- Research Article
- Abstract
- Introduction
- Prevalence of Intestinal Parasitic Infections in Army Soldiers
- Methods and Materials of the Study
- Variables
- Stool Specimen Collection and Examination
- Data Quality
- Results
- Parasitological Results Discussion
- Socio-Demographical Results Discussion
- Bivariate Results Discussion
- Conclusion
- Limitations of the Study
- Recommendations
- Acknowledgement
- References
Prevalence of Intestinal Parasitic Infections and Associated Risk Factors among Ethiopian Army Students, Health Sciences College, Bishoftu, Ethiopia, 2019
Fasil Kenea Duguma*, Tesfaye Dadi, Bethisrael Madebo Ayzo, Habtamu Gizachew, Sinimengn Menge and Mekoya Aregaw
Department of Public Health, Defense Health Science College, Ethiopia
Submission: September 12, 2019;Published: January 21, 2020
*Corresponding author: Muhammad Sarwar Khan, Centre of Agricultural Biochemistry and Biotechnology (CABB), University of Agriculture, Faisalabad, Pakistan
How to cite this article: Fasil K D, Tesfaye D, Bethisrael M A, Habtamu G, Sinimengn M, et al. Prevalence of Intestinal Parasitic Infections and Associated Risk Factors among Ethiopian Army Students, Health Sciences College, Bishoftu, Ethiopia, 2019. Adv Biotech & Micro. 2020; 15(2): 555908. DOI:10.19080/AIBM.2019.14.555908
- Research Article
- Abstract
- Introduction
- Prevalence of Intestinal Parasitic Infections in Army Soldiers
- Methods and Materials of the Study
- Variables
- Stool Specimen Collection and Examination
- Data Quality
- Results
- Parasitological Results Discussion
- Socio-Demographical Results Discussion
- Bivariate Results Discussion
- Conclusion
- Limitations of the Study
- Recommendations
- Acknowledgement
- References
Abstract
Vitamins and minerals are essential nutrients that are required for normal functioning and growth of the body. Plants are a major source of these nutrients. Grains are consumed as staple food in developing countries, and these are lacking in essential micronutrients, leading to health problems. To address such inadequacies, biofortification using transgenic approaches has long been on the wish-list of biotechnologists whereby essential micronutrients are incorporated into plants. In addition to adding nutritional elements in crops, transgenic technology has ensured the bioavailability of these micronutrients. This review summarizes the current status of nutritional deficiencies, nutritional deficiencies disorders and strategies for the biofortification of cereals, and briefly describes how novel approaches facilitate bioavailability of these micronutrients in food.
Keywords: Biofortification; Essential micronutrients; Minerals; Vitamins; Transgenic technology
Abbreviations: MNM: Micronutrient Malnutrition; DALYs: Disability-Adjusted Life Years; YLD: Years Lived With Disability; YLL: Years of Life Lost; TALENs: Transcription Activator-Like Effector Nucleases; CRISPR : Clustered Regularly-Interspaced Short Palindromic Repeats; LCY-B: Lycopene β cyclase; CHY: β-Carotene Hydroxylase; CRTI: Phytoene Desaturase; CRTW: β- Carotene Ketolase; GMO: Genetically Modified Organism
- Research Article
- Abstract
- Introduction
- Prevalence of Intestinal Parasitic Infections in Army Soldiers
- Methods and Materials of the Study
- Variables
- Stool Specimen Collection and Examination
- Data Quality
- Results
- Parasitological Results Discussion
- Socio-Demographical Results Discussion
- Bivariate Results Discussion
- Conclusion
- Limitations of the Study
- Recommendations
- Acknowledgement
- References
Introduction
Intestinal parasitic infections are a group of diseases caused by one or more species of protozoa, cestodes, trematodes and nematodes. These parasites are responsible for the major share of morbidity and mortality in those communities where there is over-crowding, poor environmental sanitation and personal hygienic practices, which make them a great concern for the developing countries. Intestinal parasitic infections are endemic worldwide and have been described as constituting the greatest single worldwide cause of illness and disease [1]. Poverty, illiteracy, poor hygiene, lack of access to potable water and warm and humid tropical climate are some factors associated with intestinal parasitic infections [2,3]. Parasitic protozoa and helminths are responsible for some of the most devastating and prevalent diseases such as intestinal or biliary obstruction, growth retardation and iron deficiency anemia that affect humans [4,5].
Intestinal parasitic infections constitute a global health burden causing clinical morbidity in 450 million people; many of these are women of reproductive age and children in developing countries [6]. Intestinal parasitic infection is one of the major health problems in developing countries. It has been estimated to affect some 3.5 billion people globally and 450 million are thought to be ill because of such infections, the majority being children [7]. Intestinal parasitic infections, mostly helminthes, have been linked with an increased risk for nutritional anemia, protein-energy malnutrition and growth deficits in children, loss of weight in pregnancy and intrauterine and growth retardation followed by low birth weight [8,9]. Approximately 3 billion people globally are infected with helminthes [10]. Epidemiological surveys have revealed that, poor sanitation and inappropriate environmental conditions coupled with indiscriminate defecation, geophagy and contamination of water bodies are the most important predisposing factors to intestinal worm infestation [11]. The prevalence and intensity of infection is especially high in developing countries, particularly among populations with poor environmental sanitation [12]. Other practices such as improper hand washing, disposal of refuse, personal hygiene and not wearing shoes may contribute to the infection [13]. Entamoeba histolytica, Giardia lamblia and Cryptosporidium parvum are the three of the most common intestinal protozoan parasites infecting humans worldwide. They are known to be the most important diarrhea-causing protozoa [14]. It is estimated that 10% of the world’s population are infected with E. histolytica with the highest prevalence in developing countries [15].
The research findings will provide data on the distribution and prevalence of intestinal parasites and assist in proposing strategies to protect those soldiers that may be at risk of intestinal parasitic infections. Accurate information on intestinal parasitic infections and risk factors for infection are needed to control and/ or eradicate the parasitic infections. As mentioned earlier, poor sanitation and overcrowded lifestyle contributes to the infestation of internal parasite. Military camp and schools are areas where large number of people live and feed together where the possibility of parasitosis is expected to be prevalent. Hence, this research was to overview the condition in the military school camp. Most of the intestinal parasites are more common and their manifestations are more severe in army soldiers next to children than adult civilians due to their mission. Infection in army soldiers is also associated with severe gastroenteritis, anemia, mental retardation and poor mission performance. An upsurge in water, sanitation and hygiene related diseases such as amoebic dysentery has been reported at army camps. Intestinal parasitic infections are highly prevalent because of low living standards, poor environmental sanitation, unsafe human waste disposal systems, inadequacy and lack of safe water supply, and low socio-economic status. This could be one reason for why intestinal parasitism has been widespread in Ethiopia. Besides the health impact, intestinal parasites have significant politico-economic impact in terms of absence from mission, diagnostic and treatment expenses. Therefore, this study was aimed to access the prevalence and associated risk factors of intestinal parasitic infections among army soldier students at Defense University College of Health Sciences at Bishoftu town school camp.
Intestinal parasitic infections in Ethiopia
The most prevalent intestinal protozoan parasites in Ethiopia are Giardia lamblia and Entamoeba histolytica/ dispar. Helminthic infection includes Ascaris lumbricoides, Trichuris trichuria and Taenia saginata. Many of these intestinal parasites usually cause asymptomatic infections or produce only mild symptoms, leading to difficulties in eradication and control [16].
- Research Article
- Abstract
- Introduction
- Prevalence of Intestinal Parasitic Infections in Army Soldiers
- Methods and Materials of the Study
- Variables
- Stool Specimen Collection and Examination
- Data Quality
- Results
- Parasitological Results Discussion
- Socio-Demographical Results Discussion
- Bivariate Results Discussion
- Conclusion
- Limitations of the Study
- Recommendations
- Acknowledgement
- References
Prevalence of Intestinal Parasitic Infections in Army Soldiers
The historical documents explained the significance of schistosomiasis that from the time of the Napoleonic conquests, schistosomiasis has proved to be a significant medical adversary for troops exposed to freshwater in tropical environs. Several hundred British and Australian troops became infected in Egypt and the Middle East during World War I [17]. During World War II, more than 1,500 British and African troops became infected in Nigeria. Perhaps the most spectacular outbreak was among U.S. service members during the liberation of the Philippine Islands, when hundreds of troops were infected during the invasion of Leyte [18]. During World War II, a high prevalence of active schistosomiasis was found among Puerto Rican nationals applying for enlistment into the U.S. Army; potential recruits were rejected based on positive stool examination findings. In addition, medical historians suspect that S. japonicum infection was the cause of Dapeco fever among prisoners of war and guards in the Davao penal colony (southern Mindanao, Philippines) [19]. Sixty-nine troops reportedly suffered from schistosomal dermatitis during operations in the Mekong Delta during the Vietnam conflict, but there was no evidence that U.S. service members were infected with human Schistosomes [20].
During the First World War, there was evidence that Ascaris, Trichuris and Taenia were present in German soldiers [21]. Hookworm (Ancyclostoma duodenale) was first discovered by Colonel Bailey K. Ashford, in Puerto Rico in1899, from an infected army soldier. His discovery led to worldwide therapeutic campaigns to cure this important parasitic disease [22-25]. A study carried out in soldiers from different countries was showed a very high prevalence for intestinal parasites; however, the prevalence and profile of intestinal parasitic infections vary widely among continents and within countries sub regions. In 2000, 2002 and 2006, in Thailand, a study was undertaken on the prevalence of intestinal parasites among army soldiers of Royal Thai Army, in Central Military Bases and Nakornrachasrima province, indicated that the prevalence of intestinal parasites were 53.9%, 55.7% and 22.4% (74/317), respectively. The most prevalent protozoa found was Blastocystis infection followed by Giardia duodenalis. Others were soil-transmitted helminthes (hookworm, Strongyloides stercoralis) and food borne trematode (Opisthorchis viverrini). Among these Blastocystis spp.14.5%, Strongyloides stercoralis 2.5%, Giardia duodenalis 1.3%, hookworm spp.1.0%, Opisthorchis viverrini 1.0%, Taenia spp.0.35%, Entamoeba coli 2.2%, and Endolimax nana 0.6%. The prevalence of S. Stercoralis in Thailand soldiers was 2.5% and for Blastocystis, 14.5%, which highlights the high fecal-oral contamination. Parasitic infections in Thai military personnel remain a public health concern. Privates had significantly higher risk of acquiring parasitic infections than noncommissioned officers and officers [26,27].
In 2012, in Latin America, in the Amazonic region of Peru, army soldiers in the local army base of Iquitos city, intestinal parasites were diagnosed in 94/104 studied army soldiers and the prevalence was 90.4%. Among these, Blastocystis spp. (54.8%), hookworm (47.1%), Strongyloides stercoralis (28.8%), Ascaris lumbricoides (27.9%), G.lamblia (18%), T. trichiura (16%), E.histolytica /dispar (6%) and H. nana (3.4%). The most common helminthes found were hookworm (47.1%), Strongyloides stercoralis (28.8%) and Ascaris lumbricoides (27.9%). The most common protozoans found were Blastocystis spp. (54.8%) and Giardia lamblia (18%) [28,29]. In Honduras, intestinal parasites were diagnosed in 9/58 for the U.S. military personnel in Soto Cano region and indicated that the prevalence of intestinal parasites was 15.5% (9/58). Among these three parasites were found Blastocystis hominis (12.1%), Entamoeba coli (1.7%), and Cryptosporidium spp. (1.7%). These worldwide cases indicate that due to the harsh environment (camp life) and crowded living condition might have contributed for the prevalence of intestinal parasitic infections in the army soldiers. This need to be checked by empirical evidence in Ethiopian army soldiers to overcome the problem.
- Research Article
- Abstract
- Introduction
- Prevalence of Intestinal Parasitic Infections in Army Soldiers
- Methods and Materials of the Study
- Variables
- Stool Specimen Collection and Examination
- Data Quality
- Results
- Parasitological Results Discussion
- Socio-Demographical Results Discussion
- Bivariate Results Discussion
- Conclusion
- Limitations of the Study
- Recommendations
- Acknowledgement
- References
Methods and Materials of the Study
Study area and period
The study was conducted from January 2019 up to May 2019 at Defence University College of Health Sciences school camp, in Bishoftu town. The College is located in Bishoftu town. Bishoftu town is found in East Shoa zone, Ada’a Liban district, Oromia Region, 47 km south-east of Addis Ababa. Geographically, it is located between latitudes of 8O 45’N and longitudes of 38O 59’E /8.750ON and 38.983OE. The mean annual minimum and maximum temperatures are 12 °C and 27 °C respectively, with an overall average of 18.7 °C. The mean relative humidity is 61.3%. It has three agro-ecological zones, namely midland (94%), highland (3%) and lowland (3%) [28]. According to the 2012 census, Bishoftu town has a total population of about 171,115, of whom 89,160 were women and 81,955 were men [29]. Defense University College of Health Sciences school camp one of the institutions in the town. During the study period there were 2000 army soldier in Defense University. Of these army scholars, 1200 were in engineering camps and other colleges, and 800 were in Health Science College at Bishoftu and Addis Ababa school camps the rest schools.
Study design
Institutional based cross-sectional study design was carried out among army soldier by using systematic probability sampling technique.
Study population
Army Soldier students of Health Sciences at Bishoftu school camp
Sample size determination
The sample size was calculated by using a single population proportion formula:
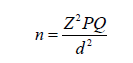
(Z= this is the critical value of the standard normal distribution (1.96 at 95% confidence interval); P = estimated prevalence of intestinal infection; Q = 0.152 - P and d = absolute precision or sampling error tolerated = 5%). The minimum calculated sample size that was used for this study is 197 participants.
The sample size was calculated using a single population proportion formula with the assumptions of 95% confidence interval and 5% of marginal error;
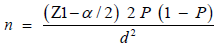
N = Source population (assume 2000 army soldier students in Defense University College)
n’ = total sample size to be studied
n= sample size was collected from finite population, n= Z2 P (q)/d2
Where, P = Prevalence of intestinal parasites to be studied was taken as 15.2% = 0.152= failure rate1-p, 1-0.152 = 0.848
d2= Absolute precision and was taken as 0.05. 0.0025
Z2 = 1.96 at 95% confidence interval. 3.8416
n =1.96*1.96*0.152(1-0.152)/0.05*0.05 =198*10/100 = 218
An initial sample size was 198. After considering 10% nonresponse, the final sample size was determined to be (198×10)/100 = 19.8 ≈ 20 then total 198+ 20= 218. So, the number of the study population was less than 10,000 then the final sample size was calculated by using finite population correction formula.
n’= n/ (1+ n/N) = 218/ (1+218/2000) = 218/ (1+0.109) = 218/1.109 = 196.57 ≈ 197 subjects.
Sampling method
Based on the defense health science college student payroll number study participant was selected systematically. Kth interval was used= K5th = N/n= 800/179= every 5th interval of student payroll number was taken to this study.
- Research Article
- Abstract
- Introduction
- Prevalence of Intestinal Parasitic Infections in Army Soldiers
- Methods and Materials of the Study
- Variables
- Stool Specimen Collection and Examination
- Data Quality
- Results
- Parasitological Results Discussion
- Socio-Demographical Results Discussion
- Bivariate Results Discussion
- Conclusion
- Limitations of the Study
- Recommendations
- Acknowledgement
- References
Variables
Dependent variable
Prevalence of intestinal parasitic infections.
Independent variable
all sociodemographic conditions such as age, sex, ranks, level of knowledge on intestinal parasitic infections, sanitation, personal hygienic practices, etc.
Inclusion and exclusion criteria
Inclusion criteria: Those volunteers who signed informed consent and deliver stool specimen. Did not receive any antiparasitic treatments in the days prior to sampling.
Exclusion criteria
The army soldier student did not have the signed informed consent
Stool sample that was not properly collected.
Did not answer the questions on the form for sample collection
Those subjects who had taken antiparasitic drugs in the last three weeks or during data collection were excluded.
Data collection tools
To determine the risk factors and outcomes of intestinal parasitic infections, standardized questionnaires concerning sociodemographic data, sanitary behaviors including cooking and eating habits, source and treatment method of drinking water, sanitary facilities, and hygiene conditions was used in the study. All participants were asked to complete the questionnaires before they provided their specimens. The pretest was done among 20 participants of similar demographic characteristics before actual field work begun.
- Research Article
- Abstract
- Introduction
- Prevalence of Intestinal Parasitic Infections in Army Soldiers
- Methods and Materials of the Study
- Variables
- Stool Specimen Collection and Examination
- Data Quality
- Results
- Parasitological Results Discussion
- Socio-Demographical Results Discussion
- Bivariate Results Discussion
- Conclusion
- Limitations of the Study
- Recommendations
- Acknowledgement
- References
Stool Specimen Collection and Examination
Collection of faecal samples
A total of 197 stool specimens were collected from army soldier students including privates, noncommissioned officers, officers, and other employees, who voluntarily were enrolled into the study with the informed consent. A single fecal specimen was collected from each army soldier students. They were instructed to collect fresh stool specimen into labelled specimen bottles and submit no more than one hour after collection. About 20- 50 grams of formed stools or 5- 6 spoon-full for watery stools were collected. All specimens were labeled with subject’s name, age, sex, rank and date of collection. The specimens were taken to the College laboratory for examination and identification of parasites. The stool samples were examined for trophozoites and cysts of protozoans and the ova and larvae of helminths under the light microscope [31].
Direct microscopy [Wet mount]
Specimens containing blood and mucus and those that were unformed were examined immediately because these may contain motile trophozoites. A drop of fresh physiological saline was placed on one end of a slide and a drop of iodine on the other end. A small amount of specimen about 2 mg was mixed with saline, and a similar amount was mixed with the iodine using a wooden applicator. Smooth, thin preparations of the specimen was made and covered with a cover glass. The entire saline preparation was examined systematically for larvae, ciliates, helminths eggs, cysts, and oocyte underx10 objective with the condenser iris was closed sufficiently was used to give good contrast. The x40 objective was used in the identification of eggs, cysts, and oocytes. The iodine preparation was used to assist in the identification of cysts.
Formol–diethyl-ether concentration method
This concentration technique was required because it was rapid and can be used to concentrate a wide range of stool parasites from fresh or preserved stool. An application stick was used to emulsify 1 g of stools in about 10 ml of normal saline contained in a tube. The emulsified stools were sieved, and the suspension was collected in another tube. The suspension was centrifuged at 3000 rpm for 5 min. The supernatant was discarded leaving the deposit. A volume of 7 ml of 10% formaldehyde was added to the deposit and mixed. A volume of 3 ml of diethyl ether was further added and mixed well by shaking. The layer of fecal debris was loosed from the side of the tube using a stick or stem of a plastic bulb pipette, and the tube was inverted to discard the ether, fecal debris, and formaldehyde. The sediment was retained. The tube was returned to its upright position, and the fluid from the side of the tube could drain to the bottom. The bottom of the tube was tapped to suspend, and the sediment was mixed. A drop of the sediment was transferred to one end of a slide and another to the other end. A drop of iodine was mixed with one of the sediment parts, and a cover glass was used to cover each preparation. The entire preparation was examined microscopically using X10 objective with the condenser iris was closed sufficiently to give good contrast while the X40 objective was used to examine small cysts and eggs.
- Research Article
- Abstract
- Introduction
- Prevalence of Intestinal Parasitic Infections in Army Soldiers
- Methods and Materials of the Study
- Variables
- Stool Specimen Collection and Examination
- Data Quality
- Results
- Parasitological Results Discussion
- Socio-Demographical Results Discussion
- Bivariate Results Discussion
- Conclusion
- Limitations of the Study
- Recommendations
- Acknowledgement
- References
Data Quality
Reliability4 The reliability of the study was measured by predictive values of positive and negative tests.
Validity
The validity of the study was measured by the sensitivity and specificity.
Quality assurance
Pretested questionnaires were used during the study and laboratory procedures including collection and handling of specimens were carried out in accordance with standard protocols (Standard Operating Procedures). All tested slides were rechecked and supervised by senior diagnostic medical laboratory professionals. The samples were examined within the 30 minutes of preparation of the sample. The filled interview question was checked at the spot to check their completeness, consistency and clarity issues.
Data analysis techniques
Data was entered and analyzed by using statistical packages for social science (SPSS) 20 version of computer software, after checking its completeness. It was summarized in percentages and presented in tables, pie chart and bare graph. Association between risk factors and parasitological test results were assessed using chi-square with 95% CI and P-value less than 0.05 was considered as statistically significant. Chi-square (crosstab) was used to determine the association between the response variables (intestinal parasitic infections) and risk factors and their levels of significance (p < 0.05).
Ethical clearance
Ethical clearance was obtained from Institutional Health Research Ethics Review Committee of the Defense University College of Health Sciences. Also, the permission was obtained from the school camp. In addition, an informed signed and consent was obtained from participants of the study subjects after explaining the importance of the study briefly. Anyone who was not being willing to take part in the study would have full right to not be included in the study and confidentiality of the study participants were also maintained. Those army soldier students who were positive for intestinal parasites were treated freely based on their laboratory findings according to national standard treatment guideline.
- Research Article
- Abstract
- Introduction
- Prevalence of Intestinal Parasitic Infections in Army Soldiers
- Methods and Materials of the Study
- Variables
- Stool Specimen Collection and Examination
- Data Quality
- Results
- Parasitological Results Discussion
- Socio-Demographical Results Discussion
- Bivariate Results Discussion
- Conclusion
- Limitations of the Study
- Recommendations
- Acknowledgement
- References
Results
General characteristics of studied population
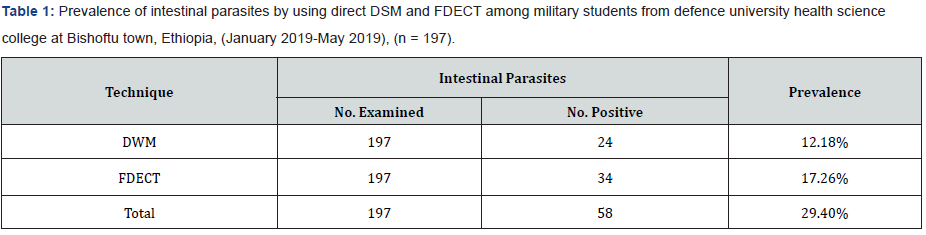
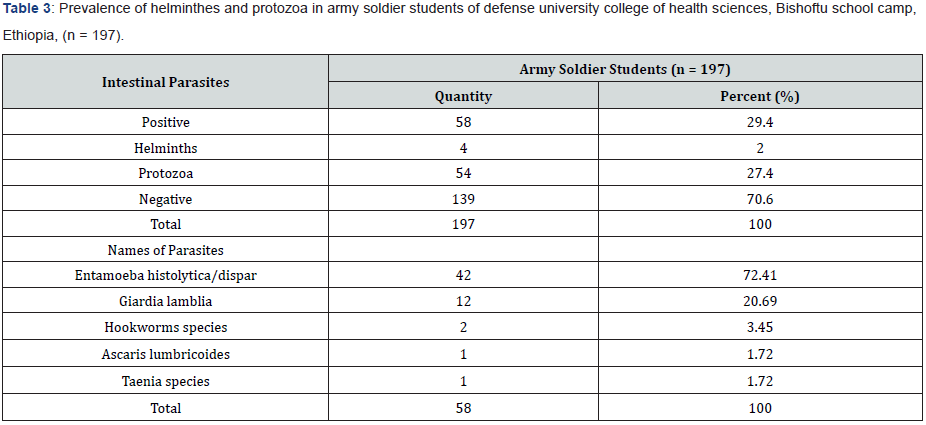
Among the 197 enrolled studied population, 159 (80.7%) male and 38 (19.3%) female were army soldier students, including civilian 39 (19.8%), non-commissioned officers 61(31.0%), and commissioned officers 97 (49.2%). Of the study subjects 58 subjects (29.4%) were infected and 139 subjects (70.6%) were not infected with parsite. In this study the intestinal parasites were diagnosed in 197 studied army soldier students and the prevalence was 29.4%. Among these, Entamoeba histolytica/ dispar (72.41%), Giardia lamblia (20.69%), Hookworms species (3.45%), Ascaris lumbricoides (1.72%) and Taenia species (1.72%). The most common protozoans found were Entamoeba histolytica/ dispar (72.41%) and Giardia lamblia (20.69%). The most common helminthes found were hookworm species (3.45%), Ascaris lumbricoides (1.72%) and Taenia species (1.72%). The prevalence of intestinal parasites in this population is presented in Tables 1 & 2. Hookworm species (1.72%), Ascaris lumbricodes (1.72%) and Taenia species (1.72%) were the most frequent helminths found by formol diethyl ether concentration technique (FDECT) (5.2%). The Direct Smear Microscopy (DSM) method did not detect any Ascaris lumbricodes (1.72%) and Taenia species (1.72%) ova in stools in any of the 197 stool specimens (Figure 1 & 2).
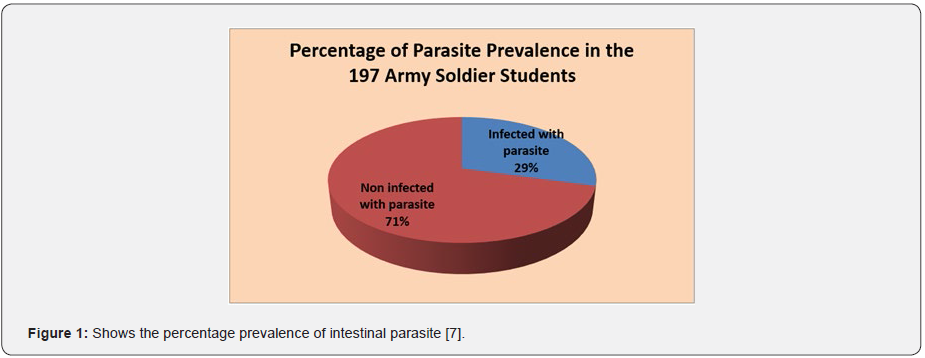
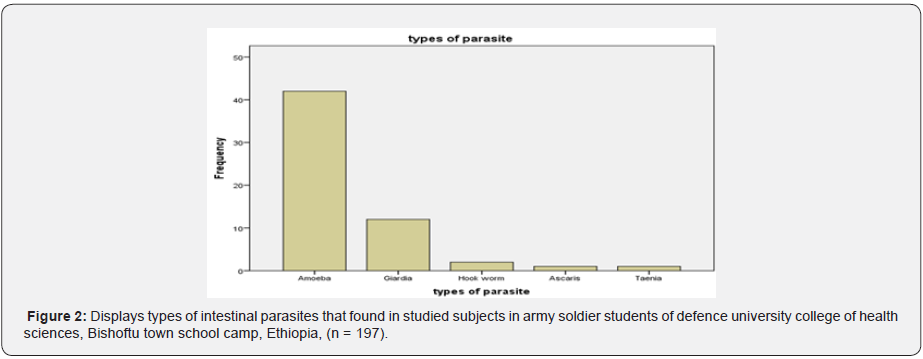
Sociodemographic characteristics of the study subjects
As shown in Tables 3 & 4, Entamoeba histolytica/dispar was the most common protozoa found in this population with the prevalence of 21.3%, followed with Giardia lamblia (6.1), Hookworm species (1.0%), Ascaris lumbricoides 0.5%, and Taenia species (0.5%).
Parasitological results of the studied subject
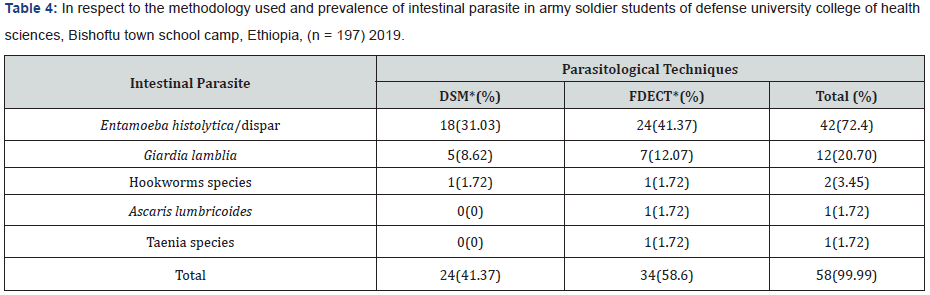
* DSM: Direct Smear Microscopy * FDECT: Formyl Diethyl Ether Concentration Technique
Associated risk factors vs intestinal parasitic infections
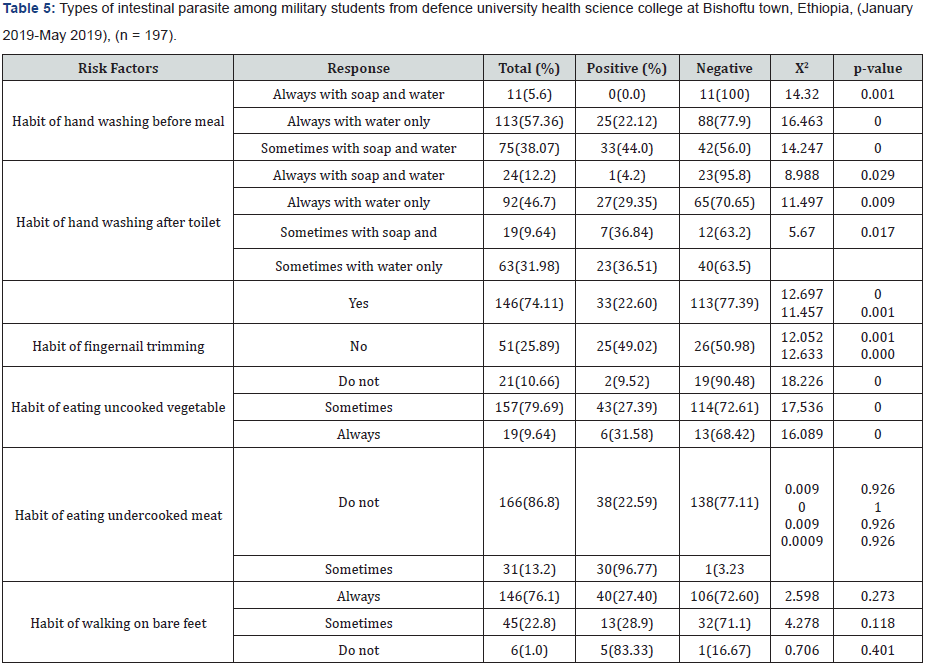
Types of intestinal parasite versus gender, age, military rank and level of education
There are many different risk factors associated with intestinal parasitic infections. The multivariate analysis for these factors was given in Tables 2 & 5 below.
Prevalence of intestinal parasite by using DSM and FDECT
Out of 197 subjects, 24 (12.18%) and 34(17.26%) were positive for intestinal parasites when using direct wet mount and FDECT respectively (Table 1).
Comparison Between Direct Wet Mount [DWM] and FDECT
Out of 197 stool samples, 24 (12.18%) were positive for intestinal parasites when using direct wet mount, 8 samples were negative by direct wet mount and positive by FDECT, while 34 (17.26%) were found to be positive by FDECT (p=0.000) (Table 6).
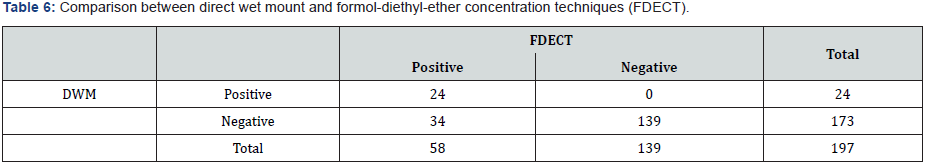
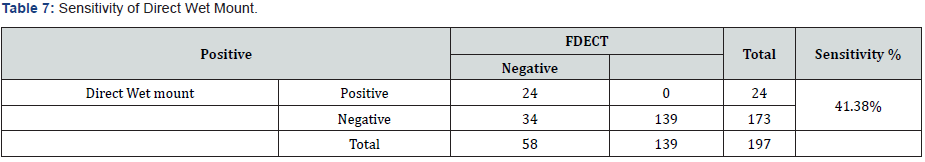
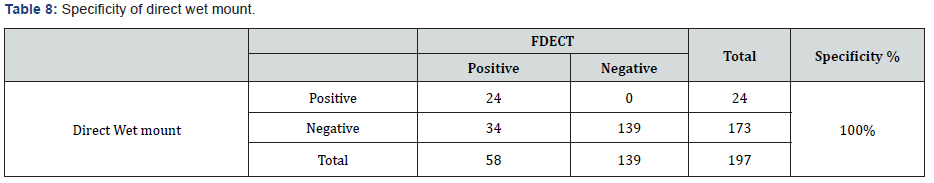
According to the formula mentioned in materials and methods the sensitivity of direct wet mount was (41.38%) (Tables 1 & 7). While the specificity was (100%) (Tables 7 & 8).
- Research Article
- Abstract
- Introduction
- Prevalence of Intestinal Parasitic Infections in Army Soldiers
- Methods and Materials of the Study
- Variables
- Stool Specimen Collection and Examination
- Data Quality
- Results
- Parasitological Results Discussion
- Socio-Demographical Results Discussion
- Bivariate Results Discussion
- Conclusion
- Limitations of the Study
- Recommendations
- Acknowledgement
- References
Parasitological Results Discussion
Intestinal parasites are a global public health problem by affecting about three billion people around the world. They present various degree of prevalence in areas where there is overcrowding, poor environmental sanitation, and personal hygienic practices especially in developing country like Ethiopia. In army communities the infections are very high next to children due to their deployment in harsh environment for the mission. In our study, five types of intestinal parasite such as Entamoeba histolytica/dispar (72.41%), Giardia lamblia (20.69%), Hookworms species (3.45%), Ascaris lumbricoides (1.72%) and Taenia species were seen (1.72%). These all five types of intestinal parasites were found by using two types of parasitological techniques such as Direct Smear Microscopy (DSM) and Formol Diethyl Ether Concentration Technique (FDECT). These techniques used in the research and its significance in identifying the parasite were indicated in Tables 1 & 5. The FDECT method was found more sensitive and specific in the finding intestinal parasites than DSM method. Especially, in the case of Ascaris lumbricoides (1.72%) and Taenia species (1.72%) identification. The FDECT is largely superior to the direct smear microscopy method for detection of intestinal parasites and these findings are confirmed in our study. Moreover, we found that it has a higher sensitivity than the DSM for detection of helminths larvae. This suggests that FDECT should be added to the screening tests when carrying epidemiological studies in highly endemic areas for helminths.
Without ruling out a possible acquisition of infection during duty activities, our study assessed the prevalence of intestinal parasitic infections and associated risk factors among the army soldier students of DUCHS at Bishoftu town school camp. The overall prevalence of intestinal parasites in this study was (29.4%), which is very low compared with that reported by [32], in Latin America of Peru army soldier(90.4%), (53.9%) and 2002 (55.7%) from Thailand army soldiers in Nakornrachasrima province. However, it was relatively higher compared with that the same study carried out by [33] (22.4%) in Thailand on army soldiers and (15.4%) in U.S. military personnel in Honduras. Defense Health College is a comparatively sanitized school camp with aware health sciences students learning in the college. Furthermore, the periodic campaign of basic health care in the college could possibly explain the lower prevalence of intestinal parasitic infections seen in this study.
In the present study, the overall prevalence of intestinal parasitic infections observed among the studied army soldier student population was (197, 29.4%) its low findings compared with the study conducted in Latin America among Peru army by, which revealed that (104, 90.4%) of the population harbored intestinal parasites. Found that 90.4% of the soldiers were infected with some pathogenic parasite. There was two parasites per person on average, which highlights the high burden of intestinal parasites in this particular population. The maximum number of different parasite species in one individual was seven. In our case, the maximum number of different parasite species in one individual was only two with a prevalence rate of 91.38% was monoparastism and 8.62% polyparasitism. This may be attributed to the fact that the studied army had access to good water supply and adequate sanitary facilities at school camp.
Global statistics on the prevalence of Entamoeba histolytica infection indicates that 90% of infected individuals remain asymptomatic carriers while the other 10% develop clinically apparent disease. The most prevalent intestinal protozoan parasites in Ethiopia are Giardia lamblia and Entamoeba histolytica/ dispar. Our study showed significantly higher prevalence of Entamoeba histolytica/dispar (72.41%) compared with that reported by in Latin America from Peru army soldiers (6%) but relatively the same prevalence of Giardia lamblia (20.69%) compared with that found from Peru army (18%). The prevalence of Hookworms infection (3.45%) in this study was lower than the study conducted by in Peru which showed very high (47.1%). The prevalence of Ascaris lumbricoidis (1.72%) compared with that studied by Maria et al. in Peru army soldiers (27.9%). Entamoeba histolytica/dispar infection was the most important intestinal protozoan recorded in our present study. The overall prevalence of Entamoeba histolytica/dispar infection among the studied army soldier students was 72.41%. This could be attributed to high transmission of the parasite due to poor water and food handling at the mess and inadequate sanitary facilities in the studied army. In relation to the individual sex and military ranks, the prevalence rates of Entamoeba histolytica/ dispar infection were 80.95%, 19.05%, 50%, 26.19% and 23.81% in male and female army, commissioned and non-commissioned officers, and privates respectively (Tables 1 & 6). The high prevalence of Entamoeba histolytica/dispar (72.41%) and Giardia lamblia (20.69%), which highlights the high fecal-oral contamination that may be related to poor handling of water and food in the mess.
The investigation also reveals a prevalence of 2.03% intestinal helminth infections which comprise Hookworm (3.4%), Ascaris lumbricoides (1.72%) and Taenia species (1.72%). The overall prevalence 2.03% was much lower than a study conducted by among army soldiers in Peru, Latin America. She recorded Hookworm (47.1%) and Ascaris lumbricoides (27.9%) prevalence of intestinal helminth infection. Being hookworm (3.45%) the most common helminthic parasite found in this population, one could speculate that the most common route of transmission in these subjects was through the skin penetration (i.e. feet in mud or contaminated water/soil with hookworm larvae). Therefore, this could be due to soil contamination in fact of that the younger army soldier students were very active to play football sometimes with bare feet on dusty poor ground; that they may come into contact with soil and water that contaminated with these parasites’ eggs/ larvae. This study showed relatively high prevalence of Taenia (1.72%) compared with that reported by in Thailand (0.35%). This attributed to that high consumption of raw meat in the studied population than that of Thai army.
- Research Article
- Abstract
- Introduction
- Prevalence of Intestinal Parasitic Infections in Army Soldiers
- Methods and Materials of the Study
- Variables
- Stool Specimen Collection and Examination
- Data Quality
- Results
- Parasitological Results Discussion
- Socio-Demographical Results Discussion
- Bivariate Results Discussion
- Conclusion
- Limitations of the Study
- Recommendations
- Acknowledgement
- References
Socio-Demographical Results Discussion
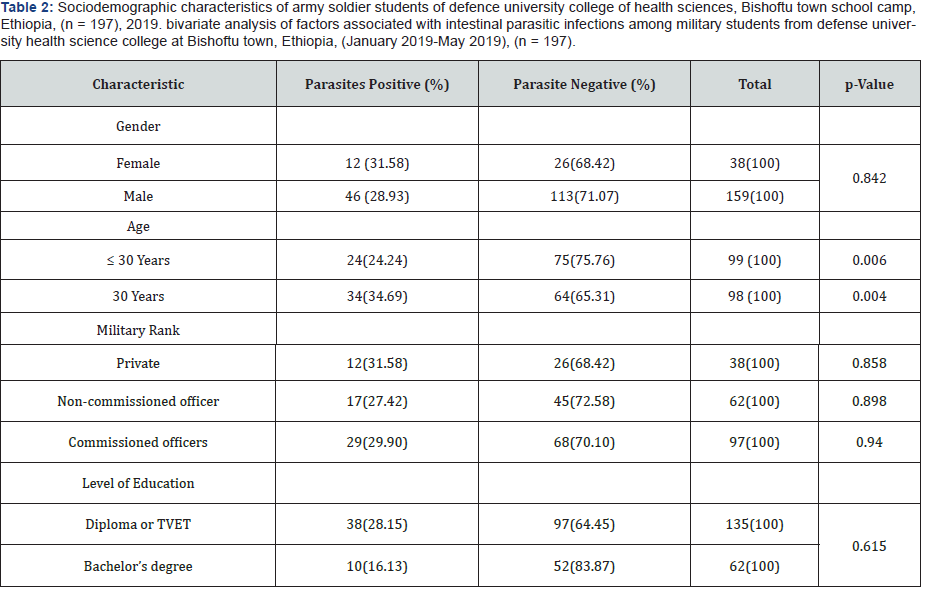
According to the gender, the prevalence of parasitic infections was seen higher in female (31.58 %) than male (28.93%) army soldier students. However, the study conducted. In Thai Army in Thailand showed that the prevalence of parasite infection was slightly higher in male army (22.6%) than female army (20.0%). This indicated that the gender may or may not play the role in intestinal parasitic infection depending up on the region and other harsh environmental or behavioral factors due to their mission. In general, the increased mobility of the male army to different duty activities increases the risk of infection among them, while female army have more contact with contaminated food items and eat raw vegetable with prepared food more often than male’s army. According to the age groups of the study subjects, the prevalence of intestinal parasite was statistically very significant p< 0.05. It was 24.24% in younger age active army soldier students (≤30- year old), especially those who enrolled recently as compared to >30 years older army members (34.69%). This may be related to good basic health care by younger active army members. This in turn attributed to grooming practice in search of mate or to be alert and neat as a military science rule.
According to the military rank, the prevalence of parasitic infection was higher among privates (31.58%) than commissioned officers (29.90%) followed by non-commissioned officers (27.42%). It was not significantly different among commissioned and non-commissioned officer (Table 1). The high prevalence of parasites in privates may be attributed to their low income which drives them to use substandard catering service providers out of the school camp, or further study is needed. Similar result has been reported by [34] in Thailand army that the privates had significantly higher risk of acquiring intestinal parasitic infections than non-commissioned and commissioned officers. Regarding to level of education of the study group the prevalence of intestinal parasite in diploma level army members was 28.15% whereas it was 16.13% in bachelor’s degree holders. The intestinal parasitic infection was significantly higher among the army soldier students whose level of education was diploma (35.55%) than those firstdegree army soldiers (16.13%). The study carried out by [35] also showed high prevalence of intestinal parasitic infection in Thai army whose education level was diploma (20.8%) than that firstdegree army (13.0%).
- Research Article
- Abstract
- Introduction
- Prevalence of Intestinal Parasitic Infections in Army Soldiers
- Methods and Materials of the Study
- Variables
- Stool Specimen Collection and Examination
- Data Quality
- Results
- Parasitological Results Discussion
- Socio-Demographical Results Discussion
- Bivariate Results Discussion
- Conclusion
- Limitations of the Study
- Recommendations
- Acknowledgement
- References
Bivariate Results Discussion
Due to the bivariate analysis, in this study the associated risk factors such as hand wash before meal and after defection, fingernail trimming and eating undercooked vegetable were statically very significant p <0.05. Among the total of 197 respondents 11 or 5.6% responded that they wash their hand always with soap and water, 113(57.36%) always with water and 75(38.07%) sometimes with soap and water. However, among those 11 responded Always with soap and water only one respondent (9.09%) was found to be infected and 90.01% not infected. Since washing with soap and water before meal is strongly related with prevention of intestinal parasite, only 9.09% are infected. Those responded Sometimes with soap and water are prone to intestinal parasite infection because these parasites are transmitted fecal to mouth route and unwashed hand would be the source of contamination. This is confirmed by the fact that out of the total 75 respondents about 33(44%) are infected with the parasite.
Among the respondents for the habit of hand washing after toilet the most significant relationship goes to those responded sometimes with water only p<0.05. Among these respondents 36.84% of them are infected with intestinal parasite. Since intestinal parasite transmission is via fecal to mouth route. Therefore, hand washing after toilet reduces contamination of hand with fecal material and hence reduces transmission of parasite. The best way of washing hand to reduce parasite infection will be always washing with soap and water. Those who wash their hand after toilet regularly indicated the lowest rate of infection (4.2%). Habit of fingernail trimming is related to intestinal parasite infection in that waste in the fingernail will be a source of fecal hand to mouth infection. Those trim their finger regularly showed lower infection (22.60%) as compared to the non-trimmers (49.02%). Those respondents who always eat uncooked vegetable 31.58% are infected with intestinal parasite infection and it was statistically significant p <0.05. Because uncooked vegetable can be contaminated with parasites either on the farmland with infected water or during handling, transportation and preparation. Out of the study subjects, those who sometimes ate undercooked meat were higher than (96.77%) those who did not eat it.
According to the army soldier students that using soap and water always before eating meal had significantly lower prevalence of intestinal parasitic infection (9.09%) when compared with hand washing only using water (22.12%) and who did wash hand sometimes with soap and water (29.35%) (Table 1). This study showed that the army soldier students washing hand with soap and water after defecation had significantly lower prevalence of intestinal parasitic infection (4.2%) when compared with washing hand only using water (29.35%) and sometimes using soap and water (36.84%). The similar studies conducted in Thailand also showed lower prevalence of parasitic infection those who were using soap and water for hand washing after defecation than using only water Regular wearing of closed-shoes had a significantly lower prevalence of intestinal parasitic infections (25.89%) than those did not wear closed-shoes (74.11%). A similar study conducted in Peru army soldiers, in Latin America also showed that regular wearing of closed shoes had a significant contribution to the low prevalence rate of intestinal parasitic infections (P < 0.05).
- Research Article
- Abstract
- Introduction
- Prevalence of Intestinal Parasitic Infections in Army Soldiers
- Methods and Materials of the Study
- Variables
- Stool Specimen Collection and Examination
- Data Quality
- Results
- Parasitological Results Discussion
- Socio-Demographical Results Discussion
- Bivariate Results Discussion
- Conclusion
- Limitations of the Study
- Recommendations
- Acknowledgement
- References
Conclusion
Our study has revealed high prevalence of intestinal protozoa (27.4%) and low intestinal helminth infection (2.0%) among the studied army soldier students at Defense Health Sciences College in Bishoftu town school camp, Ethiopia. Entamoeba histolytica/ dispar (21.32%), Giardia lamblia (6.1%) and Hookworm species (1.0%) were the predominant pathogen recorded with a lower percentage having 0.5% Ascaris lumbricoides and Tania species (0.5%).
The differences in the rates of most of the pathogenic protozoa (Entamoeba histolytica/dispar, Giardia lamblia) and helminths (Hookworm, Ascaris lumbricoides and Taenia) infections recorded among the studied army soldier students in our study school camp were statistically significant (p < 0.05) with risk factors such as age but no other factors. The age groups most affected were greater than thirty (>30, 34.69%) years followed by less than or equal thirty (≤30, 24.24%) years age groups in all the army soldier students. Female army soldier students (31.58%) recorded the highest of intestinal parasitic infections than males (28.93%) among the studied army soldier population. Those who were infected with pathogenic parasitic infection were treated with proper antiparasitic drugs. In addition, instruction to prevent themselves from acquiring intestinal parasitic infections i.e. foodborne parasite, waterborne parasite and soil-transmitted helminthes were provided to the studied population.
In general, this study shows a high prevalence of helminthes, most commonly hookworm and protozoans, most commonly Entamoeba histolytica/dispar followed by Giardia lamblia, in army soldier students from DUCHS and we propose to include the army soldier students from this school camp were relatively risk populations for intestinal parasites especially protozoans. Moreover, when we compare with other studies that carried out in army soldiers the prevalence of overall intestinal parasitic infection was low among army soldier students of Defense Health Sciences College. The lower prevalence in the current study could be attributed to the fact that most of the studied army soldiers had adequate sanitary facilities, good knowledge and attitudes being their healthy students, good water supply and practiced proper hygiene (hand washing before eating and after defecation) and the common reason to this fact that all compered study was developed country articles [36-39].
- Research Article
- Abstract
- Introduction
- Prevalence of Intestinal Parasitic Infections in Army Soldiers
- Methods and Materials of the Study
- Variables
- Stool Specimen Collection and Examination
- Data Quality
- Results
- Parasitological Results Discussion
- Socio-Demographical Results Discussion
- Bivariate Results Discussion
- Conclusion
- Limitations of the Study
- Recommendations
- Acknowledgement
- References
Limitations of the Study
Limitations of this study are the sample size, the single study setting and testing methods. Ideally, three consecutive days samples and multiple army sites, especially active army on duty outside of the camp, may be more representative of the population. Ethyl acetate and real-time PCR testing techniques and methods are the best to detect and differentiate intestinal parasites. Diagnosis of intestinal parasites is confirmed by the recovery of protozoan trophozoites and cysts, helminth eggs and larvae in the clinical parasitology laboratory. Due to the low density of the parasites in the faces, the sensitivity, specificity and accurate microscopic identification of intestinal parasitic infections will depend on the method used, the number of stools analyzed, and the quantity of parasites excreted per sample. In our current study direct smear microscopy and formyl ether concentration methods were used and the result was based on a single sample obtained from individual participants. If three stool samples from each participant had been analyzed, the sensitivity of the results would have been enhanced. Diethyl ether was used in place of ethyl acetate as faucal fat extraction solvent. This was because it was very difficult to obtain ethyl acetate on the market. Ethyl acetate is a good faucal fat extractor as compared to diethyl ether because it provides clear sediment but may also end up extracting some of the parasite. In case of Entamoeba histolytica/dispar, very wide range of prevalence (72.41%) has been reported whether they were pathogenic Entamoeba histolytica or non-pathogenic Entamoeba dispar. In fact, of their species similarity we did not differentiate their quantities by parasitological methods. They further distinguished in a real-time polymer chain reaction (PCR) with primers-based on the SSU-rRNA gene sequence.
- Research Article
- Abstract
- Introduction
- Prevalence of Intestinal Parasitic Infections in Army Soldiers
- Methods and Materials of the Study
- Variables
- Stool Specimen Collection and Examination
- Data Quality
- Results
- Parasitological Results Discussion
- Socio-Demographical Results Discussion
- Bivariate Results Discussion
- Conclusion
- Limitations of the Study
- Recommendations
- Acknowledgement
- References
Recommendations
Based on the findings from the study, it is recommended that the Defense Health Sciences College authorities in parallel with Ministry of Defense department and other stakeholders should conduct periodical health promotion in this army community and academics specifically on the modes of transmission, prevention and control of the intestinal parasites, environmental sanitations, personal hygiene and their impact on the magnitude occurrence of these intestinal parasitic infections. it needs adequate provision of sanitary facilities in college and in the army school camps. Food handlers at the school camp should need to screen periodically for protozoa and helminths to prevent the transmission of the infection through the food they handle and prepare at mess. School camp deworming programmers and health educations (especially on personal hygiene practices) should be conducted in the study school camp to minimize the spread of intestinal parasitic infections. Be that as it may, providing an effective basic health care and a massive drug administration may be the next step as intervention in this relatively risk population. To the best of our knowledge, providing effective basic health care massive drug administration against intestinal parasites in highrisk populations may be a cost-effective intervention to reduce the burden of disease. It is recommended that ethyl acetate and real time PCR should need to be used; three stool samples and multi setting samplings should need to obtain from individual participants to effectively detect the parasites if present during future study. Future studies should need to focus on asymptomatic carriers and deworming of this military population with monitoring of infections or resistance.
- Research Article
- Abstract
- Introduction
- Prevalence of Intestinal Parasitic Infections in Army Soldiers
- Methods and Materials of the Study
- Variables
- Stool Specimen Collection and Examination
- Data Quality
- Results
- Parasitological Results Discussion
- Socio-Demographical Results Discussion
- Bivariate Results Discussion
- Conclusion
- Limitations of the Study
- Recommendations
- Acknowledgement
- References
Acknowledgement
We would like to acknowledge the Department of public health, defense College of health science for the remarkable efficiency of their service and dedication to duty. Our special thanks go to all study participants for their participation, without them this study would not have been realized. Finally, I extend my appreciation to all my friends and family members for their support during my academic studies, because without them, nothing would be possible.
- Research Article
- Abstract
- Introduction
- Prevalence of Intestinal Parasitic Infections in Army Soldiers
- Methods and Materials of the Study
- Variables
- Stool Specimen Collection and Examination
- Data Quality
- Results
- Parasitological Results Discussion
- Socio-Demographical Results Discussion
- Bivariate Results Discussion
- Conclusion
- Limitations of the Study
- Recommendations
- Acknowledgement
- References
References
- Adamu H, Endeshaw T, Teka T, Kife A, Petro B (2006) Prevalence of intestinal parasite. Ethiopia J Health Div 20(1): 39-47.
- Ahmed AK, Malik B, Shaheen B, Yasmeen G, Dar JB (2003) Frequency of intestinal parasitic infestation in children of 5-12 years of age in Abbottabad. J Ayub Med Coll Abbottabad 15(2): 28-30.
- Aklilu A, Kahase D, Dessalegn M, Tarekegn N, Gebremichael S, et al. (2015) Prevalence of intestinal parasites, salmonella and shigella among apparently health food handlers of Addis Ababa University student’s cafeteria, Addis Ababa, Ethiopia. BMC Res Notes 8(1): 17.
- Allen AM, Taplin D, Legters LJ, Ferguson JA (1974) Letter: Schistosomes in Vietnam. Lancet 1(7867): 1175-1176.
- Amster LJ (1985) Bailey Ashford: prophet of tropical medicine. HospPract 20(4): 183-206.
- Ashford RW, Crewe W (1998) The parasites of Homo sapiens. Cromwell Press, Liverpool.
- Ayeh-Kumi PF, Quarcoo S, Kwakye-Nuako G, Kretchy JP, Osafo-Kantanka A (2009) Prevalence of Intestinal Parasitic Infections among Food Vendors in Accra, Ghana. J Trop Med Parasitol 32(1): 1.
- Kwa BH, Aviles R, Tucker MS, Sanchez JA, Isaza MG, et al. (2004) Surveillance for Enteric Parasites among US Military Personnel and Civilian Staff on Joint Task Force Base-Bravo in Soto Cano, Honduras and the Local Population in Comayagua and La Paz, Honduras. Mil Med 169(11): 903-908.
- Brooker S, Hotez PJ, Bundy DA (2008) Hookworm-Related Anaemia among Pregnant Women: A Systematic Review. PLoS Negl Trop Dis 2(9): e291.
- Escobedo AA, Almirall P, Alfonso M, Cimerman S, Rey S, et al. (2009) Treatment of intestinal protozoan infections in children. Arch Dis Child 94(6): 478-482
- Forman DW, Tong MJ, Murrell KD, Cross JH (1971) Etiologic study of diarrheal diseases in Vietnam. Am J Trop Med Hyg 20(4): 598-601.
- Frickmann H, Schwarz NG, Wiemer DF Fischer M, Tannich E, et al. (2013) Food and drinking water hygiene and intestinal protozoa in deployed German soldiers. Eur J MicrobiolImmunol 3(1): 53-60.
- Garcia LS, Bruckner DA (1997) Diagnostic Medical Parasitology. American Society for Microbiology, Washington, USA.
- Greenberg JH (1969) Public health problems relating to the Vietnam returnee. JAMA 207(4): 697-702.
- Huh S, Lee SU, Huh SC (1994) A follow-up examination of intestinal parasitic infections of the Army soldiers in Whachon-gun, Korea. Korean J Parasitol 32(1): 61-63.
- Kumie A, Ali A (2005) An overview of environmental health status in Ethiopia with particular emphasis to it is organization, drinking water and sanitation: A literature survey. Ethiop J Health Dev 19(2): 89-103.
- Lalkhen A, McCluskey A (2008) Clinical tests: sensitivity and specificity. British Journal of Anaesthesia 8(6): 221-223.
- Bandarsidas (2006) Park’s textbook of preventive and social medicine. Bhanot Publishers, Jabalpur, India.
- Maizels RM, Yazdanbakhsh M (2003) Immune regulation by helminths arasites: cellular and molecular mechanism. Nat Rev Immunol 3(9): 733-744.
- María S Gallardo, Marilhia Cornejo, George Vasquez, Renato Errea, Jorge Urquiaga (2015) High prevalence of intestinal parasites among soldiers in Peru: another population at risk. Peru J Parasitol 19(2): e60-e67.
- Marshall MM, Naumovitz D, Ortega Y, Sterling CR (1997) Waterborne protozoan pathogens. ClinMicrobiol Rev 10(1): 67-85.
- Matthys B, Bobieva M, Karimova G, Mengliboeva Z, Jean-Richard V, et al. (2011) Prevalence and risk factors of helminths and intestinal protozoa infections among children from primary schools in western Tajikistan. Parasit Vectors 4: 195.
- Noor Azian MY, San YM, Gan CC, Yusri MY, Nurulsyamzawaty Y, et al. (2007) Prevalence of intestinal protozoa in an aborigine community in Pahang, Malaysia. Trop Biomed 24(1): 55-62.
- Olsen A, Samuelsen H, Onyango-Ouma W (2001) A study of risk factors for intestinal helminth infections using epidemiological and anthropological approaches. J Bio Sci 33(4): 569-584.
- Ortega YR, Eberhard ML, Kris H (2008) Protozoan diseases: cryptosporidiosis, giardiasis and other intestinal protozoan diseases, pp. 354-366. In International encyclopedia of public health. Academic Press, Oxford, United Kingdom.
- Ouattara MN, Guessan NA, Yapi AN, Goran EK (2010) Prevalence and spatial Distribution of Entamoeba histolytica/dispar and Giardia lamblia among schoolchildren in Agboville area (Côte d'Ivoire). PLoS Negl Trop Dis 4: e574.
- Peterson KM, Singh U, Petri WA (2011) Enteric Amebiasis. Tropical Infectious Diseases: Principles, Pathogens, and Practice, (3rd edn), In: Guerrant R, Walker DH, Weller PF (Eds.), Elsevier, Philadelphia, pp. 614.
- Quihui L, Valencia ME, Crompton DW, Phillips S, Hagan P, et al. (2006). Role of the employment status and education of mothers in the prevalence of intestinal parasitic infections in Mexican rural schoolchildren. BMC Public Health 6: 225.
- Rodríguez-Morales AJ, Barbella RA, Case C, Arria M, Ravelo M, et al. (2006) Intestinal parasitic infections among pregnant women in Venezuela. Infect Dis Obstet Gynecol 23125.
- Sackey ME, Weigel MM, Armijos RX (2003) Predictors and nutritional consequences of intestinal parasitic infections in rural Ecuadorian children. J Trop Pediatr 49(1): 17-23.
- Leelayoova S, Siripattanapipong S, Naaglor T, Taamasri P, Mungthin M (2009) Prevalence of Intestinal Parasitic Infections in Military Personnel and Military Dogs Thailand: J Med Assoc Thai 92(Suppl 1): S53-S59.
- Sehgal R, Gogulamudi VR, Jaco JV, Atluri VSR (2010) Prevalence of intestinal parasitic infections among school children and pregnant women in a low socio-economic area, Chandigarh, North India. Reviews of infection (RIF) 1(2): 100-103.
- Stauffer WM, Weinberg M (2009) Emerging clinical issues in refugees. Curr Opin Infect Dis 22 (5): 436-442.
- Stoltzfus RJ, Chwaya HM, Tielsch JM, Schulze KJ, Albonico M, et al. (1997) Epidemiology of iron deficiency anaemia in Zanzibari school children: the importance of hookworms. Am J Clin Nutr 65(1): 153-159.
- Taamasri P, Mungthin M, Rangsin R, Tongupprakarn B, Areekul W, et al. (2000) Transmission of intestinal Blastocystosis related to the quality of drinking water. Southeast Asian J Trop Med Public Health 31(1): 112-117.
- Takafuji ET, Kelley PW, Weiner H, Milhous N, Miller R (1984) An Outbreak of Hookworm Infection in the 82nd Airborne Division following Operation Urgent Fury in Grenada, November 1983 to January 1984. Washington, DC, Epidemiology Consultant Service (EPICON) Report.
- Vandenberg O, Van Laethem Y, Souayah H, Kutane WT, van Gool T (2006) Improvement of routine diagnosis of intestinal parasites with multiple sampling and SAF-fixative in the triple-faeces-test. Acta Gastroenterol Belg 69(4): 361-366.
- WHO (2000) Conquering Suffering Enriching Humanity? Report of an Informal Consultation, WHO.CWS, Geneva, Switzerland.
- WHO (2012) Eliminating Soil-Transmitted Helminthiasis as a Public Health Problem in Children Soil-Transmitted Helminthiasis? Progress Report 2001-2010 and Strategic Plan 2011-2020.






























