Anaerobic Biodegradation of Three Aromatic Sulfur Compounds by Desulfovibrio Psychrotolerans JS1T, in Soil and Sludge Microcosms
Sasi Jyothsna TS*, Saikat Dutta and Chakradhar Bandari
Environmental Consultancy, Ramky Enviro Services Pvt. Ltd, India
Submission: June 19, 2019; Published: July 19, 2019
*Corresponding author: Sasi Jyothsna, Environmental Consultancy, Ramky Enviro Services Pvt. Ltd, Ramky Grandiose, Hyderabad, India
How to cite this article: S Jyothsna TS, Saikat D, Chakradhar B. Anaerobic Biodegradation of Three Aromatic Sulfur Compounds by Desulfovibrio Psychrotolerans JS1T, in Soil and Sludge Microcosms. Adv Biotechnol Microbiol. 2019; 14(3): 555888. DOI: 10.19080/AIBM.2019.14.555888
Abstract
The sulfate reducing bacterium, Desulfovibrio psychrotolerans, JS1T could biodegrade three aromatic Sulphur compounds namely P-Toulene Sulfonic acid (PTSA), Sulfanilic acid (SFA) and Thiophene - 2 -acetic acid (TPA) when provided as sole source of carbon under strict anaerobic conditions. The strain could grow in 25 mM and tolerate up to 50 mM of all the three test compounds supplemented as sole carbon source with optimum growth at 3 or 4 mM. Though none of the tested compounds were completely metabolized within the experimental period of three months, PTSA was degraded up to 82%, SFA up to 65.5% and TPA was degraded up to 72% in liquid culture. The soil and sludge microcosm studies revealed that strain JCM14597T could degrade the test compounds more efficiently as pure culture when compared to that with its consortium, with role of native microorganisms being insignificant. The biodegradation of PTSA & TPA was significantly reduced in sludge microcosm than in soil while SFA degradation was similar in both soil and sludge microcosms. The present work in laboratory scale is a preliminary study conducted at ambient conditions of naturally occurring soils and sludge and thus indicates the potential of D. psychrotolerans, strain JCM14597T for biodegradation of contaminated soils and sludge. To the best of our knowledge, this is the first report of a sulfate reducing bacterium capable of degrading three aromatic Sulphur compounds under anaerobic conditions and the isolate will be potentially useful in bioremediation of contaminated soils.
Keywords: Anaerobic biodegradation; Aromatic sulphur compounds; Microcosm studies; Sulfate reducing bacteria
Abbrevations: SRB: Sulfate Reducing Bacteria; PTSA: Para-Toluene Sulfonic Acid; SFA: Sulfanilic Acid; TPA: Thiophene-2-acetic acid
Introduction
Aromatic compounds form the second largest group of organic compounds in nature after carbohydrates [1]. Hazardous aromatic compounds get into the environment in the form of diverse detergents, with oil spills, sewage from petroleum refineries and chemical plants, and with municipal waste waters. Many environments are anoxic or rapidly become anoxic due to contamination with carbon rich compounds like wastes from industrial effluents, gasoline, crude oil etc [2]. Removal of such recalcitrant compounds becomes important as these potentially hazardous molecules may enter into drinking water supplies.
As a major part of natural environment has little or no access to atmospheric oxygen, anaerobic microbes hold a major role in the processing of the nutrient cycles in nature and also in waste treatment plants where the aerobic processes may not completely remove aromatic compounds, turning researchers’ interest to the study of the anaerobic metabolism of these compounds [3]. In this regard, Sulfate Reducing Bacteria (SRB) have been extensively recognized and studied due to their ubiquitous distribution and capability in anaerobic biodegradation and biotransformation of a number of environmental pollutants under sulfate reducing conditions [4].
The aim of the present investigation was to study the ability of a sulfate reducing bacterium reported from our lab, Desulfovibrio psychrotolerans, strain JS1T [5] to degrade three aromatic sulfur compounds namely Para-Toluene Sulfonic Acid (PTSA), sulfanilic acid (SFA) and thiophene-2-acetic acid (TPA) under strict anaerobic conditions. These pollutants enter into environment through effluents from textile, dye and chemical industries and petroleum products. The aerobic degradation pathway of toluene was demonstrated in Pseudomonas testosterone [6]. A 40% degradation of sulfanilic acid under aerobic conditions by fungal strains Phanerocheate chrysosporium [7] and Aspergillus niger RH19 [8] were demonstrated. Sphingomonas subartica strain was reported to utilize sulfanilic acid as sole carbon, nitrogen and sulfur source indicating its degradation by the strain [9]. Aerobic microbiological conversion of thiophenes has been studied extensively [10,11]. However, very little information is available concerning the anaerobic conversion of these aromatic sulfur compounds.
As the true fulfillment of any laboratory studies on pollutant biodegradation is accomplished only when they are applied on field for bioremediation of contaminated sites, microcosm studies on the degradation of the test compounds were conducted in sludge and soil microcosms to understand the effects of physicochemical properties of sludge and soil and other biological parameters on the survival and growth of strain JS1T and subsequently on the degradation of test compounds before switching on to onsite studies. The present study demonstrating the degradation of aromatic sulfur compounds by D. psychrotolerans, JS1T in liquid culture and in microcosms is the first such study of degradation, by any pure culture of SRB.
`
Materials and Methods
Growth medium for degradation studies
The pure culture of D. psychrotolerans strain JS1T was grown in Postgate’s B medium (PBM) [12] consisting of (gL-1) KH2PO4.7H2O, 0.5; NH4Cl, 1.0; Na2SO4, 4.5; CaCl2.2H2O, 0.06, MgSO4.7H2O, 2.0, Yeast extract, 1.0, FeSO4.7H2O, 0.004, Sodium citrate, 0.3, Sodium Lactate, 3.5, Sodium ascorbate solution (1M), 1 mL and Na2S.9H2O solution (1M), 1mL. A one percent (v/v) inoculum of pure culture of strain JS1T washed twice in sterile saline, centrifuged and the culture pellet was inoculated into Postgate’s B medium with the aromatic sulfur test compounds namely, Para-Toluene Sulfonic Acid [PTSA], Sulfanilic acid [SFA] and Thiophene 2-Acetic acid [TPA] supplemented as either sole source of Carbon (3mM) replacing lactic acid or electron acceptor (0.5mM) replacing FeSO4 respectively in 7.5 mL screw capped tubes incubated at 30±2 °C. The culture was frequently sub cultured in the same medium and used for various biodegradation studies mentioned in the present report. Turbidometry was used to monitor the growth of D. psychrotolerans, strain JS1T. Increase in optical density (OD) (turbidity) was measured for every 24 h starting from time 0 h to 96 h. Optical density of the bacterial suspension was directly measured in a Systronics make (model 112) colorimeter at 540 nm (filter 7) against un-inoculated medium as blank.
Maximum biodegradability and biosorption test
Different concentrations of the test compounds (i.e. 0, 1, 2, 3, 4, 5, 10, 25 and 50 mM) were supplemented as sole carbon source replacing lactic acid and growth as increase in optical density was measured colorimetrically at 24 h interval starting from time 0 h (i.e.0, 24,48,72, 96 and 120 h). For reading the degradation of the test compounds, 2 mL of the liquid culture was taken in 2.0 mL Eppendorf tube, centrifuged at 5008 g for 15 minutes, 0.5 mL of the culture supernatant was diluted 10 times with deionized water. The diluted sample was measured for absorbance at the λ maxima of 229nm for PTSA, 280nm for SFA and TPA on a Spectron Genesys 2 Spectrophotometer. Cells were simultaneously observed under phase contrast light microscope (Olympus BH-2) for viability from each test sample.
Test to rule-out biosorption of test compounds by JS1T
In order to see whether the aromatic sulfur compounds PTSA, SFA and TPA under test were being adsorbed or truly utilized by the strain JS1T, live and dead (heat killed) culture/biomass of strain JS1T were separately inoculated into Postgate’s B medium supplemented with 3mM of the test compound as sole carbon source and incubated at 30±2 °C. Growth in terms of increase in OD was measured colorimetrically and concentrations of each of the test compounds were estimated by U.V. absorption at their respective absorption maxima of the test compounds.
HPLC analysis for biodegradation of test compounds
The HPLC analysis of aromatic sulfur test compounds, PTSA, SFA and TPA in culture supernatants was performed at room temperature using a Shimadzu SPD-10AVP isocratic system. Luna 5μ C18 (2) 100A column (250 x 4.6 mm) was used for the detection of metabolites in a UV-VIS detector. SFA and TSA were detected in a solvent system containing methanol: Potassium Phosphate buffer 0.05M at pH 6.5 (40:60) at 1.0 mL.min-1 flow rate with the detection done at 280nm. The PTSA was detected in a solvent system containing methanol water (40:60) at 1.0 mL.min-1 flow rate with the detection done at 229nm. The retention times (TR in minutes) of SFA, PTSA and TPA were 2.6, 3.2 and 3.4 respectively in their specific solvent systems and flow rates as mentioned above. The degradation/disappearance of three aromatic sulfur compounds PTSA, SFA and TPA by the strain JS1T was determined though HPLC at regular intervals from 0 h to 15 days of incubation. Three months (90 days) old inoculated sample was also analyzed for each test compound.
Soil and Sludge microcosm studies for biodegradation
The microcosm studies were carried out in soil collected from JNTU (Jawaharlal Nehru Technological University) campus and anaerobic sludge collected from the sedimentation tank of JETL (Jeedimetla Effluent Treatment Limited), Hyderabad. The sludge used in this experiment had a pH of 7.58, EC (Electrical conductivity) of 16.42 μS, alkalinity of 440 mg L-1, COD of 4200 mg L-1 (for 10 times diluted sample), TDS (Total Dissolved Solids) of 4308 mg L-1. These parameters were analyzed according to the methods suggested in the standard methods for determination of water and wastewater. The soil used had a pH of 7.2. The carbon, nitrogen, hydrogen and sulfur content in the soil were analyzed by Elementar make CHNS analyzer comprising Vario Micro software. The soil contained 1.8% organic carbon, 0.2% hydrogen, 0.4% nitrogen and 0.02% sulfur.
The strain JS1T grown in PBM supplemented with 3 mM of the test compound as sole source of carbon was centrifuged and the cell pellet was suspended in the PBM without any carbon source. This was used as inoculum in the soil and sludge microcosms spiked with the test compound. Microcosms were carried out in test tubes (25 X 150 mm) with 20g soil and/or 20 ml sludge spiked with one aromatic sulfur test compound in each at 3 mM concentration supplemented with 2 ml PBM without any carbon source in two sets of tubes. One set was autoclaved which indicated the degradation of test compound by the inoculated strain JS1T alone and another set was left unautoclaved to assess the degradation of the test compound by the native microorganisms if any. Within the autoclaved and unautoclaved sets of tubes, one set was inoculated with 2 ml of the strain JS1T and another was left uninoculated. The culture was thoroughly mixed with the soil and sludge, then closed with rubber seal followed by flushed with Argon gas to maintain anaerobic conditions and incubated at 28 ± 2 °C for 20 days. Soil and sludge samples (1g and/or 1mL) were drawn and analyzed for concentrations of each test compound at 5 days intervals.
For extraction of PTSA, SFA and TPA from soil and for sludge, 1g of soil or sludge was suspended in 10ml of deionized water and vortexed thoroughly followed by centrifugation at 7845 g for 10 min. The supernatant was extracted and diluted 10 times (10 μL supernatant in 90 μL deionized water) and was analyzed by HPLC as mentioned in the previous segment. All experiments carried out in triplicate. Numerical data are presented as mean ± SD.
Results
Growth of JS1T on aromatic sulfur compounds
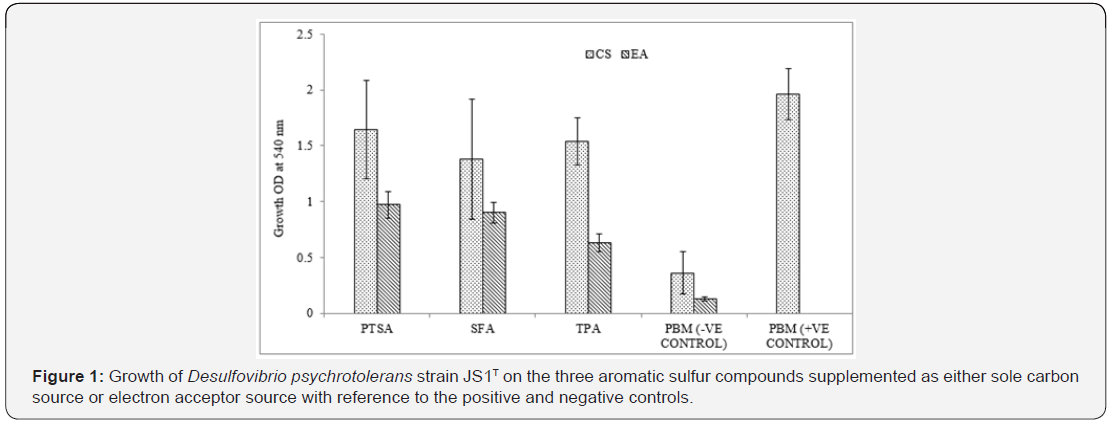
Strain JS1T showed a gradual increase in growth within the tested period of 10 days in all the three aromatic sulfur test compounds. Growth in terms of OD at 540 nm was maximum (1.98) in positive control where PBM (with 3 mM lactate as carbon source and 0.5 mM FeSO4 as electron acceptor) was used and was minimum (0.1 & 0.3) where PBM without any carbon or electron acceptor source was used respectively. Growth of JS1T was comparatively more in medium with the test compound supplemented as Carbon Source (CS) than as Electron Acceptor (EA). The decreasing order of growth in terms of OD at 540 nm after 10 days of incubation of JS1T in the test compounds was PTSA-CS (1.64) > TPA-CS (1.54) >SFA-CS (1.38) >PTSA-EA (0.97) >SFA-EA (0.90) >TPA-EA (0.63) (Figure 1).
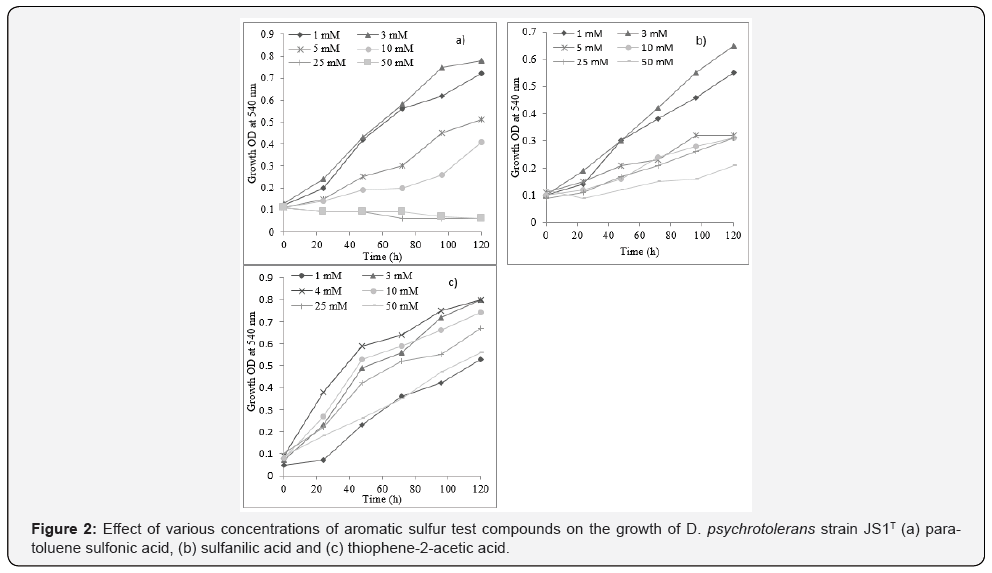
The effect of various concentrations from 0 mM to 50 mM of each of the three aromatic sulfur test compounds, i.e., PTSA, SFA and TPA when given as sole carbon source on the growth of strain JS1T within a time course of 5 days showed that the strain JS1T grew optimally in all three test compounds at 3 or 4 mM concentration. Growth was feeble at and above 25 mM concentration of PTSA, while the strain could tolerate up to 50 mM concentration of SFA and TPA (Figure 2).
Degradation of Aromatic sulfur compounds by live and dead biomass of strain JS1T
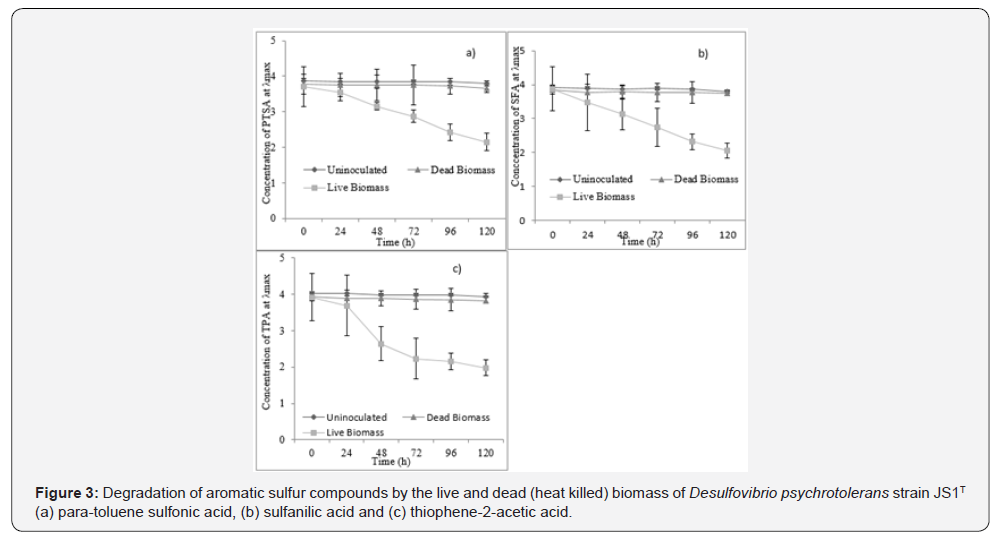
In the experiment conducted with live and dead biomass of JS1T to understand the loss of each test compound in the course of degradation, due to passive adsorption, if any, it was observed that the compound adsorbed was ignorable in all the three cases. There was no decrease in concentration of the test compounds inoculated with dead biomass of JS1T until 5 days of observation. Among the three test compounds, 42% of PTSA (Figure 3-a), 47% of SFA (Figure 3-b) and 49% of TPA (Figure 3-c) were degraded after 5 days of incubation by the strain. None of the tested compounds were completely degraded by strain JS1T within 5 days of incubation. The compounds PTSA and SFA were gradually degraded up to day 5 while the degradation of TPA was rapid up to day 2 after inoculation and then the degradation slowed down gradually till the end of day 5 (Figure 3).
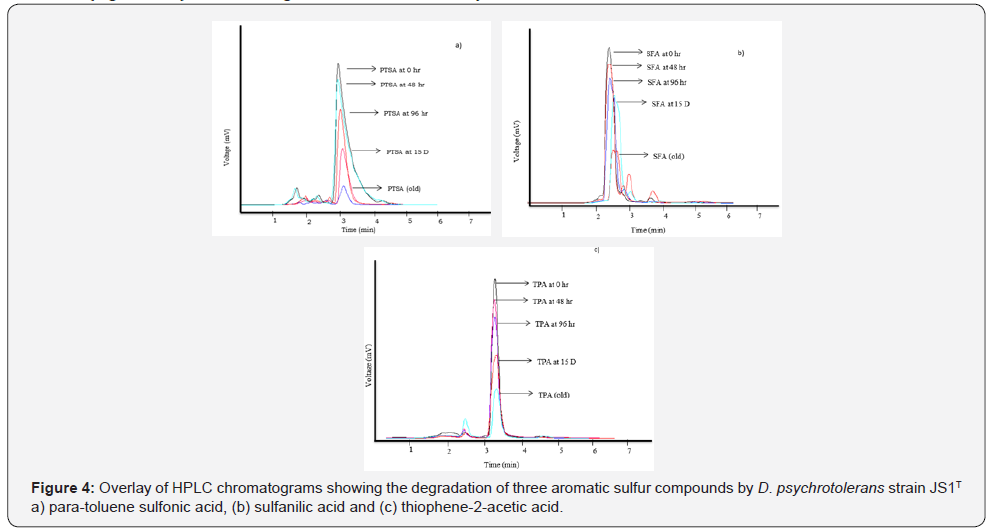
HPLC determination of degradation of aromatic sulfur compounds
The HPLC analysis of degradation of the aromatic sulfur test compounds by the strain JS1T showed a decrease of PTSA by 13%, 34% and 60% after 2, 4 and 15 days of incubation respectively and a maximum decrease of 83% after 90 days of incubation. Neither additional peaks nor peak shift was observed up to 15 minutes of run of the test compound in HPLC (Figure 4-a). A decrease in concentration of SFA by 10%, 20% and 33% after 2, 4 and 15 days of incubation respectively and a maximum decrease of 75% were observed after 90 days of incubation. The decrease in the SFA peak (TR 2.6) was associated with an increase in an unknown peak (TR 3.0). But the increase in the unknown peak was not proportional to the decreasing SFA peak (Figure 4-b). A decrease in concentration of TPA was noted by 11%, 23% and 47% after 2, 4 and 15 days of incubation respectively and a maximum decrease of 68% was observed after 90 days of incubation. The decrease in the TPA peak (TR 3.4) was associated with an increase in an unknown peak (TR 2.6). But this was not proportional to the decrease in TPA peak (Figure 4-c). None of the test compounds were degraded completely within the tested period of three months.
Degradation of the selected aromatic sulfur compounds by Desulfovibrio psychrotolerans, JS1T
The degradation of aromatic sulfur compounds in sludge microcosms due to the individual and combined activities of D. psychrotolerans, JS1T and the native microorganisms (if any, present in the sludge sample) analyzed through HPLC observed after 10 days of incubation revealed a degradation of PTSA up to 38% by strain JS1T alone (Figure 5-1a) and up to 47% by the combined activity of JS1T and native microbes of the sludge (Figure 5-1b). SFA was degraded up to 64% by strain JS1T alone (Figure 5-2a) and 58% by the combined activity of JS1T and native microbes of the sludge (Figure 5-2b). TPA was degraded up to 31% by strain JS1T alone (Figure 5-3a) and 48% degradation was observed by the combined activity of JS1T and native microbes of the sludge (Figure 5-3b). However, no significant decrease in concentrations of PTSA, SFA and TPA was observed in uninoculated sludge samples either autoclaved or unautoclaved (Figure 5).
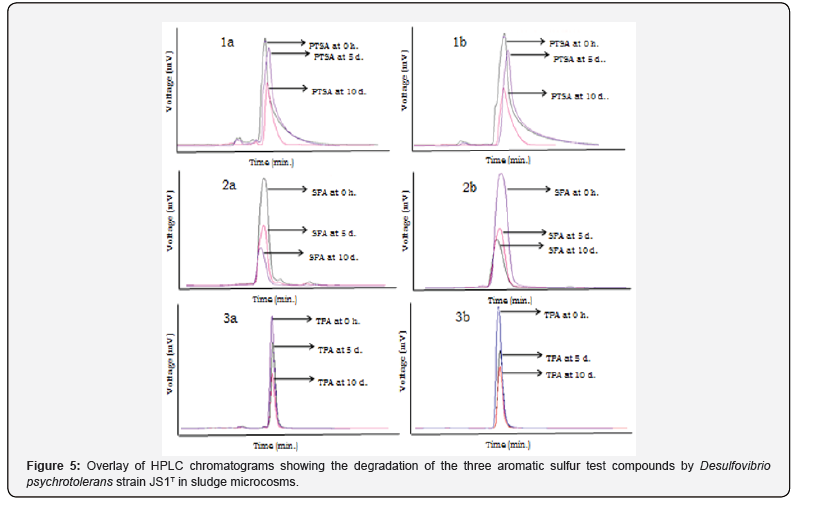
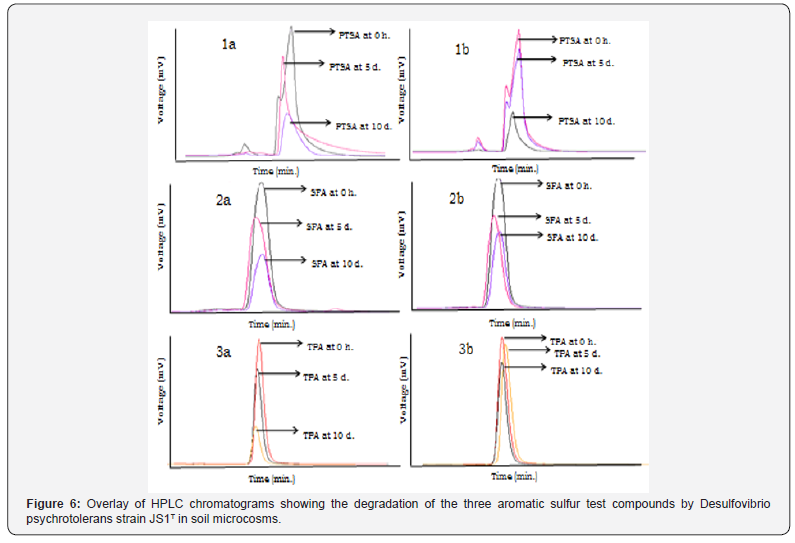
Similarly, the degradation of aromatic sulfur compounds in soil microcosms revealed a PTSA degradation of up to 66% by strain JS1T alone (Figure 6-1a) and 63% degradation by the consortium of JS1T and native microbes of the soil (Figure 6-1b). SFA was degraded up to 65% by strain JS1T alone (Figure 6-2a) and 40% degradation was observed by the consortium of JS1T and native microbes of the soil (Figure 6-2b). TPA was degraded up to 72% by strain JS1T alone (Figure 6-3a) while only 30% degradation was observed by the consortium of JS1T and native microbes of the soil (Figure 6-3b). However, no significant decrease in concentrations of PTSA, SFA and TPA was observed in uninoculated soil samples either autoclaved or unautoclaved (Figure 6).
Discussion
Sulfate reducing bacteria are phylogenetically and physiologically diverse group of bacteria, characterized by their versatile metabolic capabilities to use various electron acceptors and donors [13,14]. They are generally considered as the terminal oxidizers in the natural recycling of organic compounds to CO2 in anoxic environments. Due to these exceptional capabilities, SRB are studied and applied in various bio-degradative tasks under anoxic regions. In the present study, three test compounds were selected for biodegradation studies, namely Para-Toluene Sulfonic Acid (PTSA), Sulfanilic acid (SFA) and Thiophene-2-acetic acid (TPA). These pollutants enter into environment through effluents from textile, dye and chemical industries and petroleum products. These compounds were used as sole sulfur source or as sole carbon/electron donor source in many previous reports [15-17]. In the present study, the biodegradability of these compounds by strain JS1T was tested by supplementing them as either sole carbon source and/or sole electron acceptor. Shcherbakova, et al. [18] reported that a sulfate reducing strain Desulfovibrio sp. SR1 utilized PTSA as an electron acceptor. PTSA and SFA have reducible sulfur moiety in the chemical structure, and hence can also serve as electron acceptor in addition to being utilized as carbon source. TPA on the other hand was reported to undergo degradation in soil under oxygen limiting conditions when no other external electron acceptor was added and dibenzothiophene served as an electron acceptor in anaerobic respiration in a report by Annweiler, et al. [19]. Hence the degradation of TPA was also tested in the absence of any electron acceptor. Desulfovibrio psychrotolerans, JS1T was selected as the test organism for studying the degradation of aromatic sulfur compounds, as this was the only bacterium among 5 cultures tested, that could grow in a medium supplemented with the test compounds.
Our efforts to enrich SRB degrading these compounds from 20 different environmental samples also were not successful (data not shown). The D. psychrotolerans, JS1T could grow in medium with the aromatic sulfur compounds supplemented as either sole carbon source or as sole electron acceptor. But as D. psychrotolerans, JS1T could grow well in medium with the aromatic sulfur compounds supplemented as sole carbon source than as sole electron acceptor source, the test compounds were supplemented as sole carbon source in further degradation studies. This result also supports the earlier report that aromatic compounds are metabolized by SRB much efficiently when they are supplemented as sole carbon source [20, 21].
Hulshoff Pol [22] has reported that many SRB are quite resistant to overloads of certain organic compounds and to toxic upsets from aromatic compounds like alkanes, ethylbenzene, toluene, chloroform and other long chain fatty acids and can dominate in growth by competing with other anaerobic bacteria. A similar observation was made with respect to the tolerance of the test compounds by strain JS1T. The D. psychrotolerans, JS1T was not sensitive to even very high concentrations of the test compounds. PTSA was tolerated up to a concentration of 25 mM while SFA and TPA were tolerated even up to the highest concentration of 50 mM tested. However, optimum growth was observed when the aromatic sulfur compounds were supplemented as sole carbon sources within a concentration of 3 and 4 mM. The pattern of degradation of the test compounds by D. psychrotolerans, JS1T was similar to that of its growth on these compounds. A few SRB have been reported to adsorb certain metals and pollutants especially in activated sludge [23, 24]. Hence, it was essential to understand whether disappearance of the compound observed only in the presence of D. psychrotolerans, JS1T was due to the biodegradation or simple passive adsorption. It was concluded that passive adsorption did not play a significant role since there was no significant loss (<5% loss) in the concentration of the compounds when inoculated with dead biomass (heat killed), while there was rapid decrease in their concentration when inoculated with live biomass within 5 days of incubation.
Though there are no reports of degradation of aromatic sulfur compounds by any SRB, Desulfobacula toluolica and Desulfobacula phenolica [20,25], Desulfosarcina cetonica have been demonstrated to degrade toluene completely to CO2. The mixed cultures of SRB and other methanogenic bacteria were reported to degrade toluene. Degradation of PTSA under anaerobic conditions by mixed cultures was reported by Shcherbakova, et al. [18]. Also, anaerobic degradation of dibenzothiophene [17,26] and anaerobic desulfurization of benzothiophene and dibenzothiophene have been reported in mixed cultures of SRB earlier, no reports exist on the anaerobic degradation of either PTSA, SFA or TPA by pure cultures of SRB. Hence, the present study on degradation of aromatic sulfur compounds by D. psychrotolerans, JS1T is the first such study of degradation by any pure culture of SRB. However, none of the aromatic sulfur compounds were completely degraded within the experimental period of three months (PTSA was degraded by 82%, SFA by 65.5% and TPA by 72%).
In general, laboratory studies are not accurate predictors of field degradation rates [27]. Bioremediation via environmental introduction of degradative microorganisms requires that microbes survive in substantial numbers and effect an increase in the rate and extent of pollutant removal in these natural habitats [28,29]. Measuring biodegradative activity and efficiency of the selected microbes in natural habitats is difficult due to limited accessibility of samples as well as sorption and abiotic transformation of contaminants [30]. Due to these reasons, microcosm studies are generally used to understand these important parameters before switching on to on-site studies.
Microcosm studies are also known as the bio feasibility studies. Microcosms are artificial, simplified ecosystems that are used to simulate and predict the behavior of natural ecosystems under controlled conditions [31,32]. These studies are useful in understanding the effect caused due to a pollutant or to determine the role of microbes in eliminating or reducing the effect of these pollutants in natural systems. The microcosm studies of degradation of pollutants by SRB conducted till now have mainly concentrated on degradation by mixed populations. An example is the degradation of toluene by SRB in oil contaminated soils [33,34].
Conclusion
In the present study, the degradation of aromatic sulfur compounds and their subsequent degradation by pure culture of D. psychrotolerans, JS1T was carried out in both soil and sludge microcosms that mimic the naturally existing environments of contaminants. D. psychrotolerans JS1T was employed independently (in sterile soil and/or sludge) and also in consortium with the native microbiota (unsterile soil and/or sludge) inhabiting the soil and sludge samples. The viability of spiked pure culture of strain JS1T in soil and sludge microcosm could be checked by streaking the spiked samples on to PBM at regular time intervals during the experiment and observing for the pure colonies of the strain JS1T.
The role of native microbiota alone in the degradation of the test compounds was insignificant as observed in the unsterile and uninoculated microcosms of soil and sludge spiked with the aromatic sulfur compounds. PTSA was degraded by strain JS1T much efficiently in soil microcosm (66%) than in sludge microcosm (38%). Degradation of PTSA by indigenous soil and sludge microbiota in consortium with JS1T was not very significant, but a slight increase of degradation to 47% (from 38%) in sludge and a slight decrease of degradation to 63% (from 66%) in soil were observed. The degradation of SFA by pure culture of JS1T was almost similar in sludge and soil microcosms (64 and 65% respectively) but the degradation in consortium with soil and sludge microflora was lowered to 58% (from 64%) in sludge and 40% (from 65%) in soil microcosms respectively. The degradation of TPA was the least in sludge microcosm (31%) while the highest in soil microcosm (72%). There was a significant decrease in degradation to 30% (from 72%) due to contribution of soil consortia and an increase to 48% (from 30%) due to contribution of sludge consortia. The present work in lab scale is a preliminary study conducted at ambient conditions (pH, temperature, nutritional conditions etc.) of naturally occurring soils and sludge and thus indicates the potential of D. psychrotolerans, JS1T for bioremediation of contaminated soils and sludge.
Conflict of Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Shinoda Y, Akagi J, Uchihashi Y, Hiraishi A, Yukawa H, et al. (2005) Anaerobic degradation of aromatic compounds by Magnetospirillum strains, Isolation and degradation genes. Biosci Biotechnol Biochem 69(8): 1483-1491.
- Abdel-Shafy HI, Mansour MS (2016) A review on polycyclic aromatic hydrocarbons: source, environmental impact, effect on human health and remediation. Egypt petrol 25(1): 107-123.
- Harwood CS, Burchhardt G, Herrmann H, Fuchs G (1999) Anaerobic metabolism of aromatic compounds via the benzoyl-coa pathway. FEMS Microbiol Rev 22(5): 439-458.
- Hong Y, Gu JD (2009) Bacterial anaerobic respiration and electron transfer relevant to the biotransformation of pollutants. Int Biodeterior Biodegradation 63(8): 973-980.
- Jyothsna TSS, Sasikala Ch, Ramana ChV (2008) Desulfovibrio psychrotolerans nov, a psychrotolerant and moderately alkaliphilic sulfate-reducing deltaproteobacterium from the Himalayas. Int J Syst Evol Microbiol 58(4): 821-825.
- Locher HH, Leisinger T, Cook AM (1989) Degradation of p-Toluenesulphonic Acid via Side chain Oxidation, Desulphonation and meta Ring Cleavage in Pseudomonas (Comamonas) testosteroni T-2. J Gen Microbiol 135(7): 1969-1978.
- Paszczynski A, Pasti-Grigsby MB, Goszczynski S, Crawford RL, Crawford DL (1992) Mineralization of sulfonated azo dyes and sulfanic acid by Phanerochaete chrysosporium and Streptomyces chromofuscus. Appl Environ Microbiol 58(11): 3598-3604.
- Faryal R, Ahmed S, Hameed A (2006) Biodegradation of 4-aminobenzene sulphonic acid by a local textile mill Aspergillus niger Pak J Bot 38: 1333-1340.
- Perei K, Rákhely G, Kiss I, Polyák B, Kovács KL (2001) Biodegradation of sulfanilic acid by Pseudomonas paucimobilis. Appl Microbiol Biotechnol 55(1): 101-107.
- Hirasawa K, Ishii Y, Kobayashi M, Koizumi K, Maruhashi K (2001) Improvement of desulphurisation activity in Rhodococcus erythropolis KA2-5-1 by genetic engineering. Biosci Biotechnol Biochem 65(2): 239-246.
- Ratledge C (Ed.) (2012) Biochemistry of microbial degradation. Springer Science & Business Media.
- Postgate JR (1984) The sulphate-reducing bacteria, (2nd ed). Cambridge University Press, Cambridge, England.
- Widdel F (1998) Microbiology and ecology of sulfate - and sulfur-reducing bacteria. Biology of Anaerobic microorganisms. Zehnder AJB (ed.). John Wiley and Sons, Inc. New York. pp.469-585.
- Muyzer G, Stams AJ (2008) The ecology and biotechnology of sulphate-reducing bacteria. Nat Rev Microbiol 6(6): 441-454.
- Locher HH, Poolman B, Cook AM, Konings WN (1993) Uptake of 4-toluene sulfonate by Comamonas testosteroni T-2. J Bact 175(4): 1075-1080.
- Yamashova N, Telegina A, Kotova I, Netrusov A, Kalyuzhnyi S (2004) Decolorization and partial degradation of selected azo dyes by methanogenic sludge. Appl Biochem Biotechnol 119(1): 31-40.
- Yamada KO, Morimoto M, Tani Y (2001) Degradation of dibenzothiophene by sulfate reducing bacteria cultured in presence of only Nitrogen gas. J Biosci Bioengineer 91(1): 91-93.
- Shcherbakova VA, Chuvilskaya NA, Golovchenko NP, Suzina NE, Lysenko, et al. (2003) Analysis of the anaerobic microbial community capable of degrading p-Toluene sulfonate. Microbiology 72(6): 666-671.
- Annweiler E, Michaelis W, Meckenstock RU (2001) Anaerobic cometabolic conversion of benzothiophene by a sulfate reducing enrichment culture and in a tar-oil contaminated aquifer. Appl Environ Microbiol 67: 5077-5083.
- Harms G, Zengler K, Rabus R, Aeckersberg F, Minz D, et al. (1999) Anaerobic oxidation of o-xylene, m-xylene, and homologous alkylbenzenes by new types of sulfate-reducing bacteria. Appl Environ Microbiol 65(3): 999-1004.
- Togo CA, Mutambanengwe CCZ, Whiteley CG (2008) Decolourisation and degradation of textile dyes using a Sulphate Reducing Bacteria (SRB)–biodigester microflora co-culture. Afr J Biotechno 7(2): 1483-1491.
- Hulshoff Pol LW, Lens PN, Stams AJ, Lettinga G (1998) Anaerobic treatment of sulphate-rich wastewaters. Biodegradation 9(3-4): 213-224.
- Byrns G (2001) The fate of xenobiotic organic compounds in wastewater treatment plants. Water Res 35(10): 2523-2533.
- Mohammed AS, Kapri A, Goel R (2011) Heavy metal pollution: source, impact, and remedies. In Biomanagement of metal-contaminated soils 1-28.
- Rabus R, Nordhaus R, Ludwig W, Widdel F (1993) Complete oxidation of toluene under strictly anoxic conditions by a new sulfate-reducing bacterium. Appl Environ Microbiol 59(5): 1444-1451.
- Rodrigo J, Boltes K, Esteve-Nuñez A (2014) Microbial-electrochemical bioremediation and detoxification of dibenzothiophene-polluted soil. Chemosphere 101: 61-65.
- Di HJ, Aylmore LAG, Kookana RS (1998) Degradation rates of eight pesticides in surface and subsurface soils under laboratory and field conditions. Soil Sci 163: 404-411.
- Krumme ML, Smith RL, Egestorff J, Thiem SM, Tiedje JM, et al. (1994) Behaviour of pollutant-degrading microorganisms in aquifers: Predictions for genetically engineered organisms. Environ Sci Technolo 28(6): 1134-1138.
- Adams GO, Fufeyin PT, Okoro SE, Ehinomen I (2015) Bioremediation, biostimulation and bioaugmention: a review. Int J Environ Bioremediat Biodegrad 3(1): 28-39.
- Margesin R, Schinner F (1997) Efficiency of indigenous and inoculated cold-adapted soil microorganisms for biodegradation of diesel oil in Alpine soils. Appl Environ Micrbiol 63(7): 2660-2664.
- Kassen R, Buckling A, Bell G, Paul B (2000) Diversity peaks at intermediate productivity in a laboratory microcosm. Nature 406 508-512.
- Beyers RJ, Odum HT (2012) Ecological microcosms. Springer Science & Business Media.
- Noh SL, Choi JM, An YJ, Park SS, Cho KS (2003) Anaerobic biodegradation of toluene coupled to sulfate reduction in oil-contaminated soils: optimum environmental conditions for field applications. J Environ Sci Health 38(6): 1087-1097.
- Kobayashi M, Horiuchi K, Yoshikawa O, Hirasawa K, Ishii Y, et al. (2001) Kinetic analysis of microbial desulphurisation of model and light gas oil containing multiple alkyl dibenzothiophenes. Biosci Biotechnol Biochem 65(2): 298-304.






























