use of Waste Limestone from the Marble Industry. Mechanistic Aspects and Mass Transfer Phenomena of the Acid-Base Reaction at the Liquid-Solid Interface. Ind Eng Chem Res 48(1): 399–405.
- Lodungi FJ, Alfred BD, Khirulthzam AFM, Adnan FFRB, Tellichandran S (2016) A review in oil exploration and production waste discharges according to legislative and waste management practices perspective in Malaysia. Int J Waste Resour 7(260): 1-8.
- Patoczka J, MacDonald PH (2007) Impact of chemicals addition in water/wastewater treatment on TDS concentration and sludge generation. In Florida Water Resources Conference.
- Santhini K, Myla J, Sajani S, Usharani G (2009) Screening of Micrococcus sp from oil contaminated soil with reference to bioremediation. Bot Res Int 2(4): 248-252.
- Head I, Jones D, Roling W (2006) Marine microorganisms make a meal of oil. Nat Rev Microbio 4(3): 173-182.
- Odo EJ, Fayemi AAA (1995) The effect of oil pollution on soil germination, growth and nutrient uptake of corn. J Environ Qual 4(4): 537-540.
- Bragg J, Prince R, Wilkinson J, Atlas R (1992) Bioremediation for Shoreline Cleanup Following the 1989 Alaskan Oil Spill, Exxon Company, Houston, TX., USA.
- Karima AH, Badawi AM, Fahd MI, Nour MH (1995) Enhancement of Egyptian crude oil bioremediation using bacterial mutants. Egypt J Petroleum 4: 45-60
- Srivastava S, Thakur IS (2007) Evaluation of biosorption potency of Acinetobacter sp. for removal of hexavalent chromium from tannery effluent. Biodegradation 18(5): 637-646.
- Rajkumar K (2016) An Evaluation of Biological Approach for the Effluent Treatment of Paper Boards Industry-An Economic Perspective. J Bioremediat Biodegrad 7(5): 1-13.
- Shin D, Jang S, Hwang J (2005) Combustion characteristics of paper mill sludge in a lab-scale combustor with internally cycloned circulating fluidized bed. Waste Manag 25(7): 680-685.






























