Augmentation of Native Mycorrhizal Population and its Functionality Using Parthenium Biochar
Pallavi Suvigya Sharma and AK Sharma*
Department of Biological Sciences, Govind Ballabh Pant University of Agriculture & Technology, India
Submission: July 30, 2019; Published: September 11, 2019
*Corresponding author: AK Sharma, Department of Biological Sciences, College of Basic Sciences and Humanities, Govind Ballabh Pant University of Agriculture & Technology, Pantnagar, US Nagar, Uttarakhand, India
How to cite this article: Pallavi Suvigya Sharma, AK Sharma. Augmentation of Native Mycorrhizal Population and its Functionality Using Parthenium Biochar. Adv Biotechnol Microbiol. 2019; 14(4): 555892. DOI: 10.19080/AIBM.2019.14.555892
Abstract
Parthenium is one of the world’s deadliest weeds and its highly allelopathic nature is a major cause of crop yield reduction. There has been growing interest in production of biochar from parthenium by pyrolysis, as a weed control strategy. Biochar is the carbonaceous residue left after pyrolysis, and many studies have found its application in maintaining soil quality by improving its physical, chemical and biological properties. Arbuscular Mycorrhizal Fungi (AMF) are inseparable component of soil microbial community forming mutualistic relationship with almost 80% of plant species. They play an important role in maintaining soil fertility and plant health nutrition. The present study was carried out to study the effect of different application rates of Parthenium biochar (0g/kg, 1g/kg, 3g/kg, 5g/kg and 10g/kg) on the native AMF population of three altitudes of Kumaun Himalayas and maize plant growth under greenhouse conditions. The results indicate positive influence of biochar application on AMF population and root colonization. In addition, the integrated application of AMF and biochar also resulted in enhanced plant growth and foliar nutrient content in maize plants when compared to control plants. The study suggests the utility of Parthenium biochar as soil management practice with multifaceted ability to promote AMF population of soil as well as plant health.
Keywords: Arbuscular mycorrhiza fungi; Parthenium; Biochar; Maize (Zea mays); Plant growth
Introduction
Sustainable soil fertility management has been suggested as essential to the prosperity. Hilly regions of Uttarakhand have prevalence of rain-fed conditions, resulting in water stress which restricts the optimum plant growth. Also, soils from these regions are shallow and coarse textured thus have a poor soil structure and low water-holding capacity. Datta and Handal, highlighted the nitrogen and organic matter deficiency of soil in South and South East Asian countries. Furthermore, the organic manure used in these areas is made from oak and chir pine leaves which results in the acidification of the soil [1]. Thus, for achieving high agriculture productivity the focus should also be on improving soil health as only then sustainable agriculture system can be achieved.
External carbon input as amendment into these marginalized soil for uplifting the soil organic matter and health has been recommended by many researchers [2]. Traditional soil amendments include farmyard manure, composted manure, poultry manure and cattle manure [3,4]. However, in the recent times soil amendment with biochar has gained interest among the researchers as well as among the farmers because of the inherent advantages associated with it. Biochar is a collective term for carbon rich soil amendments of either plant or animal biomass through heating at 300 to 600 °C under limited oxygen supply [5]. Increased soil water-holding capacity has been observed on biochar addition to sandy-loamy soil [6]. Also, improvement in hydraulic conductivity of soil along with an increased rice yield in low P availability can be achieved after biochar addition [7]. Studies have indicated that plant responses to biochar are indirectly related to biochar effects on soil microbial community [8,9]. Biochar enhances populations and activity in soil by modulating metabolism and growth of soil microorganisms [10,11]. AM fungi provide their host plants with mineral nutrients and receive photosynthetically derived carbohydrates in return [12]. Biochar, especially from wood materials, typically have a large surface area due to porous nature and Cation Exchange Capacity (CEC). Therefore, its addition to soils also increases the CEC of the soil [13]. Higher CEC means more ions will be adsorbed this will prevent leaching of nutrients [14]. However, these nutrients may not be accessible by plants, as most roots are unable to reach the fine porous structure of the biochar due to their large size [15]. On the other hand, AM fungal hyphae being much finer in diameter can easily re-capture some of the adsorbed nutrients and transfer them to their host plants [16]. A combined management of AM fungi and biochar may lead to efficient fertilAbstract
water-holding capacity has been observed on biochar addition to sandy-loamy soil [6]. Also, improvement in hydraulic conductivity of soil along with an increased rice yield in low P availability can be achieved after biochar addition [7]. Studies have indicated that plant responses to biochar are indirectly related to biochar effects on soil microbial community [8,9]. Biochar enhances populations and activity in soil by modulating metabolism and growth of soil microorganisms [10,11]. AM fungi provide their host plants with mineral nutrients and receive photosynthetically derived carbohydrates in return [12]. Biochar, especially from wood materials, typically have a large surface area due to porous nature and Cation Exchange Capacity (CEC). Therefore, its addition to soils also increases the CEC of the soil [13]. Higher CEC means more ions will be adsorbed this will prevent leaching of nutrients [14]. However, these nutrients may not be accessible by plants, as most roots are unable to reach the fine porous structure of the biochar due to their large size [15]. On the other hand, AM fungal hyphae being much finer in diameter can easily re-capture some of the adsorbed nutrients and transfer them to their host plants [16]. A combined management of AM fungi and biochar may lead to efficient fertilAbstractParthenium hysterophorus L. commonly known as carrot weed, chatak chandani, Congress grass, star weed. The plant belongs to the division Magnoliophyta, class Magnoliopsida, Order Asterales and family Asteraceae. It is speculated that Parthenium gained entry in India before 1910 through contaminated cereal grain, however, its presence was first acknowledged in 1956. Since 1956, the weed has spread like wildfire throughout India. Presently, the weed is a major problem in the agriculture fields of Uttarakhand [20,21]. Zea mays showed an increase in seedling vigour index with parthenium derived biochar addition and no adverse effect on soil microbial activity was observed even at the highest rate (20g/kg) along with the removal of allelochemical ambrosin [22]. However, the effect of Parthenium based biochar on native AMF population of agriculture lands have not been tested. Therefore, the present study was undertaken in an attempt to test and optimize the dosage of Parthenium biochar for agriculture purposes that works synergistically with soil AMF population.
Material and Methods
AMF Inoculum and Biochar
Greenhouse pot cultures of the native AMF of three agricultural lands (Dwarson, Nachini and Ghorpatta) were established with suitable host plants (Maize, Sorghum and Cowpea). Description of the agricultural land is provided in Table 1. Potting mixture included 200gm soil sample with autoclaved mixture of vermiculite and gravel (2:1). After 60 days, the plants were left for drying to enhance sporulation. After five such consecutive cycles, soil of these pots was used as the AMF inoculum.
Pot experiment with Zea mays
Potting substrate consisting sand and soil mixed in ratio 5:1, respectively was used. Combination of AMF inoculum (trap soil having 2000 spores per pot) and Parthenium biochar described in the Table 2 were added to the substrate in pots. Three replicates per combination were taken. Surface sterilized maize seeds were grown in these pots. Plants were allowed to grow for 60 days in green house under controlled conditions at relative humidity 60%, temperature 28±2 °C, photoperiod: 16/8h day/night cycle and light intensity 400 Em-2 s-1 (400-700nm).
Growth parameters
Plants were harvested after 60 days, rinsed with tap water and air dried, root/shoot length and root/shoot fresh weight were recorded. Plant samples (root and shoot) were kept in paper bags, oven dried at 65 °C until constant sample weight was achieved, and dry weight was assessed. Some of the roots were stored at 4 ℃ for root colonization studies.
Mineral nutrition
Dried plant tissues were used for nutrient estimation. The nitrogen content of the leaves was estimated according to Kjeldahl method using the KJEL PLUS System (Pelican, India). Diacid digestion of the sample was carried out using 5:1 mixture of HNO3: HClO4. Phosphorus content was measured by the vando-molybdate phosphate method. Sodium, potassium, and calcium were measured using flame photometer (Systronics Flame Photometer 128).
Spore count
Spore count was done using procedure given by Gerdemann & Nicolson [23]. 1-gram air-dried soil was washed through two juxtaposed sieves of mesh size 60 and 400 respectively under tap water. 40% sucrose solution was added to the content and centrifuged at 2000 rpm for 3 minutes. The supernatant was then transferred to sieve of 400 mesh size, washed with distilled water to remove excess sucrose, transferred to Petri dish and counted on stereo-zoom microscope. The total number of spores were counted and expressed as spores per gm of soil.
Mycorrhiza frequency
Roots were first stained by trypan blue method. Root samples were cleared in KOH solution (2.5%) and stained using the Trypan Blue (0.05%) [24]. Mycorrhizal colonization was determined by examining 1 cm root segments (n = 50 per each treatment) under the microscope according to Trouvelot [25]. Results are expressed as percentage frequency.
Total Glomalin Estimation (TEG)
Total glomalin extraction was done according to Wright and Upadhyay [26]. 1g soil was autoclaved in 8ml of 50mM sodium citrate at pH 8.0 for 60 min. Immediately after autoclaving, we centrifuged the tubes at 3000g for 15-20 min, then poured off the supernatant and stored it at 4 ℃ until analysis. For TEG, the soil pellet was re-suspended in the same volume of fresh extraction solution, and the extraction repeated for one more cycle.
Results
Mycorrhizal population

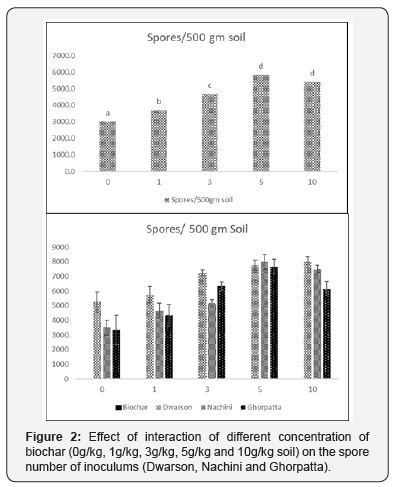
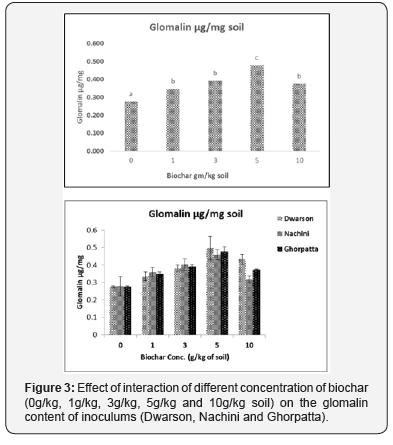
Biochar addition resulted in enhanced frequency of mycorrhiza in the root system and irrespective of the inoculum highest frequency was observed in 3 and 5 g/Kg biochar concentration (Figure 1). The effect of biochar application can also be seen in spore numbers as well, enhanced number of AMF spores/ gm of inoculum with up to 59 % increase over control (without biochar) in 5g/Kg of biochar concentration in Ghorpatta were observed (Figure 2). Similarly, glomalin conc. (representative of AMF community activity) of the inoculum after biochar application was also found to be enhanced with up to 49 % increase over control (without biochar) in 5 g/Kg biochar + Ghorpatta (Figure 3).
Growth Parameters
Shoot parameters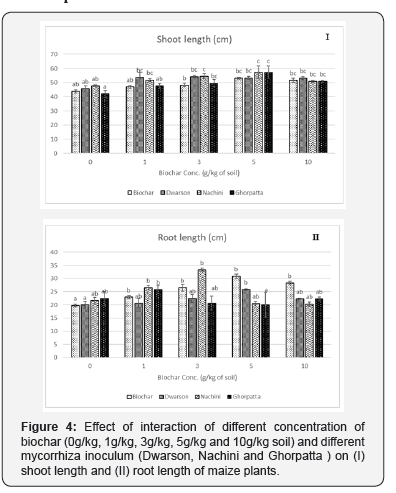
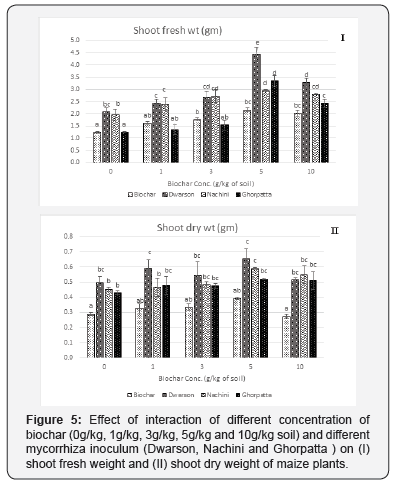

Irrespective of biochar application rate, each mycorrhiza inoculum was able to promote maize plant growth with Dwarson and Nachini significantly enhancing shoot parameters over uninoculated plants. Similarly, irrespective of mycorrhiza inoculum biochar treatment resulted in increased shoot length (Figure 4), shoot fresh weight (fwt) (Figure 5), shoot dry weight (dwt) (Figure 6) over plants grown without biochar application. This response increased with increasing application rate of biochar, however, at highest application rate of biochar (10g/kg) the effect was slightly decreased. Most effective combination was of 5g/Kg biochar application rate + Dwarson.
Root parameters

Similar to shoot parameters, irrespective of biochar application rate each mycorrhizal inoculum resulted in improved root parameters (Figure 7) except root length (2.1 II) over non-mycorrhized plants. When considering only biochar application, increasing application rate positively influenced root parameters with 3g/ kg and 5g/kg application rate were found to be optimum. When considering interaction of both biochar and mycorrhiza, 3g/kg + Dwarson was the most effective combination.
Foliar nutrient content
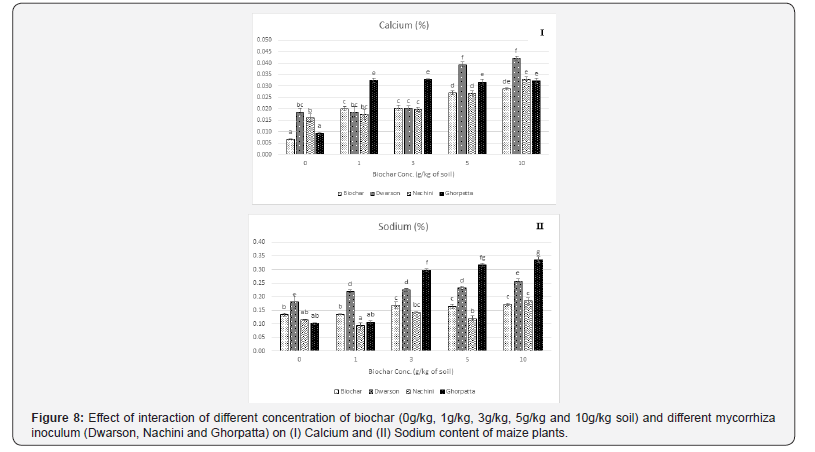
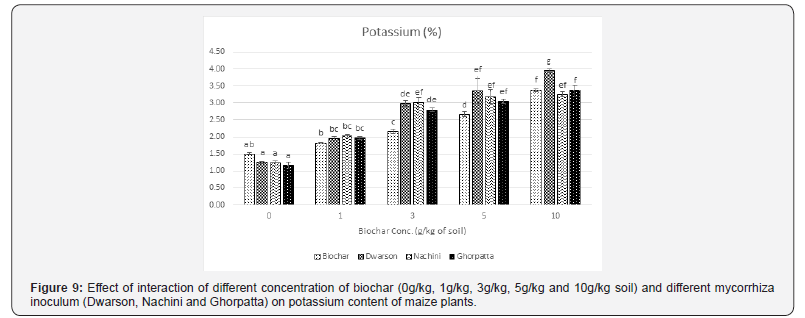
Nutrient uptake by the maize plants showed synergistic effect by the combined treatment of biochar and mycorrhiza. All the nutrient estimated showed a significant increase in all the treatment combinations over control. Also, the increase was directly proportional to the biochar application rate. Unlike growth parameters, no decrease in nutrient content of maize plants treated at 10 g/ kg application rate was observed. The effect on phosphorus uptake (Figure 8) was most prominent with increased uptake with increasing concentration of biochar indicating the enhanced functionality of AMF population. Nitrogen (Figure 9), Calcium, Sodium and Potassium uptake was also significantly enhanced. Among the mycorrhiza inoculums, Dwarson was most effective in improving nitrogen content, Nachini was most effective in improving phosphorus content, calcium and sodium content was most effectively improved by Ghorpatta. Potassium was equally improved by all the inoculums.
Discussion
In our study, we observed a positive synergistic effect of biochar and mycorrhiza amendment on maize growth however, at higher application rate (10 g/kg) of biochar a slight reduction in growth enhancement was observed. However, the nutrient uptake didn’t show any reduction. Other studies have also reported AM dependent enhanced nutrient uptake even in absence of positive growth response [27]. In a study, application of charred bark of Acacia, yield of maize and peanut significantly improved. According to researchers, increased pH, CEC, available N and P, along with increased colonization by AMF resulted in this enhanced yield [28]. In another study, enhanced root colonization by AMF in soybean on biochar addition along with yield enhancement of soybean after biochar addition [29]. Similar results were reported in wheat and clover [30]. 6 % increase in AM colonization was observed in Phaseolus vulgaris on biochar addition [31]. In an assessment of different application rate of biochar on maize growth it was noted that with increasing biochar concentration the growth improved and even the highest dose of 20g/kg biochar did not negatively affect soil microbial activity [32]. Parthenium biochar reduced the commercial fertilizer dose required without compromising on the yield and improved the soil health [33].
Conclusion
The study shows that the biochar amendment of soil can positively affect the native AMF population which in turn result in enhanced productivity and nutrient uptake of plants. Our study supports the idea of utilizing parthenium for biochar production as a weed management strategy. Results emphasize that properly optimized parthenium derived biochar can prove to be an economical and ecological substitute for the chemical fertilizers. It is capable of working alone and along with AMF its properties can be further exploited.
Acknowledgement
Pallavi acknowledges the Department of Science and Technology, India, for providing her Inspire Fellowship (IF130963).
Authors acknowledge Dr. R. N. Pateriya, Professor & PI (CRPAM) Farm Machinery and Power Engg. College of Technology, Govind Ballabh Pant University of Agriculture and Technology, Pantnagar, Uttarakhand, India for providing Parthenium biochar for the purposes of this study..
References
- De Datta SK, Hundal SS (1984) Effects of organic matter management on land preparation and structural regeneration in rice-based cropping system.
- Sherchan DP, Karki KB (2005) Plant nutrient management for improving crop productivity in Nepal. In: Proceedings of regional workshop on improving plant nutrient management for better farmer livelihood, food security and environmental sustainability, pp. 41-57.
- Bista P, Ghimire R, Chandra Shah S, Raj Pande K (2010) Assessment of soil fertility management practices and their constraints in different geographic locations of Nepal. In Forum geografic 9.
- Uddin MB, Mukul SA, Khan MASA., Hossain MK (2009) Seedling response of three agroforestry tree species to phosphorous fertilizer application in Bangladesh: growth and nodulation capabilities. Journal of Forestry Research 20(1): 45-48.
- Lehmann J, Joseph S (2015) Biochar for environmental management: science, technology and implementation. Routledge.
- Basso AS, Miguez FE, Laird DA, Horton R, Westgate M (2013) Assessing potential of biochar for increasing water‐holding capacity of sandy soils. Gcb Bioenergy 5(2): 132-143.
- Asai H, Samson BK, Stephan HM, Songyikhangsuthor K, Homma K, et al. (2009) Biochar amendment techniques for upland rice production in Northern Laos: 1. Soil physical properties, leaf SPAD and grain yield. Field Crops Research 111(1-2): 81-84.
- Thies JE, Rillig MC, Graber ER (2015) Biochar effects on the abundance, activity and diversity of the soil biota. Biochar for environmental management: science, technology and implementation 2: 327-389.
- Abujabhah IS, Bound SA, Doyle R, Bowman JP (2016) Effects of biochar and compost amendments on soil physico-chemical properties and the total community within a temperate agricultural soil. Applied Soil Ecology 98: 243-253.
- Kookana RS, Sarmah AK, Van Zwieten L, Krull E, Singh, B (2011) Biochar application to soil: agronomic and environmental benefits and unintended consequences. In Advances in agronomy (112): 103-143.
- Tong H, Hu M, Li F, Liu C, Chen M (2014) Biochar enhances the microbial and chemical transformation of pentachlorophenol in paddy soil. Soil Biol Biochem 70: 142-150.
- Smith SE and Read DJ (2008) Mycorrhizal symbiosis. 3rd. Academic Press New York, USA p. 605.
- Blackwell P, Krull E, Butler G, Herbert A, Solaiman, Z (2010) Effect of banded biochar on dryland wheat production and fertiliser use in south-western Australia: an agronomic and economic perspective. Soil Res 48(7): 531-545.
- Yao Y, Gao B, Zhang M, Inyang M, Zimmerman AR (2012) Effect of biochar amendment on sorption and leaching of nitrate, ammonium, and phosphate in a sandy soil. Chemosphere 89(11): 1467-1471.
- Fitter A (2002) Characterstics and function of root systems. In Plant roots (49-78). CRC Press.
- Hammer EC, Balogh-Brunstad Z, Jakobsen I, Olsson PA, Stipp SL, et al. (2014) A mycorrhizal fungus grows on biochar and captures phosphorus from its surfaces. Soil Biol Biochem 77: 252-260.
- Hammer EC, Forstreuter MCR, Kohler J (2015) Biochar increases arbuscular mycorrhizal plant growth enhancement and ameliorates salinity stress. Applied Soil Ecology 96: 114-121.
- Birk JJ, Steiner C, Teixiera WC, Zech W, Glaser B (2009) Microbial response to charcoal amendments and fertilization of a highly weathered tropical soil. WI Woods (Ed.), Amazonian Dark Earths: Wim Sombroeks Vision, Springer, Netherlands, pp. 309-324.
- Warnock DD, Mummey DL, McBride B, Major J, Lehmann J, Rillig MC (2010) Influences of non-herbaceous biochar on arbuscular mycorrhizal fungal abundances in roots and soils: results from growth-chamber and field experiments. Applied Soil Ecology 46(3): 450-456.
- Kumar, S (2009) Biological control of Parthenium in India: status and prospects. Indian Journal of Weed Science 41(1-2):1-18.
- Kumar M, Kumar S (2010) Effect of Parthenium hysterophorus ash on growth and biomass of Phaseolus mungo. Academia Arena 2(1): 98-102.
- Kumar S, Masto RE, Ram LC, Sarkar P, George J, et al. (2013) Biochar preparation from Parthenium hysterophorus and its potential use in soil application. Ecological Engineering 55: 67-72.
- Gerdemann JW, Nicolson TH (1963) Spores of mycorrhizal Endogone extracted from soil by wet sieving and decanting. Transactions of the British Mycological Society 46(2): 235–244.
- Philip JM, Hayman SD (1970) Improved procedures for clearing and staining parasitic and vesicular_arbuscular mycorrhizal fungi for rapid assessment of infection-trans. Br Myed 55.
- Trouvelot A, Kough J, Gianinazzi-Pearson V (1986) Evaluation of VA infection levels in root systems. V Gianinazzi-Pearson, S Gianinazzi (Eds.), Research for estimation methods having a functional significance, Physiological and Genetical Aspects of Mycorrhizae. INRA Press, Paris, France pp.217-221
- Wright SF, Upadhyaya A (1998) A survey of soils for aggregate stability and glomalin, a glycoprotein produced by hyphae of arbuscular mycorrhizal fungi. Plant and Soil 198(1): 97-107.
- Li H, Smith SE, Holloway RE, Zhu Y, Smith FA (2006) Arbuscular mycorrhizal fungi contribute to phosphorus uptake by wheat grown in a phosphorus fixing soil even in the absence of positive growth responses. New Phytol 172(3): 536-543.
- Yamato M, Okimori Y, Wibowo IF, Anshori S, Ogawa M (2006) Effects of the application of charred bark of Acacia mangium on the yield of maize, cowpea and peanut, and soil chemical properties in South Sumatra, Indonesia. Soil Science and Plant Nutrition 52(4): 489-495.
- Wathira NL, Wachira P, Okoth S (2016) Enhancement of colonisation of soybean roots by arbuscular mycorrhizal fungi using vermicompost and biochar. Agriculture, Forestry and Fisheries 5(3): 71-78.
- Solaiman ZM, Murphy DV, Abbott LK (2012) Biochars influence seed germination and early growth of seedlings. Plant and Soil 353(1-2): 273-287.
- Vanek SJ, Lehmann J (2015) Phosphorus availability to beans via interactions between mycorrhizas and biochar. Plant and Soil 395(1-2): 105-123.
- Kumar S, Masto RE, Ram LC, Sarkar P, George, J, Selvi VA (2013) Biochar preparation from Parthenium hysterophorus and its potential use in soil application. Ecological Engineering 55: 67-72.
- Shafiq M (2016) Management of the Parthenium hysterophorus through biochar formation and its application to rice-wheat cultivation in Pakistan. Agriculture, Ecosystems and Environ 235: 265-276.






























