Efficacy of Grain Spawn and Lime/Gypsum Ratio on Mycelial Growth of Oyster Mushroom
Rukhsana Afzal1*, Abida Akram1, Rehmatullah Qureshi1, Zahid Akram2 and Kishwar Nazir Sultana3
1Department of Botany, Arid Agriculture University, Pakistan
2Department of Plant Breeding and Genetics, Arid Agriculture University, Pakistan
3Institute of Biochemistry and Bioinformatics, Arid Agriculture University, Pakistan
Submission: February 21, 2019; Published: April 17, 2019
*Corresponding author: Rukhsana Afzal, Department of Botany, Arid Agriculture University, Rawalpindi, Pakistan
How to cite this article: Rukhsana A, Abida A, Rehmatullah Q, Zahid A, Kishwar N S. Efficacy of Grain Spawn and Lime/Gypsum Ratio on Mycelial Growth of Oyster Mushroom. Adv Biotech & Micro. 2019; 13(5): 555871. DOI: 10.19080/AIBM.2019.13.555871
Abstract
Efficacy of six differential grain spawn i.e. wheat, sorghum, pearl millet, barley, rye and maize were assessed against mycelial growth (mm) of eighteen samples of oyster mushroom collected from hilly areas of Sub-Himalayan range. Grains of pearl millet and sorghum revealed paramount range 2.4-6.95 mm and 2-5.75mm respectively. Five doses of lime Gypsum for spawn production technology against eighteen samples of oyster mushroom exemplified best mycelial growth of 5.5-9mm in 1:1 ratio. Thus, best quality spawn of oysterr mushroom was produced on pearl millet and sorghum with 1:1 lime, gypsum ratio.
Keywords: Mushroom; Oyster; Spawn; Grain; Mycelial growth
Introduction
Mushroom, a macro-fungus considered as indispensable and cherished food globally owing to its distinctive flavor. They have been stimulated historically by several nations as Romans acknowledged as the “God’s Food” and Chinese as “solution of existence.” They are deliberated as beneficial and profitable diet because of obvious organoleptic worth, therapeutic values and monetary significance [1]. Almost more than 14000 varieties of mushrooms belonging to miscellaneous families have been discovered encompassing 2000 edible mushroom species. Merely five to six edible mushroom species have been commercially cultivated including button and oyster mushrooms respectively [2,3]. Oyster mushroom, member of class basidiomycetes is a ubiquitous second edible mushroom having fleshy, gilled appearance like oyster’s shell termed it “oyster mushroom” [4]. It comprised 40 edible species including P. ostreatus (Jacq. Ex Fr.) P. Kumm., P. citrinopileatus Singer, P. pulmonarius (Fr.) Quel, P. eryngii (DC.) Quel and P. florida (Eger.) as protuberant one [5,6]. All of them contain esteemed nutrients, therapeutic assets and other imperious possessions [7,8]. Oyster mushrooms are cultivationally applicable in both tropical and temperate environments [9] and its culture attained attractiveness throughout the world due to varied temperature propagation as well as consumption of lignocellulosic wastes [10, 11].
Spawn has prodigious importance in mushroom cultivation technology, which prepared on any agricultural crop substrate or grain. It has an equivalency to plant seed [12]. Mushroom spawn is a propagating medium comprised of mushroom mycelium along with subsidiary medium indispensable for fungal proper development. Suitable spawn substrate played a fundamental role in crop production and grain spawn possess advantage over other substrate spawn having mushroom mycelium equally distributed on all grain surface and preserved for a long period. Different spawn grains such as corn, wheat, millet have been employed for their impact on different edible mushrooms [13]. Grains retained moisture and aid in quick prevalence of fungal mycelium but it time depends on type and size of grain used [14]. Several additives such as lime and gypsum sustenance in enhancement of fungal mycelium proliferation and expansion by providing essential nutrients. Different doses of these additives affect the fungal mycelial running and spawn production. Therefore, the present study was planned to study the efficacy of different grains and ratio of lime and gypsum on mycelial growth of oyster mushroom.
Materials and Methods
Sample collection and their maintenance
Eighteen samples of oyster mushroom collected from selected hilly areas of Sub-Himalayan Range during 2012-2014. Pure cultures of these edible mushroom samples were maintained using tissue culture technique [15]. Small tissue portion was taken by an inoculation needle and positioned in the center of petriplates comprising PDA medium and incubated at 25 °C. This yielded fungal mycelia. Small mycelial plugs of each sample was taken and placed on the medium under a laminar flow cabinet. The process was frequently carried out and until hygienic and uncontaminated mycelial cultures were obtained and preserved for further studies.
Spawn production for oyster mushroom
Grain collection and sterilization
Grains of wheat (Triticum aestivum L.), sorghum (Sorghum bicolor L.) Moench), pearl millet (Pennisetum glucam (L.) R. Br.), barley (Hordeum vulgare L.), rye (Secale cearale L.) and maize (Zea mays L.) were collected from their corresponding sources at National Agricultural Research Centre, Islamabad, Pakistan. The grains were cleaned and soaked in water for 24 hours and spreaded on newspaper to eradicate unnecessary moisture. The grains were mixed with lime (Calcium carbonate) and gypsum (Calcium sulphate) in the ratio of 1:1, which helped in balancing of grain pH. The treated grains were taken in bottles, plugged with cotton wool, covered with aluminum foil and autoclaved at 15 psi for 1 hour [16].
Mother spawn preparation
The grain-filled bottles were kept under laminar flow cabinet, inoculated with 5mm plug of pure cultures of oyster mushroom and incubated at 27 °C. The data were chronicled after two weeks on the development of mycelium of oyster mushroom [16].
Optimization of lime and gypsum ratio for spawn preparation
Five dissimilar treatments were used i.e. To (0:0, no lime and gypsum), T1 (1:0, 1 percent lime and 0 percent gypsum), T2 (0:1, 0 percent lime and 1 percent gypsum), T3 (1:1, 1 percent lime and 1 percent gypsum), T4 (1.5:1.5, 1.5 percent lime and 1.5 percent gypsum). In this, grains of Pearl millet (P. glucam) were soaked in water for 24 hours and spreaded on the newspaper to remove unnecessary moisture. After checking grain moisture, the grains were treated with lime and gypsum in diverse ratios in bottles, plugged with cotton wool, covered with aluminum foil and autoclaved at 15 psi for 1 hour. The bottles were further cooled and inoculated with mycelium of oyster mushroom in decontaminated circumstances the bottles were incubated at 27 °C and data was recorded after two weeks in term of mycelial growth (cm) on each of the treatment [17].
Statistical analysis
The data attained was statistically evaluated using CRD and MStat-C Programme [18]. Regression equation, mean and standard error were achieved using Microsoft Excel.
Results and Discussion
Effect of different grain spawn
All grains demonstrated a substantial impression on progression of all mushroom samples (df= 44, S. S= 195.664, M. S= 1.918). It was experiential from the conclusions that two grain substrates i.e. pearl millet and chickpea had a superior influence as in term of linear growth followed by the sorghum, but mycelium appeared thin and light in chickpea as related to pearl millet. Sorghum grains considered as outstanding grains for the spawn preparation as conveyed by various researchers. In case of maize, mycelial growth was between 1.5-4.9 mm and maximum was perceived in Pl-11 and Pl-2. In rye, range of linear growth was 2.35-4.5 mm with preeminent in Pl-6. In barley, growth was 1.4-3.4 mm with superior in Pl-15. In case of wheat, light growth was perceived, and this acquired numerous days for execution of running. The range was 1.35-3.65 with dominant in Pl-2. In chickpea, Pl-7 showed best linear growth and range in all was 1.95-7.25 mm. In sorghum, 2-5.75 was observed with condensed mycelial texture in minimum days and best sample was Pl-9. Pearl millet determined fastest running rates well as compact growth ranged 2.4-6.95 mm with superlative in Pl-15. Though chickpea maximum value is maximum but numbers of days for preparation of spawn were greater than pearl millet and light mycelium.
Optimization of lime and gypsum level on the growth of oyster mushroom
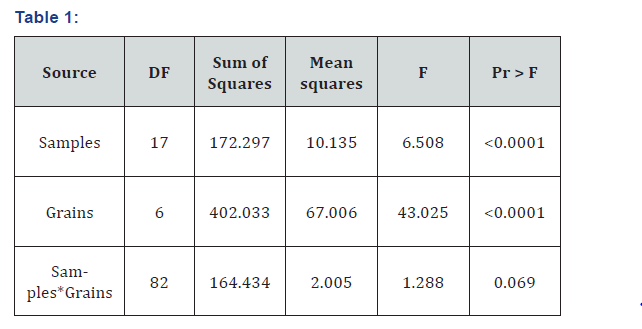
Both lime and gypsum act as a buffer for the mycelial expansion of oyster mushroom. Calcium from both of them oxidized the oxalic acid produced by the fungal mycelium. Five different treatments were experienced to assess the best lime to gypsum ratio such as T0, 0:0; T1, 1:0; T2, 0:1; T3, 1:1 and T4, 1.5:1.5 (df= 68, S.S= 445.707, M.S= 6.55). The lime to gypsum ratio 1:1 was preeminent for spawn manufacturing process and its long-term preservation. In T0, having no lime and gypsum displayed augmented mycelial growth on grains but had sticky presence in the future and there was no pH maintenance. The T1 and T2 revealed slight rise in the mycelial growth whereas T3 and T4 respond well to the mycelial advancement of oyster mushroom. In the T0, pragmatic linear growth was 2-8.5 mm with paramount samples were Pl-6 and Pl- 10. In T1, superlative growth was in Pl-11 tracked by Pl-7. In T2, maximum growth was seen in Pl-18 followed by Pl-17. In T3, the range was 5.5-9 mm with supreme increased by all samples. In T4, Pl-18 had maximum one and range was 2-7.8 mm. Though T0 had a worthy growth percentage but grain adhesiveness was observed. So, for the paramount spawn production, 1:1 lime and gypsum ratio was employed in the future. Both chemicals demonstrated a vibrant fragment in spawn preparation and storage eventually resulted in better mushroom production. It was determined that lime/gypsum ratios and samples both momentously influenced the mushroom progression rate and their collaborations exhibited inordinate importance over the fungal mycelium (Table1), (Figures1&2).


In mushroom cultivation, spawn is regarded as fermented provision of mushroom seeds on a proper grain or crop substrate under aseptic conditions. Spawn incorporates fungal mycelium and supportive medium primarily from pure mushroom culture [19]. Due to the capability of substrate strengthening and easiness of planting grain spawn is in common practice. In this, Pearl millet and sorghum grains revealed superlative mycelium running rate. Spawn running on diverse grains related to their size as extreme inoculation points was provided by small grains for completion, however larger grains with great reserved food have an capacity to maintain fungal mycelium in traumatic condition for longer period [20,21]. Quality of diverse grain and additives ratio significantly affected the spawn running [22,23]. Millet grains are best carbohydrate source certifying suitable nutritive supremacy for mycelium propagation [24]. Millet grain for spawn production of mushroom was also determined [25,26]. Extensive oyster mushroom production can be conquered using collective grain substrate of millet and sorghum in 1:3 ratios [27,28]. Improved fungal mycelium rate was observed on red sorghum followed by blending of red and white sorghum [29]. Four different grains (wheat, barley, sorghum and millet) subsidized equally in quality spawn production of oyster mushroom [30,31]. Enhancement of spawn structure and inadequacy of pH was carried out by suitable lime and gypsum ratio as unnecessary lime causes decline in fungal mycelial growth as well as nutrients taking capacity [32,33]. Mushroom mycelium can flourish efficaciously at explicit pH level, due to proper uptake of substrate’s nutrients by fungal mycelium [34]. Greatest spawn running was perceived at 1:1 lime, gypsum ratio. Accrual of lime improved the lignocellulytic enzyme production owing to neutral pH and crucial minerals, Sulphur and calcium existence [35].
References
- Chang ST, Miles PG (2008) Mushrooms: Cultivation, Nutritional value, Medicinal effect and Environmental Impact. 2nd ed. CRC Press, Boca Raton, Fla, USA. pp. 315-324.
- Chang S T, Miles PG (1986) Oyster mushroom nutritional and economic value. J Sci Food Agric 4(4): 341-330.
- Guillamon E, Garcia Lafuente A, Lozano M (2010) Edible Mushrooms: role in the prevention of cardiovascular diseases. Fitoterapia 81(7): 715-723.
- Alexopoulos CJ, Mims CW (1979) Introductory Mycology. (3rd ed.), John Wiley and Sons, New York, USA. pp. 1-613
- Eger G, Eden G, Wissing E (1976) Pleurotus ostreatus-breeding potential of a new cultivated mushroom. Theor Appl Genet 47(4): 155- 163.
- Kues U, Liu Y (2000) Fruiting body production in basidiomycetes. App Microbiol Biotechnol 54(2): 141-152.
- Jayakumar T, Sakthivel M, Thomas PA, Geraldine P (2008) Pleurotus ostreatus, an oyster mushroom decreases the oxidative stress induced by carbon tetrachloride in rat kidneys, heart and brain. Chem Biol Interac 176(2-3): 108-120.
- Jedinak A, Salva D (2008) Pleurotus ostreatus inhibits proliferation of human breast and colon cancer cells through p53-dependent as well as p53- independent pathway. Int J Oncol 33(6): 1307-1313.
- Amin S M R, Mrod CS, Moonmoon M, Khandaker J, Rehman M (2007) The Officer’s training manual. National center, Savar, Dhaka, Bangladesh. p. 7-17.
- Lee S, Bae H, Kim N, H wang S (2008) Optimization of Growth Conditions of Lentinus edodes Mycelium on Corn Processing Waste Using Response Surface Analysis. J Biosci Bioengen 105(2): 161-163.
- Rodriguez A, Soler Rivasb C, Polonia J, Wichers HJ (2010) Effect of olive mill waste supplementation to oyster mushrooms substrates on the cultivation parameters and fruiting bodies quality. Int Bioteterior Biodegrad 64(7): 638-645.
- Mohammadi E, Purjam E (2003) Principles of Mushroom Cultivation. Tarbiat Modarres University Press, UK, pp. 604.
- Nwanze PI, Khan AU, Ameh AU, Umoh VJ (2005) The effect of the interaction of various spawn grains with different culture medium on carpophores dry weights and stipe and pileus diameters of Lentinus squarrosulus (Mon.) Singer Afr J Biotechnol 4: 615-619.
- Kashi A, (1996) Edible Mushroom Cultivation. Agriculture Education Press, UK, pp. 454.
- Stamets P, Chilton J S (1983) The Mushroom Cultivator: A practical guide to growing mushrooms at home. Agarikon Press, Olympia, Washington. pp. 61-107.
- Jain AK (2005) Thesis on cultivation technology of Pleurotus spp with special reference to marketing potential in Sagar region 42: 65-81.
- Iqbal S M, Altaf Z, Iqbal U (2000) Oyster Mushroom Ki Kasht NARC, Islamabad, Pakistan. 1-20.
- Russel DF (1986) MSTAT-C package programme. Crop and Soil Science Department, Michigan State University, USA.
- Pathak VN, Yadav N, Gour M (2000) Mushroom Production and Processing Technology. Agrobios, Bangalore, India. 1-192.
- Fritsche G (1988) Spawn: properties and preparation, In: The Cultivation of Mushrooms. Darlington Mushroom Laboratories, Sussex, UK p. 1-99.
- Mamiro DP, Royse DJ (2008) The influence of spawn type and strain on yield, size and mushroom solids content of Agaricus bisporus produced on non-composted and spent mushroom compost. Biores Technol 99(8): 3205-3212.
- Shah ZA, Ashraf M, Ishtiaq MC (2004) Comparative study on cultivation and yield performance of Oyster mushroom (Pleurotus ostreatus) on different substrates (Wheat straw, Leaves and Sawdust). Pak J Nutri 3(3): 158-160.
- Pokhrel CP, Sumikawa S, Iida S, Ohgajko S (2006) Growth and Productivity of Lyophyllum decastes on compost enriched with various supplements. Micologia Appli Int 18(2): 21-28.
- Kumbhar CT (2012) Effect of spawn substrate on yield of Pleurotus eous (Berk.) Sacc Int J Pl Sci 7(2): 224-229.
- Mbogoh JM, Anjichi VE, Rotich F, Ahoya NK (2011) Substrate Effects of Grain Spawn Production on Mycelium Growth of Oyster Mushroom. Acta Hortic 9(11): 469-471.
- Kumar P, Kumar K, Kumar S (2010) Evaluation of media and substrates for spawn production of straw mushroom (Volvariella volvacea). Res. Environ. Life Sci 9(4): 446-449.
- Stanley O (2010) Effect of substrates of spawn production on mycelial growth of oyster mushroom species. Agric Biol J North Amer 1: 817- 882.
- Narh DL, Obodai M, Baka D, Dzomeku M (2011) The efficacy of sorghum and millet grains in spawn production and carpophore formation of Pleurotus ostreatus (Jacq Ex Fr) Kummer Int Food Res J 18(3): 1092- 1097.
- Jiskani MM, Bhatti MI, Wagan KH, Pathan MA, Bhatti AG (2007) Determination of Sorghum Grains for Spawn Growth of Oyster Mushroom, Pleurotus ostreatus (Jacq. Ex. Fr) Kummer Pak J Bot 39(7): 2681-2684.
- Khare KB, Mutuku JM, Achwania OS, Otaye DO (2010) Production of two oyster mushrooms, Pleurotus sajorcaju and P. florida on supplemented and unsupplemented substrates. Bot J Agric Appl Sci 6 (1): 4-11.
- Oei P, Van Nieuwenhuijzen B (2005) Small-scale mushroom cultivation: oyster, shiitake and wood ear mushrooms. Wageningen.
- Gibriel AY, Ahmed M, Rasmy N, Rizk I, Abdel-Rehem NS (1996) Cultivation of Oyster Mushrooms (Pleurotus spp.): Evaluations of Different Media and Organic Substrates. In: Mushroom Biology and Mushroom Products, Royse (ed.) Penn State Univ pp. 415-421.
- Khan S M, Nawaz A, Malik W, Javed N, Yasmin T, et al. (2011) Morphological and Molecular Characterization of Oyster Mushroom (Pleurotus spp). Afr J Biotechnol 10(14): 2638-2643.
- Sarker NC, Hossain MM, Sultana N, Mian IH, Karim AJMS, Amin SMR (2007) Effect of different levels of pH on growth and yield of Pleurotus ostreatus (Jacq ex. Fr.) Kummer. Bangladesh J Mush 1(1): 57-62.
- Curvette N, Figlaz D, Pelmastro L (2002) Sunflower seed hulls as substrate for the cultivation of Shiitake mushroom. Hort Technol 2: 652-655.






























