Nosemosis In Laboratory Mass-Rearing of Two Braconid Parasitoids, Microplitis Rufiventris and Cotesia Marginiventris
Esmat Hegazi1*, Ahlam Afazairy1, Wedad Khafagi2 and Yasien G Alabed1
1Department of Entomology, Alexandria University, Faculty of Agriculture, Egypt
2Plant Protection Research Institute, Alexandria University, Egypt
Submission: January 20, 2020; Published: January 28, 2020
*Corresponding author: Esmat Hegazi, Department of Entomology, Alexandria University, Faculty of Agriculture, Egypt
How to cite this article: Esmat H, Ahlam A, Wedad K, Yasien G A. Nosemosis In Laboratory Mass-Rearing of Two Braconid Parasitoids, Microplitis Rufiventris and Cotesia Marginiventris. Adv Biotech & Micro. 2020; 15(3): 555911. DOI:10.19080/AIBM.2019.14.555911
Keywords: Microplitis rufiventris; Cotesia marginiventris; Hymenoptera: Braconidae; Spodoptera littoralis; Nosema; Parasitoid samples
Short Communication
The larval endoparasitoids, Microplitis rufiventris Kok. and Cotesia marginiventris (Cresson) (Hymenoptera: Braconidae) are small wasps. Females of both parasitoids bear short ovipositors and parasitize young larvae. They are important wasps attacking some important noctuid pests. The two parasitoids have successfully been mass-reared at the biological Control lab, Alexandria University, for decades for the first wasp and several years for the 2nd one. Suddenly, nosemosis was obviously found in both male and female of the 2 wasps, as well as their larval hosts of S.littoralis. Nosema–infection rate was, in general, higher among adult females of both M.rufiventris (88%) and C.marginiventris (70 %) parasitoids and their infected larval hosts were short-lived and all harbored massive numbers of Nosema spores. Insectary mass rearing’s of the two parasitoids and their larval host populations were totally collapsed within few days.
M. rufiventris and C.marginiventris are sexually reproducing koinobiont end parasitoids of important noctuid pests including some Spodoptera spp [1-5]. In the field, these parasitoids prefer to attack earlier instars of their hosts when they still live in clusters near the place of egg deposition. However,1st-2nd instars for C.marginiventris and third instars for M. rufiventris are optimal stages for insectary rearing purposes [5,6]. Both wasps were reared from. several year-old colonies maintained on young S.littoralis larvae. Recently, both wasps and hosts (S.littoralis larvae) were severely infected by Nosema spp.. In the present work, Nosema infection was carefully examined. Cultures of the parasitoids were kept on young larvae of S. littoralis at 27 ± 1 °C, 65 ± 5% RH and a photoperiod of 12.00:12.00 hours L: D. Both parasitoids were reared following the methods developed in the Department of Economic Entomology (Faculty of Agriculture, Alexandria University) [7] for rearing M.rufiventris wasp. C. marginiventris (Cresson) used in this study were kindly obtained (Dr. Ted Turlings) from Institute of Zoology, University of Neuchatel, Switzerland. For decades and to date, S.littoralis larvae have been used as hosts for the subject parasitoids. Recently, the productivity of Microplitis or Cotesia mass rearing’s has severely been decreased as a result of a protozoan disease, nosemosis (Microsporidia: Nosematidae). The causative entomopathogen is a member of the genus Nosema (Figure 1), phylum Microspora, kingdom Protista [8,9]. Hence, this protozoan disease would be considered as a great threat for the biological control of some economically important insect pests. The observations revealed that both the infected parasitoid adults and the S.littoralis-host larvae were obviously short-lived. Samples (n=80) of these short-lived Nosema-infected parasitoid adults, M.rufiventris and C.marginiventris, and their host larvae, S.littoralis were checked up microscopically for presence of Nosema spores, as well as estimation of infection rates. On a microscopic slide, a wet-mount smear for each examined individual was prepared in Ringer solution. The smears were examined by a light microscope at a magnification of 400-1000x.
The microscopic examinations revealed that ca. 86 and 60 % of Microplitis and Cotesia adults, respectively, were heavily infected with Nosema spores (Figure 1). In terms of sex differences, presence of Nosema infection was also examined in adult male and female parasitoids. Infection rate in Microplate females and males was, in respect, ca. 88 and 86 %; whereas the corresponding rates in Cotesia females and males were ca. 70 and 57%, respectively. In the meantime, the prevalence of Nosema disease in the host population, S.littoralis larvae, was very marked, as the infection rate ranging from 70 to 87 %. Moreover, Nosema– infection rate was, in general, higher among adult females of both C.marginiventris (88%) and M.rufiventris (70%) than their males (86 and 57 %,respectively).
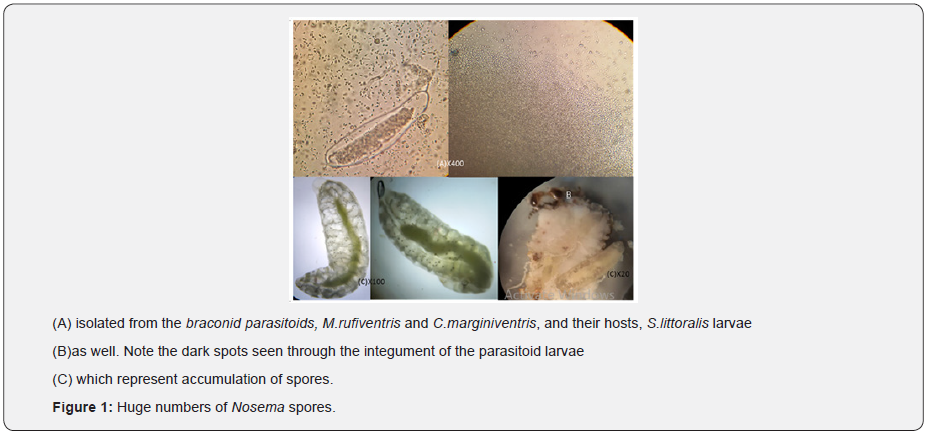
On the other hand, Nosema spores, per adult parasitoid, male and female, and larval host, were counted (n= 16-40) using a hemocytometer and a light microscope, at a magnification of 400x. The adopted statistical analyses which based on t-test and Duncan’s Multiple range test, significance at 5 % level, indicate that, in average, Nosema-infected C.marginiventris adult females were found harboring, significantly, less spores (19.04 +0.70 x 106 spores per female) than their males (23.38 + 0.41 x 106 spores per male) or M. rufiventris males and females (23.38 + 0.90 and 22.84 + 0.68 x 106 spores per adult parasitoid, in respect), and their larval hosts (23.0 +0.89 x 106 spores per S. littoralis larva). In the available literature, no reports for nosemosis in M. rufiventris or C. marginiventris, but [5] reported that Nosemainfected host larvae of Plutella xylostella (L.) had severe undesired effects on the braconid parasitoid, C. vestalis (Haliday). Hence, the present observations seem to be the first record for nosemosis on the two braconid parasitoids, M. rufiventris and C. marginiventris. Also, the disease is found in both male and female parasitoid samples. The nosemosis has previously been reported to cause a major problem in the laboratory or the insectary of the mass rearing of insect hosts and their parasitoids, where the Nosemainfected host populations have a negative effect on parasitoid populations [5, 9,10].
Conclusion
In summary, the present observations had recorded high rates (60 – 87 %) of nosemosis among insectary mass rearing’s of populations of promising two braconid parasitoids, M. rufiventris and C.marginiventris, as well as their hosts, S. littoralis larvae. The disease does not significantly prefer any sex of the studied two parasitoids where nosemosis was obviously found in both male and female parasitoid samples, as well as their larval hosts of S.littoralis. Adults of diseased parasitoids and their larval hosts were short-lived and harbored massive numbers of Nosema spores. Insectary mass rearing of the two parasitoids and their larval host populations were totally collapsed; but soon, this problem will be overcome.
Acknowledgement
Dr. Hegazi wishes to thank the Alexander-Von-Humboldt- Foundation for the research scientific donation used in this work. The authors warmly thank the anonymous reviewers for their suggestions on improving the article.
References
- Kokujev N (1914) Hymenoptera parasitic nove fauna tyrannical Platnikov Collecta (parasitic Hymenoptera new to the fauna of Turkestan, collected by V.I. Plotnikov). Revue Russe D Entomolgie St. Ptersburg XIII, pp. 513-514.
- Hammad SM, AM El-Minshawy, A Salama (1965) Studies on Microplitis rufiventris (Hym, Braconidae). Bull Entomol Soc Egypt 49: 215-219.
- Gerling D (1969) The parasites of Spodoptera littoralis (Boisd.) (Lepidoptera : Noctuidae) eggs and larvae in Israel. Israel J Entomol 4: 73-81.
- Sourakov A, Mitchell ER (2001) Effects of cool temperatures on oviposition and development of Cotesia marginiventris (Hymenoptera : Braconidae). Florida Enotomologist 84 (2): 308-309.
- Kermani N, Abu Hassan, ZA Suhaimi A, Abuzid, I, Ismail NF (2014) Parasitism performance and fitness of Cotesia vestalis (Hymenoptera: Braconidae) infected with Nosema sp. (Microsporidia: Nosematidae): Implications in integrated pest management strategy. PLOS One 9(6): e 100671.
- Hegazi EM, Schopf A, Fuhrer E, Fouad SH (1988) Developmental synchrony between Spodoptera littoralis and its parasite Microplitis rufwentris. J Insect physiol 34: 773-778.
- Hegazi EM AM, El-Minshawy, SM Hammad (1977) Mass rearing of the Egyptian cotton leafworm, Spodoptera littoralis (Boisd.) on semi-artificial diet. Proceedings of second Arab Pesticide Conference, Tanta University, Tanta, Egypt, p. 61-70.
- Solter LF, Becnel JJ (2007) Entomopathogenic microsporidia. In: Lacey LA, Kaya K (Eds.), Field Manual of Techniques in Invertebrate Pathology. Application and evaluation of pathogens for control of insects and other invertebrate pests. (2nd), Springer, pp. 199-221.
- Sajap AS, Lewis LC (1988) Effects of the microsporidium Nosema pyrausta (Microsporida: Nosematidae) on the egg parasitoid, Trichogramma nubilale (Hymenoptera: Trichogrammatidae). J Invertebr Pathol 52(2): 294-300.
- Geden CJ, Long SJ, Rutz DA, Becnel JJ (1995) Nosema disease of the parasitoid Muscidifurax raptor (Hymenoptera: Pteromalidae): prevalence, patterns of transmission, management, and impact. Biological Control 5: 607-614.






























