Production of Pectinases and Pectinolytic Enzymes: Microorganisms, Cultural Conditions and Substrates
Palagiri Samreen, Mayukha Mangipudi, Sagar Grover, Chourasiya Rajan and Sibi G*
Department of Biotechnology, Indian Academy Degree College-Autonomous, India
Submission: April 10, 2019; Published:June 21, 2019
*Corresponding author: Sibi G, Head, Department of Biotechnology, Indian Academy Degree College-Autonomous, Bengaluru, India
How to cite this article:Palagiri Samreen, Mayukha Mangipudi, Sagar Grover, Chourasiya Rajan, Sibi G. Production of Pectinases and Pectinolytic Enzymes: Microorganisms, Cultural Conditions and Substrates. Adv Biotechnol Microbiol. 2019; 14(2): 555884. DOI: 10.19080/AIBM.2019.14.555884
Abstract
Pectinase comprises a heterogeneous group of enzymes that catalyze the breakdown of pectin containing substrates. The most important enzymes of the pectinase complex are polygalacturonase, pectin lyase, pectate lyase and pectin esterase. Solid state fermentation is considered as an efficient method for the synthesis of pectinolytic enzymes over submerged fermentation. Microorganisms that are particularly suitable for pectinase production through fermentation are the filamentous fungi followed by bacteria. This article aims to present an overview of potential pectinolytic microorganisms, their fermentation conditions, factors involved in maximum pectinase activity and interesting summary of substrates used in the fermentation processes. In conclusion, recent developments in industrial biotechnology offer several opportunities for the utilization of low-cost substrates for the pectinolytic enzyme production using fungi and bacteria through fermentation.
Keywords: Pectinase; Pectinolytic enzymes; Polygalacturonase; Aspergillus; Bacillus; Solid state fermentation
Introduction
Enzymes have found their way into many new industrial processes. Demand is increasing to replace some traditional processes such as chemical bleaching with biotechnological processes involving microorganisms. Use of microbial enzymes has been an increasing trend in different industries. Fermentation technique is considered as a powerful tool for the synthesis of valuable products by the help of microbes. Pectin comprises of D-galacturonic acid occurred in α-1,4 chain, naturally esterifies with methoxy groups and natural sugars occupy the side chains. Pectinase comprises a heterogeneous group of enzymes that catalyze the breakdown of pectin-containing substrates. The most important enzymes of the pectinase complex are polygalacturonase, pectin lyase, pectate lyase and pectin esterase. Pectin esterase catalysis the de-esterification of the methoxy group of pectin, forming pectic acid. Hydrolases (Polygalacturonases and Polymethyl galacturonases) catalysis the hydrolytic cleavage of α-1,4-glycosidic linkage in pectic acid and pectin, respectively, while Lyases (Polygalacturonate Lyase and Poly methyl galacturonate Lyase)-Catalysis the cleavage of α-1,4-glycosidic linkage in pectic acid and pectin, respectively by trans-elimination reaction and forming unsaturated galacturonates and methyl galacturonates, respectively. Pectinases are widely used in the food industry to improve the cloud stability of fruit and vegetable nectars, for production and clarification of fruit juices and for haze removal from wines [1]. Pectinases are also involved in clarification of wine, oil extraction, removal of citric fruit peels, and degumming fibres [2-4]. Pectinase usage accelerates tea fermentation and also destroys the foam forming property of instant tea powders by destroying pectins. They are also used in coffee fermentation to remove mucilaginous coat from coffee beans [5-7]. Considering the advantages of pectinase enzymes in food industries, this article aims to present an overview of potential pectinolytic microorganisms, their fermentation conditions, factors involved in maximum pectinase activity and interesting summary of substrates used in the fermentation processes.
Microorganisms
The most important factors to be considered during the pectinase production are the choice of microorganisms. Microorganisms that are particularly suitable for pectinase production through fermentation are the filamentous fungi, since the technique simulates their natural habitat. During fermentation, they are able to synthesize considerable amounts of enzymes and other metabolites. Although filamentous fungi (Aspergillus and Penicillium) are considered the most appropriate microorganisms for pectinase production, there are also some species of bacteria (Bacillus, Erwinia, Enterobacter) which have been reported to successfully produce pectinases.
Pectinase was produced by solid state fermentation with Aspergillus niger using sugar beet pulp as carbon source and the wastewater from monosodium glutamate production as nitrogen and water source [8]. Aspergillus oryzae was cultivated in a pilotscale packed-bed bioreactor containing citrus pulp and sugarcane bagasse [9]. Pectinase yields of 33-41 U g-1 were obtained and indicated the potential for using solid-state fermentation to produce pectinases in a citrus waste biorefinery. Banana peel was used as substrate to cultivate A. niger for pectinase production by Barman, et al. [10]. The optimum substrate concentration, incubation time and temperature of incubation were 8.07 %, 65.82 h and 32.37 °C respectively, and the polygalacturonase activity achieved was 6.6 U/ml for crude pectinase. The use of three Aspergillus species for pectinolytic enzyme production in solid state fermentation was studied by Heerd et al. [11]. All strains produced pectinases with the highest yield reached between the fourth and fifth day of cultivation. The highest exopectinolytic activity with 33.4 U/g Polymethylgalacturonases and 28.3 U/g polygalacturonase, as well as the highest endoenzyme activity of 32.9 U/g PMG and 30.1 U/g PG was observed by A. sojae ATCC 20235. A. oryzae showed considerable prospective for the production of industrially important pectin lyase in SSF of lemon peel waste (residual waste from citrus field crop) with maximal 875 6.2 U/mL activity [12]. Pectinase production from A. niger on orange peel waste by submerged fermentation was studied by Ahmed et al. [13]. The maximum enzyme activity of 99.4 ± 1.1 μM/mL/min was observed under optimum (4%) substrate concentration. pH of 5 and 50°C was found optimum for maximum activity of pectinase enzyme produced from A. niger.
Fermentation conditions
There are two fermentation methods that can be used for pectinases production, which are Solid State Fermentation (SSF) and Submerged Fermentation (SmF). Solid State Fermentation (SSF) is a microbial involved process which takes place under minute moist conditions or in the complete absence of free-flowing water contents in the growth/fermentation media. In contrast, in submerged fermentation (SmF) the nutrients and microorganisms are both submerged in water. Solid state fermentation due to its low water activity has advantage over submerged fermentation for pectinase production by fungi. Other advantages include reduced wastewater output, simpler fermentation technology and higher product concentration [14-16].
SSF processes are interesting for countries with abundant agricultural and industrial solid wastes. Agro industrial waste materials can be used both as source of energy for growth and as carbon for synthesis of cell biomass and other products. A wide range of agricultural/ agro-industrial wastes and by-product residues such as sugar cane bagasse, oranges, rice, banana and coffee are potentially suitable feedstocks for their possible bioconversion into a range of value-added product of interests like enzymes. With this regard, solid state fermentation permits the use of agricultural and agro industrial residues as substrates which are converted into bulk chemicals and fine products with high commercial value. In Asian countries, application of SSF technology in industrial scale for enzyme production is considered as a reliable fermentation process. However, the selection of a substrate for enzyme production depends on several factors including cost and availability of the substrate.
Factors affecting microbial pectinases production pH
Acidic pH was found optimum for the production of fungal pectic enzymes [17,18]. For example, polygalacturonase produced from Lentinus edodes has a relatively lower pH of 5.0 [19]. Similarly, [20] & Silva, et al. [4] observed that Pencillium griseoroseum and P. viridicatum had produced higher levels polygalacturonase and pectin esterase at pH of 4.5 and 5. Rasheedha, et al. [21] found that P. chrysogenum exhibited maximum polygalacturonase production at initial pH of 6.5. Aspergillus niger had the optimum pectolytic activity at pH 5 [22] whereas Debing, et al. [23] reported pH 6.5 as optimal for pectinase production. Maximum pectinase and polygalacturonase (1116 and 1270 Ug-1) activity was at pH 4.0 5.0 respectively by Aspergillus fumigatus [24]. Both polygalacturonase and pectin lyase from A foetidus using mango peel as substrate had optimum activities at pH 5 and 5.5 [25]. Reda, et al. [26] found that the polygalacturonase productivity by Bacillus firmus reached its maximum at initial pH 6.0 and 6.2. Similarly, for Bacillus sphaericus, the optimal pH for polygalacturonase production was found to be 6.8 [27].
Cultivation time
The fermentation period for the production of pectinase varied among earlier reports and had a profound effect on product formation. Maximum production of pectic enzyme from different moulds varies from 1 to 6 days [28]. Castilho, et al. [29] reported that the highest polygalacturonase activities were obtained by A. niger after 70 h of fermentation period. Fawole & Odunfa [22] reported that optimum production of pectin methylesterase by A. niger was obtained after 4 days of fermentation under submerged fermentation condition. Patil & Dayanand [30] observed a gradual increase in the production of pectinase by A. niger after 72 h of fermentation period in submerged and up to 96 h in solid-state conditions. In a study by Dhillon et al. [31], maximum pectinase activity by A. niger was obtained after 120 h of fermentation. The optimum production of pectinase enzyme by a fungus Aspergillus niger was observed at 48 h of fermentation as reported by Khatri et al. [32]. Aspergillus foetidus has produced polygalacturonase and pectin lyase after 120 h and 96 h in solid state and submerged fermentations, respectively [25]. In agitated cultures supplemented with 0.5% citrus pectin and initial pH of 2.5, extracellular pectinase was produced by Penicillium frequentans after 48 h at 35 °C [33]. Reda et al. [26] found that the level of polygalacturonase increased gradually with increasing the incubation period up to a maximum of 96 h by Bacillus firmus. Maximal quantities of polygalacturonase were produced by Bacillus sphaericus when a 16-hours-old inoculum was incubated in shaking condition for 72 hours [27]. Chryseobacterium indologenes strain was found to produce maximum pectinase at 37°C with pH 7.5 upon incubation for 72 hours, while cultured in production medium containing citrus pectin and yeast extract as carbon and nitrogen sources [34].
Nitrogen source
The effects of organic and inorganic nitrogen sources on the production of pectinase were extensively studied. Both ammonium phosphate and ammonium sulphate did influence production of pectinase positively [24,30,35,36]. Rasheedha et al. [21] found that ammonium sulphate has enhanced the production of P. chrysogenum pectinase. In contrast, Sapunova [37] found that ammonium salts stimulated the pectinolytic enzyme production in Aspergillus alliaceus. Fawole & Odunfa [22] found that ammonium sulphate and ammonium nitrate were good nitrogen sources for pectic enzyme production from A. niger while glycine and tryptophan did not support enzyme production. On the other hand, report of Aguilar et al. [38] showed yeast extract as the best inducer of exopectinases by Aspergillus sp. Yeast extract, peptone and ammonium chloride were found to enhance pectinase production up to 24% and addition of glycine, urea and ammonium nitrate inhibited pectinase production [39]. For Bacillus firmus, peptone has resulted in the maximum value of polygalacturonase productivity (350 U m L-1) [26]. Vivek et al. [40] found that organic nitrogen sources showed higher endo, exo pectinases activities than inorganic nitrogen sources. Soybean meal (4%) showed the maximum Exopectinase activity of 5128 IU g-1 and endo-pectinase activity of 793 IU g-1.
Carbon source
Aguilar & Huitron [41] reported that the production of pectic enzymes from many moulds is known to be enhanced by the presence of pectic substrates in the medium. Fawole & Odunfa [22] found that pectin and poly galacturonic acid promoted the production of pectic enzyme. Phutela et al. [24] stated that wheat bran supported maximum pectinase production (589 U g-1) while pure pectin give the maximum production of polygalacturonase (642 Ug-1). Patil & Dayanand [30] reported that glucose (4-6%) increase the production of pectinase in submerged condition whereas 6-8% sucrose gives better yield of pectinase in solid-state condition. Reda et al. [26] reported that Solanum tuberosum peels was the best carbon source for polygalacturonase production by Bacillus firmus. In a study, 88 ± 9 IU/mL pectinase was produced by Bacillus pumilus after inoculating into media containing 2% each of wheat bran and Citrus limetta peel, 0.5% peptone, 10 mM MgSO4, pH 7.0 [42-65].
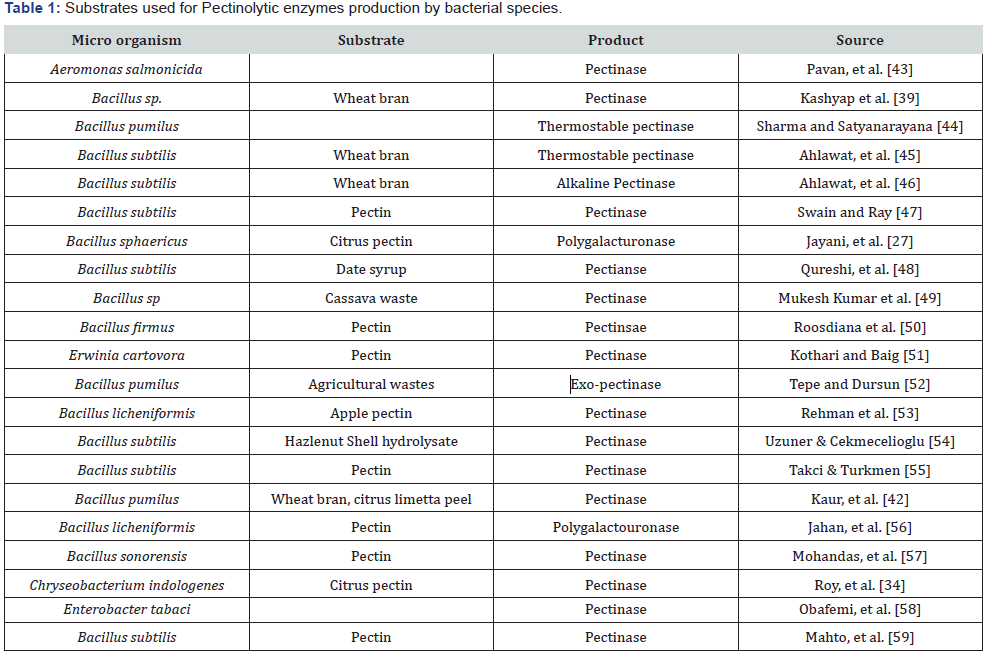
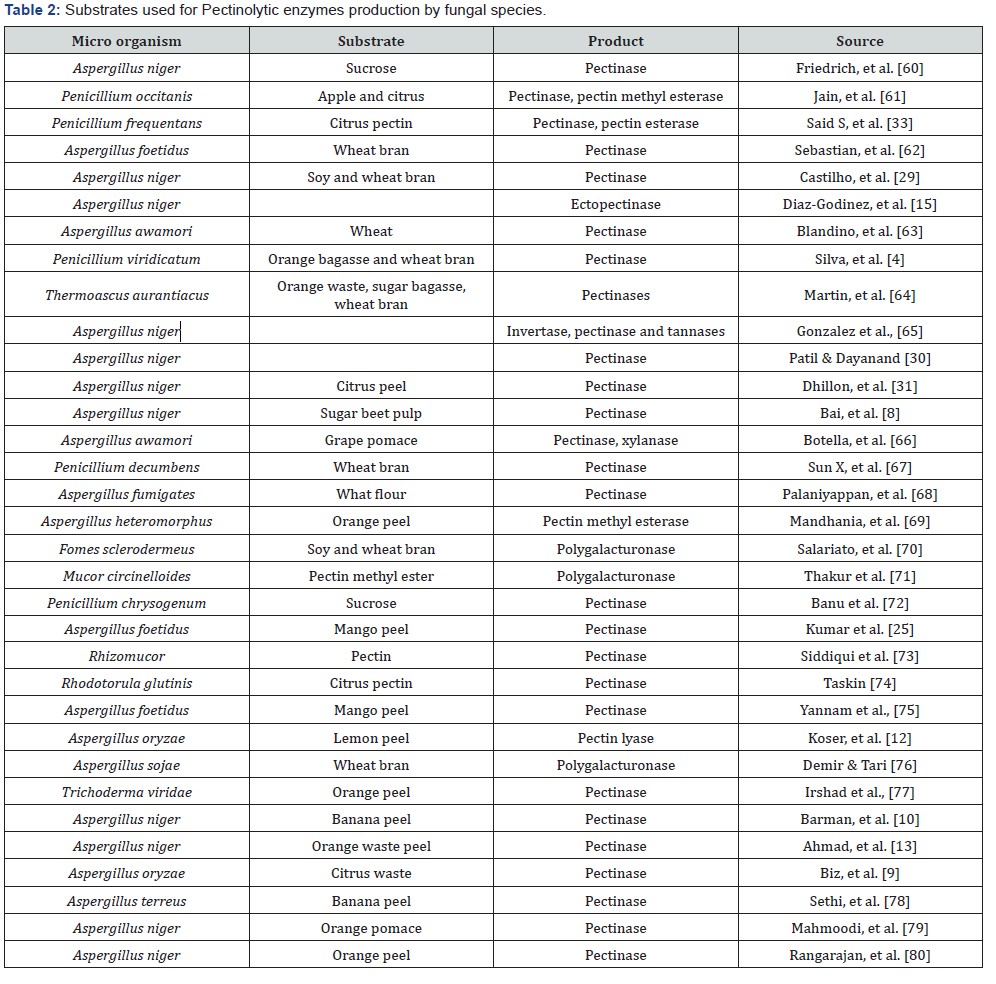
Substrates
The most promising residues for pectinase activity include agricultural residues and fruit peels. The choice of the most appropriate microorganisms (Fungi and bacteria) to be cultivated in the agro residue depends much on its composition. Usually, these agro residues are not only a solid support for nutrients absorption and biomass growth, but they are also a source of carbon and nutrients. Sometimes, supplementation is needed in order to provide all necessary nutrients for optimum growth [66- 75] (Table1&2).
Conclusion
Microbial fermentation allows the re-use of agro-industrial and/or sub-products as substrate support for biotechnological production of pectinase group of enzymes. However, it is clear that some limitations for the scale-up are still observed. In this case, the choice of the microorganism, substrate and other factors which influence the pectinase production are predominant. The economic viability industrial fermentation of pectinolytic enzymes depends on a careful selection of the microorganism and the substrate used. Recent developments in industrial biotechnology offer several opportunities for the utilization of low-cost substrates for the pectinolytic enzyme production using fungi and bacteria through fermentation [76-80].
References
- Cavalitto SF, Arcas JA, Hours RA (1996) Pectinase production profile of Aspergillus foetidus in solid state cultures at different acidities. Biotechnology Letters 18(3): 251-256.
- Jayani RS, Saxena S, Gupta R (2005) Microbial pectinolytic enzymes: a review. Process Biochemistry 40(9): 2931-2944.
- Mutlu M, Sarioglu K, Demir N, Ercan MT, Acar J (1999) The use of commercial pectinase in fruit juice industry. Part I. Viscosimetric determination of enzyme activity. Journal of Food Engineering 41(3-4): 147-150.
- Silva D, Da Silva Martins E, Da Silva R, Gomes E (2002) Pectinase production by Penicillium viridicatum RFC3 by solid state fermentation using agricultural wastes and agro-industrial by-products. Braz J Microbiol 33(4): 318-324.
- Hoondal G, Tiwari R, Tewari R, Dahiya N, Beg Q (2002) Microbial alkaline pectinases and their industrial applications: A review. Appl Microbiol Biotechnol 59(4-5): 409-418.
- Oumer OJ, Abate D (2017) Characterization of pectinase from Bacillus Subtilis strain Btk 27 and its potential application in removal of mucilage from coffee beans. Enzyme Res 2017: 7686904.
- Sieiro C, Garcia Fraga B, Lopez Seijas J, Da Silva AF, Villa TG (2012) Microbial pectic enzymes in the food and wine industry. Food Industrial Processes - Methods and Equipment 2: 1-18.
- Bai ZH, Zhang HX, Qi HY, Peng XW, Li BJ (2004) Pectinase production by Aspergillus niger using wastewater in solid state fermentation for eliciting plant disease resistance. Bioresour Technol 95(1): 49-52.
- Biz A, Finkler ATJ, Pitol LO, Medina BS, Kreiger N, et al. (2016) Production of pectinases by solid-state fermentation of a mixture of citrus waste and sugarcane bagasse in a pilot-scale packed-bed bioreactor. Biochemical Engineering Journal 111: 54-62.
- Barman S, Sit N, Badwaik LS, Deka SC (2015) Pectinase production by Aspergillus niger using banana (Musa balbisiana) peel as substrate and its effect on clarification of banana juice. J Food Sci Technol 52(6): 3579-3589.
- Heerd D, Yegin S, Tari C, Fernandez Lahore M (2012) Pectinase enzyme-complex production by Aspergillus spp. in solid-state fermentation: A comparative study. Food and Bioproducts Processing 90(2): 102-110.
- Koser S, Anwar Z, Iqbal Z, Anjum A, Aqil T, et al. (2012) Utilization of Aspergillus oryzae to produce pectin lyase from various agro-industrial residues. Journal of Radiation Research and Applied Sciences 7(3): 327-332.
- Ahmad I, Zia MA, Hussain MA, Akram Z, Naveed MT, et al. (2016) Bioprocessing of citrus waste peel for induced pectinase production by Aspergillus niger; its purification and characterization. Journal of Radiation Research and Applied Sciences 9(2): 148-154.
- Pandey A, Socco CR, Mitchell D (2000) New developments in solid state fermentation: I. Bioprocesses and products. Process Biochemistry 35(10): 1153-1169.
- Diaz Godinez G, Soriano-Santos J, Augur C, G Viniegra-Gonzalez (2001) Exopectinases produced by Aspergillus niger in solid-state and submerged fermentation: a comparative study. Journal of Industrial Microbiology and Biotechnology 26(5): 271-275.
- Favela Torres E, Volke Sepulveda T, Viniegera Gonzalez G (2006) Production of hydrolytic depolymerising pectinases. Food Technol Biotechnol 44(2): 221-227.
- Zetelaki Horvath K (1980) Factors affecting pectinase activity. Acta Alimentaria 10: 371-378.
- Shin I, Dowmez S, Kilic O (1983) Study on pectolytic enzyme production from some agricultural wastes by fungi. Chem Microbiol Technol Lebensm 8: 87-90.
- Piccoli Valle RH, Passos FML, Passos FJV, Silva DO (2001) Production of pectin lyase by Penicillium griseoroseum in bioreactors in the absence of inducer. Braz J Microbiol 32(2): 135-140.
- Zheng Z, Shetty K (1999) Solid state production of polygalacturonase by Lentinus edodes using fruit processing wastes. Process Biochemistry 35(8): 825-830.
- Rasheedha AB, Kalpana MD, Gnanaprabhal GR, Pradeep BV, Palaniswamy M (2010) Production and characterization of pectinase enzyme from Penicillium chrysogenum. Ind J Sci Technol 3: 377-381.
- Fawole OB, Odunfa SA (2003) Some factors affecting production of pectic enzymes by Aspergillus niger. International Biodeterioration and Biodegradation 52(4): 223-227.
- Debing J, Peizun L, Stagnitti F, Xianzhe X, Li L (2005) Pectinase production by solid fermentation from Aspergillus niger by a new prescription experiment. Ecotoxicol Environ Saf 64(2): 244-250.
- Phutela U, Dhuna V, Sandhu S, Chadha BS (2005) Pectinase and polygalacturonase production by Thermophilic Aspergillus fumigatus isolated from decomposting orange peels. Braz J Microbiol 36(1): 63-69.
- Kumar YS, Kumar V, Obulam VSR (2012) pectinase production from mango peel using Aspergillus foetidus and its application in processing of mango juice. Food Biotechnology 26(2): 10-123.
- Reda AB, Yassin HM, Swelim MA, Ebtsam, Abdel Z (2008) Production of bacterial pectinase(s) from agro-industrial wastes under solid state fermentation conditions. Journal of Applied Sciences Research 4(12): 1708-1721.
- Jayani RS, Shukla SK, Gupta R (2010) Screening of Bacterial Strains for Polygalacturonase Activity: Its Production by Bacillus sphaericus (MTCC 7542). Enzyme Research 306785.
- Ghildyal NP, Ramakrishna SV, Nirmala P, Lonsane BK, Asthana HN (1981) Large scale production of pectolytic enzyme by solid state fermentation. J Food Sci Technol 18(6): 243-251.
- Castilho LR, Medronho RA, Alves TLM (2000) Production and extraction of pectinases obtained by solid state fermentation of agroindustrial residues with Aspergillus niger. Bioresource Technology 71: 45-50.
- Patil SR, Dayanand A (2006) Production of pectinase from deseeded sunflower head by Aspergillus niger in submerged and solid-state conditions. Bioresour Technol 97(16): 2054-2058.
- Dhillon SS, Gill RK, Gill SS, Singh M (2004) Studies on the utilization of citrus peel for pectinase production using fungus Aspergillus niger. International Journal of Environmental Studies 61(2): 199-210.
- Khatri BP, Bhattarai T, Shreshtha S, Maharjan J (2018) Alkaline thermostable pectinase enzyme from Aspergillus niger strain MCAS2 isolated from Manaslu Conservation Area, Gorkha, Nepal. Springerplus 9(4): 488.
- Said S, Fonseca MJV, Siessere V (1991) Pectinase production by Penicillium frequentans. World Journal of Microbiology and Biotechnology 7(6): 607-608.
- Roy K, Dey S, Uddin MK, Barua R, Hossain MT (2018) Extracellular Pectinase from a Novel Bacterium Chryseobacterium indologenes Strain SD and Its Application in Fruit Juice Clarification. Enzyme Res 3859752.
- Galiotou-Panayotou M, Rodis P, Kapantai M (1993) Enhanced polygalacturonase production by Aspergillus niger NRRL-364 grown on supplemented citrus pectin. Lett Appl Microbiol 17(4): 145-148.
- Sapunova LI, Lobanok AG, Mickhailova RV (1997) Conditions of synthesis of pectinases and proteases by Aspergillus alliaceus and production of a complex macerating preparation. Appl Biotechnol Microbiol 33(3): 257-260.
- Sapunova LI (1990) Pectinohydrolases from Aspergillus alliaceus Biosynthesis Characteristic Features and Applications. Institute of Microbiology Belarussian Academy of Science, Minsk.
- Aguilar G, Trejo BA, Garcia JM, Huitron C (1991) Influence of pH on endo and exo- pectinase production by Aspergillus species CH-Y-1043. Can J Microbiol 37(12): 912-917.
- Kashyap DR, Soni SK, Tewari R (2003) Enhanced production of pectinase by Bacillus sp. DT7 using solid state fermentation. Bioresour Technol 88(3): 251-254.
- Vivek R, Rajasekharan M, Ravichandran R, Sriganesh K, Vaitheeswaran V (2010) Pectinase production from orange peel extract and dried orange peel solid as substrates using Aspergillus niger. Int J Biotechnol Biochem 6: 445-453.
- Aguilar G, Huitron C (1987) Stimulation of the production of extracellular pectinolytic activities of Aspergillus sp. by galactouronic acid and glucose addition. Enzyme and Microbial Technology 9(11): 690-696.
- Kaur A, Singh A, Dua A, Mahajan R (2018) Cost-effective and concurrent production of industrially valuable xylano-pectinolytic enzymes by a bacterial isolate Bacillus pumilus Prep Biochem Biotechnol 47(1): 8-18.
- Pavan ME, Abbott SL, Zorzopulos J, Janda JM (2000) Aeromonas salmonicida subsp. pectinolytica subsp. Nov.., a new pectinase-positive subspecies isolated from a heavily polluted river. Int J Syst Evol Microbiol 3: 1119-1124.
- Sharma DC, Satyanarayana T (2006) A marked enhancement in the production of a highly alkaline and thermostable pectinase by Bacillus pumilus dcsr1 in submerged fermentation by using statistical methods. Bioresour Technol 97(5): 727-733.
- Ahlawat S, Battan B, Dhiman SS, Sharma J, Mandhan RP (2007) Production of thermostable pectinase and xylanase for their potential application in bleaching of kraft pulp. J Ind Microbiol Biotechnol 34(12): 763-770.
- Ahlawat S, Mandhan RP, Dhiman SS, Kumar R, Sharma J (2008) Potential application of alkaline pectinase from Bacillus subtilis SS in pulp and paper industry. Appl Biochem Biotechnol 149(3): 287-293.
- Swain MR, Ray RC (2010) Production, characterization and application of a thermostable exo polygalacturonase by Bacilus subtilis Food Biotechnol 24: 37-50.
- Qureshi AS, Bhutto MA, Chisti Y, Khushk I, Dahot MU, et al. (2012) Production of pectinase by Bacillus subtilis EFRL 01 in a date syrup medium. Afr J Biotechnol 11(62): 12563-12570.
- Mukesh Kumar DJ, Saranya GM, Suresh K, Priyadharshini DA, Rajakumar R, et al. (2012) Production and optimization of Pectinase from Bacillus sp. MFW7 using cassava waste. Asian Journal of Plant Science Research 2(3): 369-375.
- Roosdiana A, Prasetyawan S, Mahdi C, Sutrisno S (2013) Production and characterization of Bacillus firmus Journal of Pure and Applied Chemistry Research 2(1): 35-41.
- Kothari MN, Baig MMV (2013) Production and characterization of extracellular polygalacturonase by Erwinia carotovora MTCC 1428. International Journal of Advanced Biotechnology Research 4(1): 981-998.
- Tepe O, Dursun AY (2014) Exo-pectinase production by Bacillus pumilus using different agricultural wastes and optimizing of medium components using response surface methodology. Environ Sci Pollut Res Int 21(16): 9911-9920.
- Rehman HU, Siddique NN, Aman A, Nawaz MA, Baloch AH, et al. (2015) Morphological and molecular based identification of pectinase producing Bacillus licheniformis from rotten vegetable. Journal of Genetic Engineering and Biotechnology 13(2): 139-144.
- Uzuner S, Cekmecelioglu D (2015) Enhanced pectinase production by optimizing fermentation conditions of Bacillus subtilis growing on hazelnut shell hydrolysate. Journal of Molecular Catalysis B Enzymatic 113: 62-67.
- Takci HAM, Turkmen FU (2016) Extracellular pectinase production and purification from a newly isolated Bacillus subtilis International Journal of Food Properties 19(11): 2443-2450.
- Jahan N, Shahid F, Aman A, Mujahid TY, Qader SA (2017) Utilization of agro waste pectin for the production of industrially important polygalacturonase. Heliyon 3(6): e00330.
- Mohandas A, Raveendran S, Parameswaran B, Abraham A, Athira RSR, et al (2018) Production of pectinase from Bacillus sonorensis Food Technol Biotechnol 56(1): 110-116.
- Obafemi YD, Ajayi AA, Taiwo OS, Olorunsola SJ, Isibor PO (2019) Isolation of polygalacturonase-producing bacterial strain from tomatoes (Lycopersicon esculentum). Int J Microbiol 750566.
- Mahto RB, Yadav M, Sasmal S, Bhunia B (2019) Optimization of process parameters for production of pectinase using Bacillus Subtilis 1. Recent Pat Biotechnol 13(1): 69-73.
- Friedrich J, Cimerman A, Steiner W (1990) Production of pectolytic enzymes by Aspergillus niger, effect of inoculum size and potassium hexacianoferrate II-trihydrate. Applied Microbiology and Biotechnology 33(4):377-381.
- Jain S, Durand H, Tiraby G (1990) Production of extracellular pectinase enzymes by a mutant (Pol6) of Penicillium occitanis. Appl Microbiol Biotechnol 34(3): 308-312.
- Sebastian FC, Jorge AA, Roque AH (1996) Pectinase production profile of Aspergillus foetidus in solid-state cultures at different acidities. Biotechnol Lett 18(3): 251-256.
- Blandino A, Iqbalsyah T, Pandiella SS, Cantero D, Webb C (2002) Polygalacturonase production by Aspergillus awamori on wheat in solid-state fermentation. Appl Microbiol Biotechnol 58(2): 164-169.
- Martin N, de Souza SR, da Silva R, Gomes E (2004) Pectinase production by fungal strains in solid-state fermentation using agro-industrial bioproduct. Brazilian Archives of Biology and Technology 47(5): 813-819.
- Gonzalez GV, Torres EF, Aguilar CN, Romero-Gomez S J, Diaz-Godina G, et al. (2003) Advantages of fungal enzyme production in solid state over liquid fermentation systems. Biochemical Engineering Journal 13(2-3): 157-167.
- Botella C, Diaz A, Ory I, Webb C, Blandino A (2007) Xylanase and pectinase production by Aspergillus awamori on grape pomace in solid state fermentation. Process Biochemistry 42(1): 98-101.
- Sun X, Liu Z, Qu Y, Li X (2008) The effect of wheat bran composition on the production of biomass-hydrolyzing enzymes by Penicillium decumbens. Appl Biochem Biotechnol 146(1-3): 119-128.
- Palaniyappan M, Vijayagopa V, Viswanathan R, Viruthagiri T (2009) Statistical optimization of substrate, carbon and nitrogen source by response surface methodology for pectinase production using Aspergillus fumigatus MTCC 870 in submerged fermentation. Afr J Biotechnol 8(22): 6355-6363.
- Mandhania S, Jain V, Malhotra SP (2010) Culture optimization for enhanced production of microbial pectin methylesterase under submerged conditions. Asian Journal of Biochemistry 5(1): 12-22.
- Salariato D, Diorio LA, Mouso N, Forchiassin F (2010) Extraction and characterization of polygalacturonase of Fomes sclerodermeus produced by solid-state fermentation. Rev Argent Microbiol 4(1): 57-62.
- Thakur A, Pahwa R, Singh S, Gupta R (2010) Production, Purification, and Characterization of Polygalacturonase from Mucor circinelloides ITCC 6025. Enzyme Research 170549.
- Banu RA, Devi MK, Gnanaprabhal GR, Pradeep BV, Palaniswamy M (2010) Production and characterization of pectinase enzyme from Penicillium chrysogenum. Indian Journal of Science and Technology 3(4): 377-381.
- Siddiqui MA, Pande V, Arif M (2013) Polygalacturonase production from Rhizomucor pusillus isolated from fruit markets of Uttar Pradesh. Afr J Microbiol Res 7(3): 252-259.
- Taskin M (2013) Co-production of tannase and pectinase by free and immobilized cells of the yeast Rhodotorula glutinis MP-10 isolated from tannin-rich persimmon (Diospyros kaki L.) fruits Bioprocess Biosyst Eng 36(2): 165-172.
- Yannam SK, Shetty PR, Obulum VSR (2014) Optimization, purification and characterization of polygalacturonase from mango peel waste produced by Aspergillus foetidus. Food Technol Biotechnol 52(3): 359-367.
- Demir H, Tari C (2014) Valorization of wheat bran for the production of polygalacturonase in SSF of Aspergillus sojae. Industrial crops and Products 54: 302-309.
- Irshad M, Anwar Z, Zahed M, Aqil T, Mehmood S, et al. (2014) Bioprocessing of agro-industrial waste orange peel for induced production of pectinase by Trichoderma viridi; its purification and characterization. Turkish Journal of Biochemistry 39(1): 9-18.
- Sethi BK, Nanda PK, Sahoo S (2016) Enhanced production of pectinase by Aspergillus terreus NCFT 4269.10 using banana peels as substrate. 3 Biotech 6(1): 36.
- Mahmoodi M, Najafpour GD, Mohammadi M (2017) Production of pectinases for quality apple juice through fermentation of orange pomace. J Food Sci Technol 54(12): 4123-4128.
- Rangarajan V, Rajasekharan M, Ravichandran R, Sriganesh K, Vaitheeswaran V (2010) Pectinase production from orange peel extract and dried orange peel solid as substrates using Aspergillus niger. Int J Biotechnol Biochem 6(3): 445-453.






























