Action of Oroxylin to Membrane Permeability of Rat Liver Mitochondria
Nurali Ergashev1, Esokhon Komilov1, Muzafar Asrarov1, Sabina Gayibova2, Komila Eshbakhova2 and Ulugbek Gayibov2*
1Institute of Biophysics and Biochemistry, National University of Uzbekistan, Uzbekistan
2Institute of Bioorganic chemistry, Academy of Sciences, Uzbekistan
Submission: October 14, 2019; Published: December 06, 2019
*Corresponding author: Ulugbek Gayibov, Institute of Bioorganic chemistry, Academy of Sciences, 83, Mirzo Ulugbek Str, Tashkent, Uzbekistan
How to cite this article: Nurali Ergashev, Esokhon Komilov, Muzafar Asrarov, Sabina Gayibova, Komila Eshbakhova, Ulugbek Gayibov. Action of Oroxylin to Membrane Permeability of Rat Liver Mitochondria. 2019; 15(1): 555905. DOI: 10.19080/AIBM.2019.14.555905
Abstract
The effect of the flavonoid oroxylin A on the state of PTP (permeability transition pore) and oxidative phosphorylation of mitochondria was studied. It was shown that a flavonoid dose-dependently inhibits the discovery of PTP mitochondria, which is possibly due to its antioxidant properties.
Keywords: Scutellaria guttata; Transition pore; Megapore; Membranes; Antioxidant activity
Introduction
Mitochondria are a mobile intracellular integrator of metabolic and ionic signaling. They generate adenosine triphosphate by oxidative phosphorylation, integrate signaling cascades leading to the release of proapoptotic factors, and form cellular signals, absorbing and releasing Ca2+ ions [1]. Thus, mitochondria regulates many physiological and biochemical processes that occur in cells and tissues of a living organism. Pathology reveals functional disorders of these structures involved in the regulation of permeability of mitochondrial membranes [2,3] and can be corrected with biologically active compounds. In this regard, it is relevant at present and is considered as one of special significance, aimed at elucidating the mechanism of action of potential pharmacological agents. Biologically active compounds, including flavonoids, have a very wide spectrum of action on biological objects, demonstrating antioxidant, cytoprotective, hepatoprotective, antihypoxic, membranotropic effects, etc. [4-7]. In the manifestation of some physiological effects of flavonoids on a cell, a certain role can be played by their ability to influence the energy and permeability of mitochondrial membranes. The purpose of this work was to study the effect of the flavonoid oroxylin A on the state of Ca2+-dependent PTP of rat liver mitochondria in in vitro experiments.
Oroxylin A was isolated from the Scutellaria guttata plant. Mitochondria from the liver of white outbred rats were isolated by differential centrifugation [8]. The protein content of mitochondria was determined by the Biuret method [9]. The parameters of the state of Ca2+-dependent PTP of mitochondria were recorded by the change in light scattering of a suspension of mitochondria (0.3-0.4 mg protein/ml) at 540 nm [10]. The Incubation Medium (IM) contained (in mM): sucrose - 200, KH2RO4 - 1, succinate - 5, Ca2+ -EDTA buffer 0.02 - μM, Hepes - 20, Tris-HCl - 20, rotenone - 0.002, oligomycin - 1 μg/ml, pH 7.2. The data obtained were processed using the Origin 6.1 software package. A value of P <0.05 was considered as a criterion an indicator of the significance of differences.
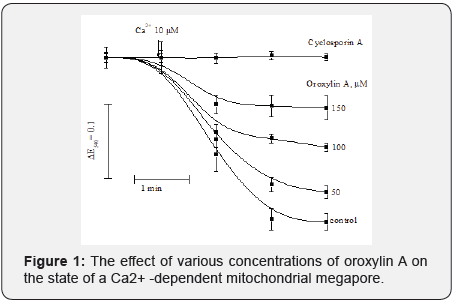
The introduction of the PTP inducer into the incubation medium of Ca2+ ions at a concentration of 10 μM causes mitochondrial swelling (Figure 1, control.) that indicates its transition to the open state. Cyclosporin A (CsA), a specific PTP inhibitor, prevents mitochondrial swelling under these conditions (Figure 1, CsA), i.e. PTP, despite the presence of Ca2+ ions, remains closed. Oroxylin A thus, at a concentration of 50 μM in the presence of Ca2+ ions, inhibits the opening of PTP by 18.6%, and when the concentration increases to 100 and 150 μM, it inhibits its opening by 45.6 and 69.7%, respectively, compared with the control. Thus, the results obtained indicate that oroxylin A has a concentration-dependent inhibitory effect on rat liver PTP.
Based on the results obtained in the previous series of experiments, it could be assumed that the induction of passive membrane permeability in the presence of various concentrations of oroxylin A will entail swelling of the organelles, which will cause a decrease in the membrane potential and the discovery of PTP. However, our results contradict this assumption. The experiments showed that oroxylin A simultaneously with an increase in passive permeability has an inhibitory effect on PTP. The observed effect of a flavonoid is probably related to its antioxidant properties [11], which manifest a stabilizing effect on membranes.
Conclusion
The transition of PTP to the open state is accompanied by swelling of mitochondria, a decrease in the membrane potential and rupture of the outer membrane, as a result of which the proteins of the intermembrane space, including proapoptotic ones, end up in the cytosol, where they can trigger one of the apoptosis pathways [12]. In human colon cancer cells, oroxylin A induces apoptosis by regulating uncoupling protein 2 (UCP2, regulating uncoupling protein 2). Moreover, its inhibition using siRNA UCP2 significantly increases the ROS-mediated activation of PTP in Caco-2 cells. Inhibition of UCP2 by oroxylin A led to a block of Bcl- 2 transfer into the mitochondria, but PTP was in this state open [13]. Oroxylin A initiates apoptosis of HepG2 cancer cells along the mitochondrial pathway, but this mechanism involves the Mitochondrial Apoptotic Channel (MAC), but not PTP [14]. As our results showed, because under in vitro conditions, oroxylin A inhibits the discovery of rat liver PTP, it can be assumed that in the mechanism of oroxylin A-induced apoptosis, its effect on PTP is not significant. Thus, a dose-dependent inhibition of rat liver PTP opening by oroxylin A was shown, which is presumably associated with the manifestation of its antioxidant properties.
References
- Poburko D, Santo Domingo J, Demaurex N (2011) Dynamic regulation of the mitochondrial proton gradient during cytosolic calcium elevations. J Biol Chem 286(13): 11672-1184.
- Jiao YH, Zhang Q, Pan LL, Chen XY, Lei KL, et al. (2015) Rat liver mitochondrial dysfunction induced by an organic arsenical compound 4-(2-nitrobenzaliminyl) phenyl arsenoxide. J Memb Biol 248(6): 1071-1078.
- Lai L, Jin JC, Xu ZQ, Ge YS, Jiang FL, et al. (2015) Spectroscopic and microscopic studies on the mechanism of mitochondrial toxicity induced by CdTe QDs modified with different ligands. J Memb Biol 248(4): 727-740.
- Asrarov MI (2015) The mechanism of action of flavone luteolin on the function of rat liver mitochondria. Problems of biol med and pharm chem 18(12): 38-43.
- Dorkina EG (2004) Investigation of the hepatoprotector action of natural flavonoids. Eksp Klin Farmakol 67(6): 41-44.
- Nikitina NA, Sobenin IA, Myasoedova VA, Korennaya VV, Mel Nichenko AA, et al. (2006) Antiatherogenic effect of grape flavonoids in an ex vivo Bull Exp Biol Med 141(6): 712-715.
- Kinoshita T, Lepp Z, Kawai Y, Terao J, Chuman H (2006) An integrated database of flavonoids. Biofactors 26(3): 179-188.
- Hageboom GH, Schneider WC, Pallade GE (1948) Cytochemical studies of mammalian tissues; isolation of intact mitochondria from rat liver; some biochemical properties of mitochondria and submicroscopic particulate material. J Biol Chem 172(2): 619-635.
- Gornall G, Bardawill C, David M (1949) Determination of serum proteins by means of the biuret reaction. J Biol Chem 177(2): 751-766.
- He L, Lemasters JJ (2003) Heat shock suppresses the permeability transition in rat liver mitochondria. J Biol Chem 278(19): 16755-1660.
- Su YL, Leung LK, Bi YR, Huang Y, Chen ZY (2000) Antioxidant activity of flavonoids isolated from Scutellaria rehderiana. Journal of the American Oil Chemists' Society 77(8): 807-813.
- Skulachev VP (2006) Bioenergetic aspects of apoptosis, necrosis and mitoptosis. Apoptosis 11(4): 473-485.
- Qiao C, Wei L, Dai Q, Zhou Y, Yin Q, et al. (2015) UCP2-related mitochondrial pathway participates in oroxylin A-induced apoptosis in human colon cancer cells. J Cell Physiol 230(5): 1054-1063.
- Liu W (2009) MAC-related mitochondrial pathway in oroxylin-A-induced apoptosis in human hepatocellular carcinoma HepG2 cells. Cancer Lett 284(4): 198-207.






























