The Efficacy of Antioxidant (Vitamin C) in the Treatment and Management of Malaria
Mgbemena IC* and Ifeyinwa Celestina
Department of Biotechnology, Federal University of Technology, Nigeria
Submission: March 10, 2020; Published: April 14, 2020
*Corresponding author:Mgbemena IC, Department of Biotechnology, Federal University of Technology, P. M. B. 1526, Owerri, Imo State, Nigeria
How to cite this article: Mgbemena I, Ifeyinwa C. The Efficacy of Antioxidant (Vitamin C) in the Treatment and Management of Malaria. Adv Biotech & Micro. 2020; 15(4): 555919. DOI:10.19080/AIBM.2020.15.555919
Abstract
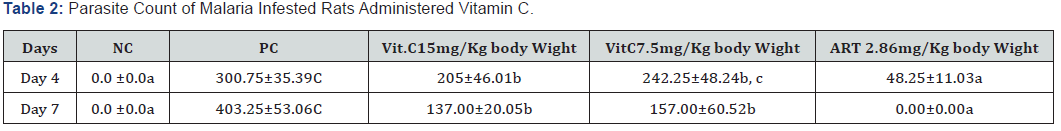
The study examines the effects of Vitamin C on malaria parasitamae and the biochemical parameters which is an indicative of the liver function. Twenty wistar Albino rats were by average weight of 100 - 250g divided into 5 groups, Groups 1) served as Normal Control (NC), group 2 as experimental groups (PC), (Group 3 & 4 ) served as Vitamin C and group 5 served as Artesunate control. All the groups were inoculated with a chloroquine-sensitive strain of Plasmodium berghei NK-65 following the administration of vitamin C and artesunate drugs. The Animals were treated for 4 days after 24 hours, post infection with P. berghei. Parasitemia levels were assessed by examining Giemsa-stained thin blood smears from each animal via an optical microscope on days 4 and 7. Blood samples collected from the animals via cardiac puncture with the aid of a capillary tube, was allowed to clot for 45 min at room temperature. Serum was separated by centrifugation at 6,000rpm for 15 min and analyzed for liver function parameters. There was a significant suppression (P<0.05) in total parasite count (205.00 & 137.00 and 242.25 & 157.00) on days 4 and 7 when compared with the other controls (300.75 & 403.25), as well as a reduction in liver function parameters with Vitamin C at 15mg & 7.5mg. This study however shows that vitamin C administration to P. berghei infected rats resulted in reducing parasite count as well as reduced malaria related alteration of liver function markers.
Keywords: Vitamin C; Plasmodium berghei; Malaria; Parasitemia; Antioxidant
Introduction
Malaria is a mosquito-borne infectious disease affecting humans and other animals caused by parasitic single-celled microorganisms belonging to the Plasmodium group [1]. The five important species of the parasite that cause this disease are Plasmodium falciparum, P. malariae, P. knowlesi (this plasmodium causes malaria in long-tailed macaques [Macaca fascicularis] but it may also infect humans, either naturally or artificially), P. ovale and P. vivax. In Nigeria, human malaria is mostly caused by Plasmodium falciparum and Plasmodium malariae and transmitted by female anopheles mosquito. Malaria causes symptoms that typically include fever, tiredness, vomiting, and headaches, shivering, joint pain, vomiting, hemolytic anemia, jaundice, hemoglobin in the urine, retinal damage, and convulsions. In severe cases it can cause yellow skin, seizures, coma, or death [2]. The prevention of this fatal disease can possibly be achieved through the control of malaria vector and usage of antimalarial drugs. Vector control as the main way to prevent and reduce malaria transmission involves two control strategies - insecticide-treated mosquito nets and indoor residual spraying are effective in a wide range of circumstances. Also, antimalarial drugs have come in handy in the fight against malaria. A couple of antimalarial drugs have been used in clearing parasitaemae and they include quinolones, antifolates, antibiotics, artemisinin and their derivatives.
Recent developments in biomedicals point to the involvement of free radicals in many diseases [3]. In spite of all the efforts geared in the fight against malaria, oxidative damage still remains one of the most important pathological consequences of malarial infections and recent reports suggest that generation of reactive oxygen species and associated oxidative stress play crucial role in the development of systemic complications in malaria [4]. It affects vital organs of the body manifesting in changes such as splenomegaly, hepatomegaly, endothelial and cognitive damages. Free radicals particularly reactive oxygen species have a greater impact on humans. Free radicals attack the unsaturated fatty acids in the bio membranes resulting in membrane fluidity. Antioxidants can greatly reduce the damage due to oxidants by neutralizing the free radicals before they attack the cells to prevent damages to the lipids, proteins, enzyme, carbohydrate and DNA. Antioxidants plays important role in inhibiting and scavenging free radicals, thus providing protection against infections and degenerative diseases. For these reasons antioxidants are of interest for the treatment of many kinds of cellular degeneration [3]. Some research investigations have focused attention on the use of antioxidants, alone or in combination with antimalarials, as a viable therapeutic strategy aimed at alleviating plasmodiuminduced oxidative stress and its associated complications. The most frequently investigated antioxidants are vitamins C and E, N-acetylcystein, folate and deferoxamine [5]. This work is aimed at determining the antioxidant effects of Vitamin C on Albino rats infected with Plasmodium berghei.
Materials and Methods
This practical work was carried out at Silver Presh Laboratories, No. 25 Mbaise Road, Owerri, Imo state.
Experimental animals
Twenty (20) male wistar albino rats weighing 100 - 250g were purchased from an animal breeding station, Animal friend farms, No. 92 Royce road Owerri, Imo State. The animals were maintained under standard laboratory condition in a stainlesssteel cage with free access to standard animal feed (Pelletised, Vital finisher) and portable drinking water Ad libitum. They were acclimatized for four days prior to use. The twenty wistar albino rats were divided into five groups of four animals each to serve for the in vivo suppressive test with Plasmodium berghei.
Parasite, P. berghei (NK65)
P. berghei parasite was obtained from the Department of Veterinary Medicine, University of Nigeria, Nsukka. The infected blood suspension containing 1 × 105 P. berghei NK65 was transported in an ice-pack and used immediately for passaging on arrival. Each rat was inoculated intraperitoneally (IP), with a parasite load of 1×105 P. berghei NK65 pRBCs.
In vivo suppressive test
Evaluation of the in vivo antimalarial activity was performed based on the Peters 4-day suppressive test [6] with modifications [7]. Briefly, 0.2 ml of infected blood suspension containing 1×105 P. berghei NK65 pRBCs was inoculated intraperitoneally in the rats. The animals were randomly divided into groups of four animals each as follows
Group 1: or control group (NC) received normal rat diet and water orally for 7days.
Group 2: or experimental group received 0.2 ml of infected blood suspension containing 1 × 105 P. berghei NK65 pRBCs by intraperitoneal route in a single dose, in addition to normal rat diet and water throughout the 7 days.
Group 3: or test group 1 was administered with 0.2 ml of infected blood suspension containing 1×105 P. berghei NK65 pRBCs by intraperitoneal route in a single dose, 15mg/kg body weight of the Vitamin C (1000mg ,Nature Field U.S.A), (obtained from Sure - Care Pharmacy LTD, Plot OB/5 Housing Area E, Opposite All Season Hotels, New Owerri, Imo State, Nigeria, for 7 days in addition to normal rat diet and water.
Group 4: or test group 2 received 0.2 ml of infected blood suspension containing 1×105 P. berghei NK65 pRBCs administered intraperitoneally in a single dose, 7.5mg/kg body weight of the Vitamin C for 7 days in addition to normal rat diet and water.
Group 5: or the standard group received 0.2 ml of infected blood suspension containing 1×105 P. berghei NK65 pRBCs which was also inoculated intraperitoneally in a single dose, 2.86mg/kg body weight of the artesunate( Mekophar chemical pharmaceutical joint company, Vietnam), (obtained from Sure - Care Pharmacy LTD, Plot OB/5 Housing Area E, Opposite All Season Hotels, New Owerri, Imo State, Nigeria), for 7 days in addition to normal rat diet and water. All the Animals were treated for 4 days after 24 hours, post infection with P. berghei. Parasitemia levels were assessed by examining Giemsa-stained thin blood smears from each animal via an optical microscope on days 4 and 7. The difference between the average parasitemia of negative-control groups (100%) and test groups was calculated as the percentage of parasite growth suppression (PGS) according to the following equation: PGS = 100 × [(A - B)/A], Where, A is the average parasitemia of the negativecontrol group and B corresponds to the parasitemia of the test group. Each sample was tested in 3 independent experiments.
Biochemical Assay
After administration for 7 days, all the animals were fasted for 24 hours and sacrificed under light anesthesia. Blood samples was collected via cardiac puncture with the aid of a capillary tube and allowed to clot for 45 min at room temperature. Serum was separated by centrifugation at 6,000rpm for 15 min and analyzed for liver function parameters.
Assay of Serum Aspartate Aminotransferase (AST) Activity
Determination of AST activity was based on the method of [8]. The spectrophotometer was set at wavelength of 546nm and incubation temperature at 37 °C. Just like the procedure for the determination of ALT activity, this test is carried out in two steps.
Step1 Measurement against reagent blank
A pipette was used to measure appropriately, 0.1ml of serum, 0.5ml of buffer (phosphate buffer 100mmol/l, L-aspartate 100mmol/l, α- oxoglutarate 2mmol/l. These chemicals were mixed and incubated for exactly 30minutes at 37 °C. 0.5ml of 2, 4-dinitrophenylhydrazine was added, mixed and allowed to stand for exactly 20min at 200C. Finally, 5.0ml of sodium hydroxide was added, mixed and the absorbance of the sample read against the blank after five minutes.
Step 2 Measurement against sample blank
A pipette was used to appropriately measure 0.1ml of serum and buffer. They were mixed and incubated for exactly 30 minutes at 37°C. 0.5ml of 2, 4-dinitrophenylhydrazine and an additional 0.1ml of serum were added, mixed and allowed to stand for exactly 20minutes at 20°C. Finally, 5ml of sodium hydroxide was added and the absorbance of the sample read against the sample blank after 5minutes. Activity of AST in the serum was obtained from the table below
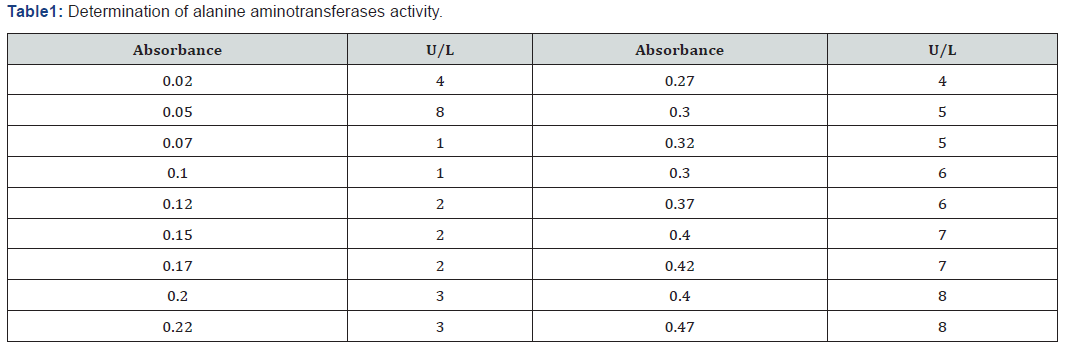
Determination of Alanine aminotranferases activity
The spectrophotometer was set at wavelength 546nm and incubation temperature at 370C. This test was carried out in two steps
Step 1: Measurement against reagent blank
Serum was mixed appropriately with 0.5ml buffer and 0.1ml of distilled water. The mixture was incubated for exactly 30minutes at 37°C. 0.5ml of 2, 4-dinitrophenylhydrazine was added, mixed and allowed to stand for 20 minutes at 25°C. Finally, 5ml of sodium hydroxide was added, mixed and the absorbance of the sample read against the reagent blank after 5 minutes.
Step 2: Measurement against sample blank
0.1ml of serum was mixed with buffer and incubated for exactly 30 minutes at 37°C. 0.5ml of 2, 4-dinitrophenylhydrazine was added with an additional 0.1ml of serum. They were mixed and allowed to stand for exactly 20 min at 25°C. Finally, sodium hydroxide was added and mixed. The absorbance of the sample was read against the sample blank after 5 minutes. The activity of Alanine aminotransferases (ALT) was extrapolated from the table below
Assay of Serum Alkaline Phosphatase (ALP) activity
ALP activity was determined according to the method described by [9].
Procedure: The reagents were brought to room temperature, and reagents were pipetted into labeled cuvettes as follows: 1.0ml of the ALP reagent was pipetted into a cuvette serving as blank and sample cuvette. Into the blank, 0.02ml of distilled water was added and used to zero the spectrophotometer. Into another cuvette 1.0ml of ALP reagent was pipetted and 0.02ml of serum was added, mixed and initial absorbance read. The timer was started simultaneously, and the absorbance read after 1 minute, 2- and 3-minutes interval respectively.
The ALP activity was calculated as follows
Calculations
ALP activity (U/L) = (ΔA 405nm/min) X 2760
Where, ΔA= Change in absorbance, min = minutes
Estimation of Total bilubirin
The method that exploits the use of diazotized sulphanilic acid as described by [10-11] was used. Colorimetric method based on that described by [12] was used. Direct bilubirin reacts with diazotized sulphanilic acid in alkaline medium to form blue coloured complex. Total bilubirin is determined in the presence of caffeine, which releases albumin bond bilubirin, by the reaction with diazotized sulphanilic acid. The colorimeter was set at wavelength of 578nm; temperature at 25°C and measurement was made against sample blank. 200ul sulphanilic acid, 500ul sodium nitrite and 1000ul caffeine were mixed with 200ul of serum and allowed to stand for 10minutes at 20°C. Tartrate was added, mixed and allowed to stand for 5-30min at 25°C and then the absorbance of the sample read against the sample blank.
Results
Parasite Count of Malaria Infested Rats Administered with Vitamin C
Result showed that administration of Vitamin C had a suppressive effect on parasite count at day 4 and day the parasite count were significantly (P<0.05) increased in PC groups compared to other groups. Administration of artesunate and Vitamin C at 15mg & 7.5mg dosages produced significant suppression of parasite load in P. berghei infected rats.
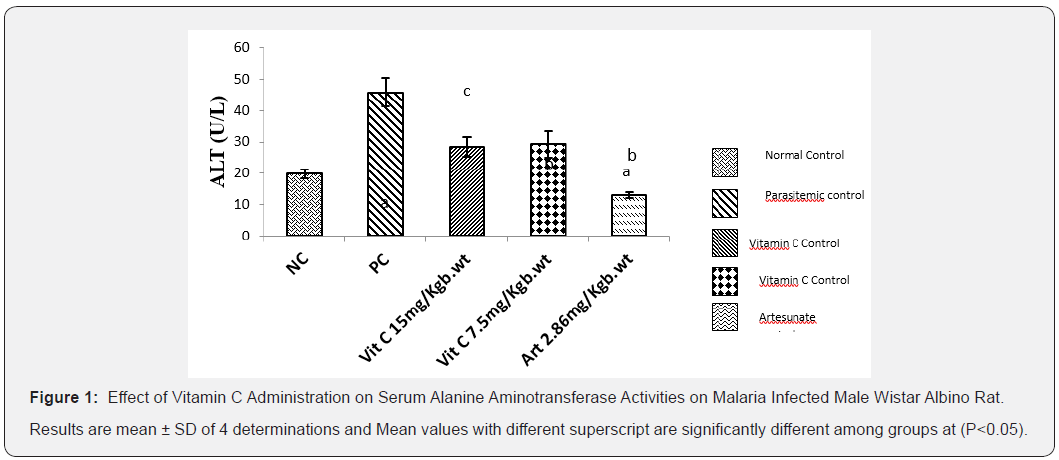
Effect of Vitamin C administration on serum Alanine Aminotransferase (ALT) activity on male wistar albino rats administered for 7 days
The result below (Figure 1) illustrates the effect of vitamin C on the liver ALT activity of treated albino rats with Plasmodium berghei. Results showed that ALT activity was significantly increased (P<0.05) in parasitemic control group. However, administration of vitamin C at 7.5mg/kg Body weight and 15mg/ kg body weight resulted in significant (P<0.05) reduction in ALT activity when compared to PC.
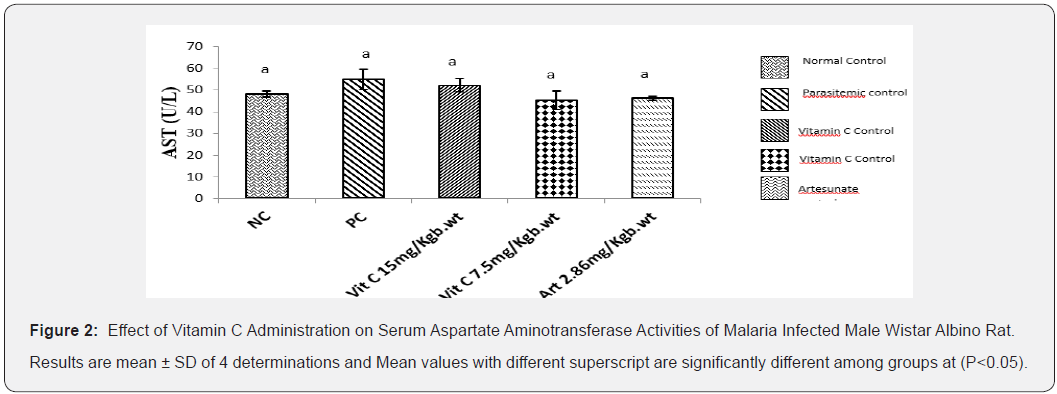
Ant
Results (Figure 2) showed that AST activity was slightly increased in parasitemic control group and parasitemia induced groups. However, administration of vitamin C at 7.5mg/kg body weight and 15mg/kg body weight and Artesunate 2.86mg/kgb.wt did not significantly reduce AST activity when compared to PC and NC.
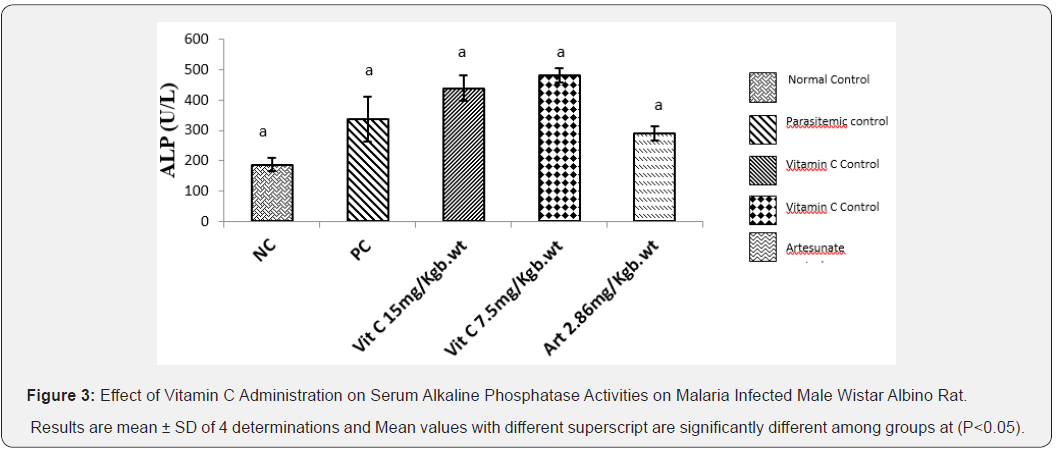
Effect of Vitamin C Administration on Serum Alkaline Phosphatase (ALP) Activity on Male Wistar Albino Rats Administered For 7 Days
The result below (Figure 3) illustrates the effect of vitamin C on the Liver ALP activity of infected albino rats with Plasmodium berghei. That ALP activity was increased in parasitemic control group, Vitamin C 15mg/kg body weight, Vitamin C 7.5mg/kg body weight, artesunate 2.86mg/kg body weight compared to normal control. However, this increase were not significant (P<0.05).
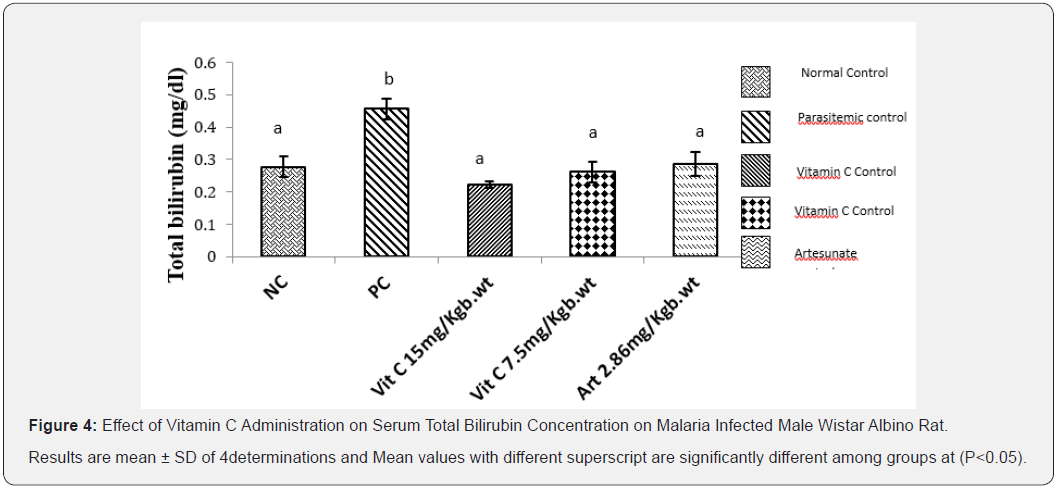
Effect of Vitamin C Administration on Serum Total Bilirubin (T/Bil) Activity on Male Wistar Albino Rats Administered for 7 Days
Result obtained from this study (Figure 4) showed that the effect of vitamin C on the total bilirubin concentration of albino rats infected with Plasmodium berghei. That total bilirubin activity was significantly increased (P<0.05) in parasitemic control group but administration of vitamin C at both 7.5mg/kg body weight and 15mg/kg body weight resulted in significant reduction in total bilirubin activity when compared to PC.
Discussion
Treatment of malaria can be affected by the use of a variety of antimalarial drugs which are readily available and can be purchased over the counter. ACTs are currently relied upon as first-line therapy for effective malaria treatment in most regions of the world in which the disease is endemic since they are effective, less toxic and well tolerated [13-14] and their continuing efficacy is crucial if control and elimination programs are to succeed. The loss of effectiveness of artemisinin and its derivatives to drug resistance constitutes a major disaster in the fight against malaria. This work studied the efficacy of Vitamin C in the treatment of malaria and its protective activity on the liver of rat infected with plasmodium berghei. Vitamin C (Ascorbic Acid) is a watersoluble vitamin that acts as an antioxidant, especially protecting the immune system cells from free radicals generated during their assault on invaders [15]. It has, therefore been suggested that vitamin C supplementation may have a role in case management of malaria [16]. Indeed, the plasma concentration of this antioxidant has been reported to be significantly decreased in chronic and acute malaria infections [17 -18].
However, analysis of malaria parasite count showed that administration of vitamin C to malaria infested rat significantly suppressed parasite count. Result obtained (Table 1&2) showed that parasite count in groups treated with vitamin C 7.5mg/ kg and 15mg/Kg body weight caused significant reduction in parasite count when compared to the parasite control group. Vitamin C had a suppressive effect on parasite count after fourand seven-day administration. Furthermore, the results showed that liver function indices (ALT, ALP) activities were significantly increased (P<0.05) by P. berghei infestation as well as total bilirubin concentration. However, the treatment of infested rats with artesunate and vitamin C significantly (p<0.05) reduced liver function enzymes activity and total bilirubin concentration. However, AST activity was not significantly altered across the groups. The liver is a primary site for xenobiotics detoxification, its metabolism is readily altered by toxicity as well as malaria parasitemia. Liver transaminases (ALT, AST) activity, alkaline phosphatases, total bilirubin are employed as indicators of hepatocellular damage. Damage to the liver results in increase in their activities in the plasma, increase in their activities is roughly to the extent of tissue damage [19]. Administration of vitamin C reduced alteration of liver function in malaria parasitemia. Hyperbilirubinaemia is characteristic of impaired bilirubin metabolism involving metabolic disturbances in the liver resulting to defective conjugation, transport and, or excretion of bilirubin or overproduction of bilirubin caused by an excessive breakdown of red cells which may be due to severe falciparum malaria, sickle cell disease, haemolysis associated with glucose- 6-phosphate dehydrogenase deficiency and toxins from bacteria, snake venoms, drugs or herbs etc. The measurement of serum bilirubin is performed to investigate the causes of liver disease and jaundice. In the present study, vitamin C administration resulted in normalization of bilirubin concentration.
Conclusion
The present study investigated the use of antioxidant vitamin C in the treatment and management of malaria. The findings showed that vitamin C administration to P. berghei infected rats resulted in a significant suppression of parasitemia as well as reduced malaria related alteration of liver function markers.
Acknowledgement
The author wishes to express her deep and heartfelt gratitude to the staff and management of Silver Presh laboratories, No. 25 Mbaise Road, Owerri, Imo state for their immense contribution in the analysis of samples.
References
- Mueller I, Zimmerman PA, Reeder JC (2007) Plasmodium malariae and Plasmodium ovale the ‘bashful’ malaria parasites, 23(6): 278 - 283.
- Beare NA, Taylor TE, Hardin SP, Lawallen S, Molyneux ME (2006) Malarial Retinopathy: A newly established diagnostic sign in severe malaria. American Journal of Tropical Medicine and Hygiene 75(5): 790-797.
- Behera BC, Verma N, Sonone A, Makhija U (2006) Determination of antioxidant potential of Lichen Usnea ghattensis in vitro LWT 39: 80- 85.
- Pabon A, Carmona J, Burgos LC, Blair S (2003) Oxidative stress in patients with non - complicated malaria. Clinical Biochemistry 36: 71 -78.
- Isah MB, Ibrahim MA (2014) The role of antioxidants treatment on the pathogenesis. Parasitology Research 113(3): 801-809.
- Peter W, Portus H, Robinson L (1995) The four-day suppressive in vivo antimalarial test. Ann Trop Med Parasitol 69: 155-171.
- Rocha eSilva LF, Pinto ACS, Pohlit AM, Quignard ELJ, Vieira PPR, et al. (2011) In vivo and in vitro antimalarial activity of 4-nerolidylcatechol. Phytother Res 25:1181-1188.
- Reitman S, Frankel S (1957) A colorimetric method for the determination of serum oxaloacetatic and glutamic pyruvic transaminases. American Journal of Clinical Pathology 28(1): 56–63.
- Englehardt A (1970) Measurement of alkaline phosphatase. Aerztl Labor 16: 42.
- Pearlman FC, Lee RT (1974) Detection and measurement of total bilirubin in serum, with use of surfactants as solubilizing agents. Clinical chemistry 20: 447-453.
- Zoppi FPA, Felini D, Marcovina S, Ramella C (1976) Metodo per la determinazione della bilirubin totale e conlugata. Uso di un tensioaltivo cationico come agentte solubilizzantte. Giorn ital chim clinica 1: 343-359.
- Jendrassik L, Grof P (1938) Estimation of total serum bilirubin level by spectrophotometrically in serum and plasma. Biochemische Zeitschrift 297: 81-89.
- Nanyunja M, Orem JN, Kato F, Kaggwa M, Katureebe C, Saweka J (2011) Malaria treatment policy change and implementations: The case of Uganda. Malaria Research and Treatment.
- Cui L, Su XZ (2009) Discovery, mechanisms of action and combination therapy of artemisinin. National Institute of Health: Expert Rev Anti Infect Ther 7(8): 999-1013.
- Wintergerst ES, Maggini S, Hornig DH (2006) Immune enhancing role of vitamin C and zinc and effect on clinical conditions. Ann Nutr Met 50: 85-94.
- Marva E, Golenser J, Cohen A, Kitrossky N, Harel R (1992) The effects of ascorbate induced free radicals on Plasmodium falciparum. Tropical Medical Parasitology 43: 17-23
- Mohammad A (2012) Effect of serum antioxidant ascorbic acid concentration by malarial infection Man. The Experiment 3(4): 214-215.
- D’Souza V, D’Souza B (2006) Comparative study on lipid peroxidation and antioxidant vitamins E and C in falciparum and vivax malaria. Indian Journal Clinical Biochemistry 21(2): 103-106.
- Gaw A, Cowman RA, O'Reilly DS, Shepherd J (1995) Clinical Biochemistry: An Illustration color Text. Clinical Biochemistry, New York.






























