Antimicrobial Activity of Fourteen Chinese Herbal Extracts
Huan Chen1, Lan Lin1, Renlin Zheng1, Liangchun Li1, Wenjiao Zhao2, Tianzhi Dai2 and Dequn Sun1*
1School of Life Science and Engineering, Southwest University of Science and Technology, China
2Marine College, Shandong University at Weihai, China
Submission: September 16, 2019; Published: October 30, 2019
*Corresponding author: Dequn Sun, School of Life Science and Engineering, Southwest University of science and technology, Mianyang, 621010, PR China
How to cite this article: Huan Chen, Lan Lin, Renlin Zheng, Liangchun Li, Wenjiao Zhao, Tianzhi Dai, Dequn Sun. Antimicrobial Activity of Fourteen Chinese Herbal Extracts. Adv Biotechnol Microbiol. 2019; 14(5): 555898. DOI: 10.19080/AIBM.2019.14.555898
Abstract
Drug resistance has drawn great attention from all over the world since it was put forward in 1900s. Infections caused by drug resistance have cost billions of dollars in clinic. Chinese herbs have been used as medicine in China for thousands of years with few adverse effects and drug resistance, it is very likely to find new drug candidates and medicine from Chinese herbs. In our work, fourteen commonly used Chinese herbal extracts were tested for their antibacterial activity against six typical bacteria. Most of them showed good inhibition to test bacteria. Especially, extracts 6, 14 exhibited very strong inhibition (MIC = 50, 10 μg/mL respectively) against S. aureus and S. epidermidis, which deserves further investigation in order to find potential activity compounds from the extracts.
Keywords: Antimicrobial activity; Chinese herbs; Extract; Gentiana scabra; Sophorae Flavescentis; Artemisia annua; Polygonum bistorta L; Agrimonia Pilosa; Chrysanthemum indicum; Hottuynia cordata Thunb; Gardeniae jasminoides; Viola philippica Cav; Gardeniae jasminoides Ellis; Astragalus membranaceus Bunge
Introduction
Nowadays, using antibiotics is the main method to protect people from infection of bacteria [1]. But the antimicrobial agents were limited, and resistance caused by antibiotics abuse has become a public health problem. The abuse of antibiotics which not only used in human life, but also widely used in aquaculture and livestock breeding has induced the production of super bacteria, meanwhile it has caused the water body of pollution, which is further harmful to human health [2]. And China is one of the countries with the most serious problem of antibiotic abuse, therefore, it is always meaningful to find new effective and low toxic antimicrobial candidates. Traditional Chinese Medicine (TCM) is a valuable resource in China, which has a long history in the treatment of various infectious diseases. And it also plays a fundamental role in traditional medicine in Korea, Japan, India, Egypt and other countries [3]. With the development of economy, Chinese herbal medicine has also gone abroad, which is favored by countries because of its low adverse reactions and non-pollution. After Tu You you won the Nobel Prize for discovering artemisinin which has a good anti-malaria effect, traditional Chinese herb has received more attention in the scientific community. For example, Ma, Feng et al researched the activity of H. pylori using 50 kinds of Chinese herbal medicine [4]. Su Pai-Wei et al. [5] studied the antibacterial activity and mechanism of Polygonum cuspidatum extracts direct ing at resistant pathogenic bacteria. In fact, many Chinese herbs have significant and broad-spectrum antibacterial activity. Some plants are also proved to be capable of improving health and be a gentle therapy of infection caused by virus without any significant side effects [6].
Here are some of the advantages of Chinese herbs
a. Traditional Chinese medicine possess good therapeutic effect for human according to the symptoms of the disease, especially for chronic disease or enhancing the body’s resistance to diseases. And complementary and alternative medicine use was more and more prevalent.
b. Herbal medicines could be used for complementary therapies, which aims to treat the entire person, because of its high value of studies [7].
c. The herbal extracts used as medicines have many potent or synergistic ingredients with diverse chemical structures. When they are used to treat disease, less resistance may be found than synthesized drugs such as antibiotics [8].
d. During the history of drug discovery, novel drug candidates were often exploited from herbal medicines [9]. In antibacterial area, the vast majority of the new chemical entities are natural products or derived molecules.

The long history of Chinese herbal medicine demonstrates the potential of plants as important sources of lead compounds. In order to have a further explore of Chinese medicinal herbs for the treatment of bacterial infections, it is highly necessary to separate the active components and find new lead compounds, or develop new Chinese medicine preparation, which could be used as anti-bacteria reagent. In this work, the antibacterial activity of 14 Chinese medicinal herbal extracts on six typical bacteria were measured. Extract of Magnolia officinalis Rehd. et Wils (Figure 1) is widely used clinically with many effects such as calming the central nervous and working as clinical antimicrobial agents for bacterial dysentery or inflammation with high safety, low side effects and easy to be excreted from the body [10]. Kyu reported the significant inhibitory activities of Magnolol and Honokiol against several human pathogenic fungi [11]. It could be used as lead compounds for the development of novel antifungal agents. Magnolol and Honokiol also have a marked antimicrobial effect against Micrococcus luteus and Bacillus subtilis [12]. We chose Magnolol (98%) as the positive control in our initial test. In order to look for agents with good antimicrobial activity, 14 commonly used Chinese medicinal herbs with great distribution in Sichuan Province were chosen for their antimicrobial activity evaluation. As a part of a research program directed at the isolation of active agents from plants grown in Sichuan Province, this paper describes the preliminary investigations on the antimicrobial activity of the extracts.
Material and Methods
Extracts preparation
All herbs: (Figure 1) and magnolol (98%) were gained from Sichuan Sanxingdui Pharmaceuticals Co., Ltd.
Preparation of extract from Radix Sophorae Flavescentis: Raw roots of Radix Sophorae Flavescentis (5.0 g) were wetted with 10.0 mL of 2.0% ammonium hydroxide for 4 h. Then the wet roots were crushed by a high-speed disintegrator. The crushed Radix Sophorae Flavescentis was extracted three times with 10.0 mL of the mixture of chloroform and methanol (5:5, v/v) successively. The combined extracts were evaporated to dryness under reduced pressure.
Preparation of extract from Gentiana scabra Bunge: Pulverized herbal Gentiana scabra Bunge was extracted with the appropriate amount of 50% aqueous ethanol (being capable of immersing the solid sample) by stirring at room temperature for 30 min, then centrifugated at a speed of 4000 rpm for 10 min. Extraction was repeated three times. The extracts were combined and filtered through a 0.45 mm cellulose acetate membrane filter. The final combined extracts were evaporated to dryness under reduced pressure.
Preparation of extract from Artemisia annua Linn: The air-dried powdered herbs of Artemisia annua Linn (5 kg) were extracted three times with methanol under refluxing. The resultant extract was combined and concentrated under reduced pressure to afford the residue.
Preparation of extract from Polygonum bistorta L.: The plants were finely powdered and 100 g of powder was extracted with 70% aqueous ethanol (500 mL) using a Soxhlet extraction apparatus. The extracts were filtered hot using a Whatman No. 1 filter paper and then concentrated under vacuum (40-60 ˚C) and finally freeze-dried to yield extract.
Preparation of extract from Ilex chinensis sims: The fresh leaves of Ilex chinensis Sims (2 kg) were extracted three times with MeOH (50 L) at room temperature for a week, and the solvent was removed under reduced pressure.
Preparation of extract from Prunella vulgaris: The dry inflorescence (250 g) was washed with water and then soaked in distilled water (2.5 L) in two 5.0 L flasks and boiled under refluxing for 2 h. The mixture was cooled to ambient temperature, left to be separated, and the aqueous extract was filtered through a Whatman No. 1 filter paper to give 1.6 L of clear solution. Freeze drying of 200 ml of this extract gave a fibrous dark brown residue (2.20 g) accounting for 7.1% of the total dry weight of the herb.
Preparation of extract from Agrimonia pilosa Ledeb: The dried leaves of Agrimonia pilosa Ledeb (10 kg) were cut into small pieces and extracted three times with 80% MeOH at room temperature for 7 days. The MeOH extract were filtered and concentrated under reduced pressure.
Preparation of extract from Chrysanthemum indicum: Dried flowers of Chrysanthemum indicum (5.8 kg) were finely cut and extracted with methanol under refluxing. Evaporation of the solvent under reduced pressure gave the MeOH extract (1650 g, 28.4%).
Preparation of extract from Artenmisia capillaris Thunb: The flowers of Artenmisia capillaris Thunb were collected and crushed with a grinder, 0.1993 g was extracted in 30 ml methanol for 30 min, and the extract evaporated was dried using nitrogen gas.
Preparation of extract from Epimedium brevicornum Maxim: The dried powder of Epimedium brevicornum Maxim (30 g) was refluxed three times for 1.5 h with 75% ethanol. The extracted solutions were combined, ethanol was removed under reduced pressure to give extract.
Preparation of extract from Hottuynia cordata Thunb: Hottuynia cordata Thunb 50% ethanol extracts (yield: 6.73% of dry wt.) were obtained by 48 h maceration at room temperature. The ethanol extract was filtered through a 0.45μm filter, lyophilized and kept at 4oC.
Preparation of extract from Violaphilippica Cav: Air-dried and powdered plant material of Violaphilippica Cav. (874 g) was extracted with 1:1 CH2Cl2/MeOH at room-temperature and filtered through a cotton wool plug, CH2Cl2 and MeOH were removed under reduced pressure.
Preparation of extract from Gardeniae jasminoides Ellis: Stir-baked Gardeniae jasminoides Ellis (1 kg) was boiled in water three times, and the resulting decoctions pooled. This solution was then clarified by centrifugation and filtration, lyophilized and kept at 4℃.
Preparation of extract from astragalus membranes bunge:The fresh plant material (300 g) was extracted twice with water (2 L) for 2.5 h at 100℃. The combined extracts were concentrated to 250 mL using a rotary evaporator at 65℃ under vacuum. The proteins in the extract were removed by Savage reagent. After removal of the Savage reagent, 100 mL of anhydrate ethanol was added before the mixture was maintained overnight at 4℃ to precipitate polysaccharides. The crude polysaccharides (25 g) was obtained by centrifugation at a speed of 3860 rpm for 15 min. The herbal extracts included Radix Sophorae Flavescentis Extract (2, 98% matrine),[13] Gentiana scabra Bunge Extract (3, main ingredients were terpenoids, containing 2.5% gentiopicroside), [14] Artemisia annua Linn Extract (4, 99% Artemisinin), [15] Polygonum bistorta L. Extract (5, 99% Ginsenoside),[16] Ilex chinensis Sims Extract (6, contains the volatile oil, the flavanone, the original catecha phenol and so on),[17] Prunella vulgaris Extract (7, 90% prunellin),[18] Agrimonia pilosa Ledeb Extract (8, 90% Agrimophol),[19] Chrysanthemum indicum Extract (9, contains Buddleog Lucside, Essential oil, Flavone and so on),[20] Artemisia capillaris Thunb Extract (10, Capillarisin ,Chlorogenic acid and so on), [21] Epimedium brevicornum Maxim Extract (11, 50% Icariin),[ 22] Houttuynia cordata Thunb Extract (12, contains Decanoy acetaldehyde, lauric aldehyde, a-pinene, linlool and so on),[23] Violaphilippica Cav. Extract (13, mainly contains organic acid, flavonoids and their glycosides, phenols, sugars, amino acids, peptides and proteins, saponins, phytosterols, tannins and ten other active ingredients),[23] Gardeniae jasminoides Ellis Extract (14, mainly contains gardenoside, crocetin and crocin),[24] Astragali Poly saccharoses (15, 80%). [25] The tested clinical microbes included four representative gram-positive bacteria (S. aureus, S. epidermidis, S. lutea and Bacillus), two representative gram-negative bacteria (E. coli and Pseudomonas)
Bioactivity Assay
All of the extracts were dissolved in dimethylsulfoxide (DMSO), H2O milliQ and the mixture of DMSO/H2O milliQ (1:1, v: v) respectively for test. The antimicrobial activity was evaluated in Laboratory for Medicinal Chemistry of Katholieke Universiteit Leuven using a growth-inhibition plate assay. Bacterial cell cultures were grown overnight in Luria Bertani (LB) broth at 37℃. The next morning, 5μL of these cultures were used to inoculate in 5 mL of LB medium. Bacterial cultures were grown at 37℃ until OD600 = 0.1 (5×107 cells/mL) and diluted 1/10 (OD600 = 0.01, 5×106 cells/mL). 10 μL of the diluted bacterial cultures was spotted on agar plates containing tested extracts. Controls containing solvent were also given. After overnight incubation of the plates at 37℃, the growth of the bacterial were observed. The MIC value was defined as the lowest concentration of each extract that showed no detectable bacterial growth.
Results
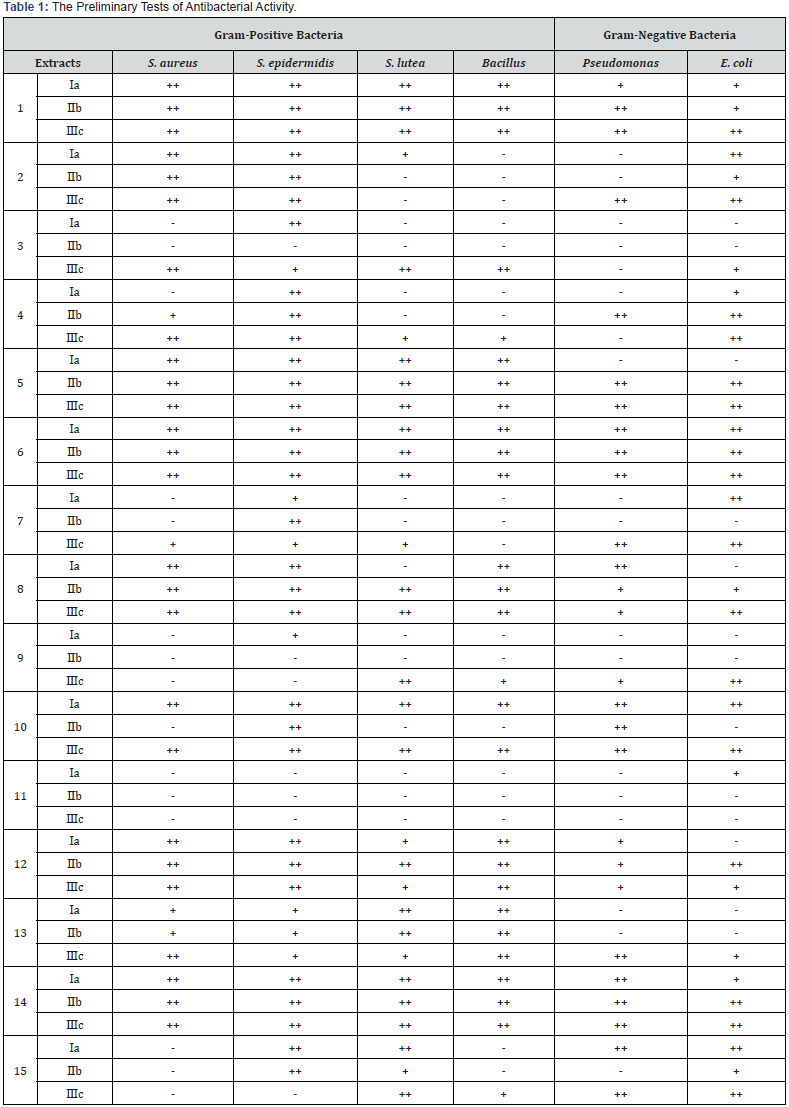
a. The extracts were dissolved in H2O milliQ;
b. The extracts were dissolved in the mixture of DMSO/H2O milliQ (1:1, v:v);
c. The extracts were dissolved in dimethylsulfoxide (DMSO);
-: no inhibition; +: intermediate inhibition; ++: total inhibition.
The results of the preliminary tests were listed in Table 1. The results exhibited the certain inhibition of all the extracts to tested bacteria’s except 9 and 11, which displayed very weak inhibition to the tested bacteria’s. Six extracts (2,3,4,7,15) demonstrated low to intermediate inhibition. Seven extracts (5, 6, 8, 10, 12, 13, 14) displayed moderate to strong inhibition to all tested bacteria’s. Remarkably, extracts 6 and 14 exhibited total inhibition to most of the tested bacteria both dissolved in water and DMSO. For most of the tested extracts (3, 4, 5, 7, 8, 9, 12, 13), better inhibition rates were obtained when the extracts were dissolved in DMSO than in H2O milliQ. Since two herbal extracts (6 and 14) inhibit the growth of the tested bacterias well. The MIC of these two extracts were tested. The results were showed in Table 2, indicating that 6 and 14 had better activity to gram-positive bacteria (especially S. aureus and S. epidermidis) than to gram-negative bacteria (Pseudomonas, E. coli).
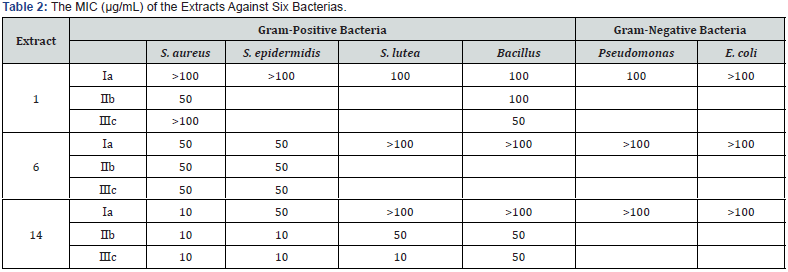
a. The extracts were dissolved in H2O milliQ;
b. The extracts were dissolved in the mixture of DMSO/H2O milliQ (1:1, v:v);
c. The extracts were dissolved in dimethylsulfoxide (DMSO);
-: no inhibition; +: intermediate inhibition; ++: total inhibition.
Discussion
Four extracts (3, 5, 12, 13) Table 1 displayed stronger inhibition to gram-positive bacterias than to gram-negative bacteria’s, while for extract 15, having better inhibition rate for gram-negative bacteria, which means Astragalus membranous Bunge 15 might contains some ingredients specifically for gram-negative bacteria’s. Eight extracts (3, 4, 5, 7, 8, 9, 12, 13,) had better inhibition rates when they were dissolved in DMSO than in H2O milliQ, this might revealed their active ingredients have better solubility in DMSO than in water.
From the information’s of MIC for 6 and 14 extracts, Extract 6 displayed strong inhibition (MIC = 50 μg/mL) to gram-positive bacteria (S. aureus and S. epidermidis) whatever it was dissolved in water or in DMSO, while it showed weak activity (MIC > 100 μg/mL) to gram-negative bacteria’s (Pseudomonas, E. coli). This result might reveal that extract 6 contains some ingredients with good inhibition activity to gram-positive bacteria’s. While magnolol had much less effect on S. epidermidis (MIC > 100 μg/mL), and it had a little better effect on S. aureus (MIC = 50 μg/mL) when it was dissolved in mixture of DMSO/H2O. Extract 14 exhibited low inhibition (MIC >100 μg/mL) to the tested gram-negative bacteria (Pseudomonas and E. coli). when 14 was dissolved in DMSO or in the mixture of DMSO/H2O, it had much excellent antimicrobial activity (MIC = 10 μg/mL) to gram-positive bacterias (S. aureus and S. Epidermidis) than magnolol (MIC ≥ 50 μg/mL) and 6 (MIC ≥ 10 μg/mL), but activity decreased sharply when it was dissolved in water, this could explains that active ingredients in 14 are more liposoluble.
Through the preliminary antibacterial experiment, we screened out the two Chinese herbal medicine extracts 6 and 14 with the best antibacterial activity. However, the Chinese herbal medicine extract generally contains a variety of effective components, whether one of them plays an antibacterial role or the joint synergistic effect of multi-components, it needs to be further studied. Ilex chinensis Sims Extract 6 contains the volatile oil, the flavanone, the original captcha phenol and so on. Specially the flavanone has efficacy of antioxidant [26] and anti-tumor activity [27]. The main components of Fructus Gardenia Extract 14 has gardenoside, crocetin and crocin. Gardenia has been used in many preparations of Chinese patent medicine with anti-inflammatory effect, [28] anti-metastatic and anti-angiogenic activities, [29] anti- diabetic [30] and so on. The action and mechanism of gardenia was preliminary reported [31]. Crocetin and crocin possess hypolipidemic effect [32] and a significant anti-tumor effect both in vitro and in vivo on pancreatic cancer [24]. But there are few studies on antibacterial activity. So, it is meaningful that we separate and purify the active ingredients and study their activity respectively to discover the lead compounds. Screening of the compounds or their derivatives may be of great importance to develop novel drug for preventing infection of bacteria with low side-effect.
Acknowledgment
The authors own lots of thanks to Professor Piet Herdwijn from K.U. Leuven for the determination of the bioactivity.
Conflict of Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Kambaroudis AG, Papadopoulos S, Christodoulidou M, Gerasimidis T (2010) Perioperative use of antibiotics in intra-abdominal surgical infections. Surg Infect (Larchmt) 11(6): 535-544.
- Guo T, Lou C, Zhai W, Tang X, Hashmi MZ, et al. (2018) Increased occurrence of heavy metals, antibiotics and resistance genes in surface soil after long-term application of manure. Sci Total Environ 635: 995-1003.
- Uniyal SK, Singh K, Jamwal P, Lal B (2006) Traditional use of medicinal plants among the tribal communities of Chhota Bhangal, Western Himalaya. J Ethnobiol Ethnomed 2(1): 14.
- Ma F, Chen Y, Li J, Qing HP, Wang JD, et al. (2010) Screening test for anti-Helicobacter pylori activity of traditional Chinese herbal medicines. World J Gastroenterol 16(44): 5629.
- Su PW, Yang CH, Yang JF, Su PY, Chuang LY (2015) Antibacterial activities and antibacterial mechanism of Polygonum cuspidatum extracts against nosocomial drug-resistant pathogens. Molecules 20(6): 11119-11130.
- Chung CY, Liu CH, Burnouf T, Wang GH, Chang SP, et al. (2016) Activity-based and fraction-guided analysis of Phyllanthus urinaria identifies loliolide as a potent inhibitor of hepatitis C virus entry. Antiviral Res 130: 58-68.
- Yue GGL, Lee JKM, Chan BCL, Kwok HF, Hoi SWH, et al. (2018) An innovative anti-cancer Chinese herbal formula exhibited multi-targeted efficacies in metastatic breast cancer mouse model. Chin Med 13(1): 64.
- Brown ED, Wright GD (2016) Antibacterial drug discovery in the resistance era. Nature 529(7586): 336-343.
- Rodrigues T, Reker D, Schneider P, Schneider G (2016) Counting on natural products for drug design. Nat Chem 8(6): 531-541.
- Sarrica A, Kirika N, Romeo M, Salmona M, Diomede L (2018) Safety and toxicology of magnolol and honokiol. Planta Med 84(16): 1151-1164.
- Bang KH, Kim YK, Min BS, Na MK, Rhee YH, et al. (2000) Antifungal activity of magnolol and honokiol. Arch Pharmacal Res 23(1): 46-49.
- Ho KY, Tsai CC, Chen CP, Huang JS, Lin CC (2001) Antimicrobial activity of honokiol and magnolol isolated from Magnolia officinalis. Phytother Res 15(2):139-141.
- Lai JP, He XW, Jiang Y, Chen F (2003) Preparative separation and determination of matrine from the Chinese medicinal plant Sophora flavescens Ait by molecularly imprinted solid-phase extraction. Anal Bioanal Chem 375(2): 264-269.
- Yang J, Long H, Liu H, Huang A, Sun Y (1998) Analysis of tetrandrine and fangchinoline in traditional Chinese medicines by capillary electrophoresis. J Chromatogr A 811(1-2): 274-279.
- Kim KS, Lee S, Lee YS, Jung SH, Park Y, et al. (2003) Antioxidant activities of the extracts from the herbs of Artemisia. J Ethnopharmacol 85(1): 69-72.
- Demiray S, Pintado M, Castro P (2009) Evaluation of phenolic profiles and antioxidant activities of Turkish medicinal plants: Tilia argentea, Crataegi folium leaves and Polygonum bistorta World Academy of Science, Engineering and Technology 54: 312-317.
- Zhao H, Wang M, Zhou G (1993) Studies on constituents of Ilex chinensis Sims. Zhongguo Zhong yao za zhi Zhongguo zhongyao zazhi China journal of Chinese materia medica 18(4): 226-228.
- Tabba HD, Chang RS, Smith KM (1989) Isolation, purification, and partial characterization of prunellin, an anti-HIV component from aqueous extracts of Prunella vulgaris. Antiviral Res 11(5-6): 263-273.
- Jung M, Park M (2007) Acetylcholinesterase inhibition by flavonoids from Agrimonia pilosa. Molecules 12(9): 2130-2139.
- Matsuda H, Morikawa T, Toguchida I, Harima S, Yoshikawa M (2002) Medicinal flowers. VI. Absolute stereostructures of two new flavanone glycosides and a phenylbutanoid glycoside from the flowers of Chrysanthemum indicum L: their inhibitory activities for rat lens aldose reductase. Chem Pharm Bull 50(7): 972-975.
- Wang H, Zou H, Ni J, Kong L, Gao S, et al. (2000) Fractionation and analysis of Artemisia capillaris Thunb. by affinity chromatography with human serum albumin as stationary phase. J Chromatogr A 870(1-2): 501-510.
- Li F, Lu X, Liu H, Liu M, Xiong Z (2007) A pharmaco‐metabonomic study on the therapeutic basis and metabolic effects of Epimedium brevicornum Maxim. on hydrocortisone‐induced rat using UPLC‐MS. Biomed Chromatogr 21(4): 397-405.
- Zhang Y, Li Sf, Wu Xw (2008) Pressurized liquid extraction of flavonoids from Houttuynia cordata Sep Purif Technol 58(3): 305-310.
- Ding L, Luo XB, Tang F, Yuan JB, Guo M, et al. (2008) Quality control of medicinal herbs Fructus gardeniae, Common Andrographis Herb and their preparations for their active constituents by high-performance liquid chromatography–photodiode array detection–electrospray mass spectrometry. Talanta 74(5): 1344-1349.
- Ma XQ, Shi Q, Duan J, Dong TT, Tsim KW (2002) Chemical analysis of Radix Astragali (Huangqi) in China: a comparison with its adulterants and seasonal variations. J Agric Food Chem 50(17): 4861-4866.
- Hu Xl, Han Zx, Chen Y, Xu L, Bian Hl (2006) Extraction and Activity Determination of Flavones in Holly [J]. Food Science 12.
- Zheng Z, Chen L, Zheng G, Tong YX, Zhang SF (2011) Preliminary study on screening anti-tumor active fraction of extracts from seven Chinese herbal medicines. Subtrop Plant Sci 40(3): 31-35.
- Park SH, An JE, Jang S, Kim JY, Lee JW, et al. (2019) Gardenia jasminoides extract without crocin improved atopic dermatitis-like skin lesions via suppression of Th2-related cytokines in Dfe-induced NC/Nga mice. J Ethnopharmacol 241: 112015.
- Im M, Kim A, Ma JY (2016) Ethanol extract of baked Gardeniae Fructus exhibits in vitro and in vivo anti-metastatic and anti-angiogenic activities in malignant cancer cells: Role of suppression of the NF-κB and HIF-1α Int J Oncol 49(6): 2377-2386.
- Yu Q, Takahashi T, Nomura M, Ikeda-Matsuo Y, Kobayashi S (2017) Mechanism of Gardeniae Fructus for the anti-diabetic action in high-fat diet-fed and streptozotocin-treated diabetic mice. 12(1): 19-30.
- Toriizuka K, Kamiki H, Ohmura NY, Fujii M, Hori Y, et al. (2005) Anxiolytic effect of Gardeniae Fructus-extract containing active ingredient from Kamishoyosan (KSS), a Japanese traditional Kampo medicine. Life Sci 77(24): 3010-3020.
- Lee IA, Lee JH, Baek NI, Kim DH (2005) Antihyperlipidemic effect of crocin isolated from the fructus of Gardenia jasminoides and its metabolite crocetin. Biol Pharm Bull 28(11): 2106-2110.






























