Effect of Crude Cellulase From Aspergillus Fumigatus on Extraction 0f Shea Fat
Christopher Ojomugbokenyode Shaibu1*, Ojochenemi Ejeh Yakubu1, Vivian Ebere Shaibu1* and Obinna Amos Eze2
1Department of Biochemistry, Federal University Wukari, Nigeria
2Department of Biochemistry, University of Nigeria Nsukka, Nigeria
Submission: January 18, 2019; Published: March 255, 2019
*Corresponding author: Christopher Ojomugbokenyode, Department of Biochemistry, Federal University Wukari, Nigeria
How to cite this article: Christopher O S, Ojochenemi E Y, Vivian E S, Obinna A E. Effect of Crude Cellulase From Aspergillus Fumigatus on Extraction 0f Shea Fat. Adv Biotech & Micro. 2019; 13(3): 555863. DOI: 10.19080/AIBM.2019.13.555863
Abstract
This study considered the use of crude cellulase sourced locally to improve the yield of fat extracted from shea kernels. The enzyme was produced by submerged fermentation using Aspergillus fumigatus with palm frond as carbon source. Shea fat was extracted with the aid of crude enzyme (cellulase) and compared with fat extracted from shea kernels treated with water and fat extracted by solvent (n-hexane) extraction. The crude cellulase was harvested on day four of fermentation which was the day of maximum cellulase activity. The protein concentration of the crude cellulase was found to be 1.23mg/ml while the maximum activity of the crude enzyme was 0.27 U/mg. The extraction yields of fat were 47.67, 41.37 and 43.37% respectively for enzyme assisted, water treated and solvent extraction. The physicochemical analysis showed that the specific gravity, acid value, peroxide value, saponification value and iodine value of fat extracted by enzyme assisted extraction were 0.83, 11.78 mg KOH/g, 5.05 me q/Kg, 174.19 mg KOH/g and 48.26I2 g/100g respectively. Also, those for water treated were 0.85, 15.34 mg KOH/g, 6.41 me q/Kg, 176.39 mg KOH/g and 47.55I2 g/100g respectively and 0.83, 10.51mg KOH/g, 5.25 me q/Kg, 170.82 mg KOH/g and 50.30I2 g/100g respectively for fat extracted by solvent extraction method. The colour of the extracted fat was yellow and was consistent in all the samples. This study suggests the possible use of crude cellulase from Aspergillus fumigatus for the extraction of shea fat.
Keywords: Aspergillus fumigatus; Crude cellulase; shea fat
Introduction
Globally, there has been an increase in the demand for vegetable fats and oil [1]. This can be attributed to their increasing relevance in commerce and nutrition where they serve as sources of dietary energy, antioxidants and as raw materials for the manufacture of industrial products especially food, cosmetics, pharmaceuticals and chemicals [1]. A lot of vegetable oils are available, example: palm oil, coconut oil, groundnut oil, rubber seed oil, cotton seed oil, olive oil, soya bean oil, shea fat [2]. In spite of this, there exist an imminent acute shortage of edible and industrial oil due to the increasing demands for vegetable fats and oil and a consequent pressure on the current amount of vegetable oil produced. The exploration of unexploited and underexploited oil-bearing seed like shea kernel to enhance its oil/fat yield is an idea to be considered. Fat extracted from shea (Vitellaria paradoxa) kernel has been found to have numerous uses, some of which include; its use as cooking and frying fat, Cocoa Butter Substitute (CBS) in chocolate and confectionary [3]. It is also used in the preparation of ointments for the treatment of inflammations, rashes, ulcers, for wrinkles and dry skin, and also for cosmetic moisturizing creams [4]. Other uses of the fat include its use as an antiseptic for the treatment of wounds, as hair oil and as rub for rheumatic pains [5].
Vegetable oils and fats are normally obtained from fruits, nuts or seed kernel by extraction using mechanical press or solvent extraction methods [6]. Considering the enormous potentials of shea fat, and the long duration it takes for shea tree to begin production of fruits of commercial quantities, which is about 20 to 50 years [3], the need for an efficient method of shea fat extraction in terms of percentage yield and cost effectiveness cannot be over emphasized. Pre-treatment of oil-bearing seed either physically with heat or biochemically with enzymes such as cellulase which as biocatalysts, have the potential to digest oil-shielding walls and membranes in plant cells exposing the oil-bearing oleosomes [7] to increase oil yield has been employed.
Enzyme-assisted oil extraction has been reported to show significant effects on the yield [8-12]. Enzymes that have been used in this technique include commercial enzymes (purified) and crude enzymes. Examples of commercial enzymes which have been successfully used for this purpose are cellulase, protease, glucanase,amylase, hemicellulases and pectinases [13-15]. The use of commercial enzyme in oil extraction is however capital intensive due to the high cost of commercial enzymes. In view of this, crude enzymes sourced from plants or microorganism may be considered.
Production of cellulase
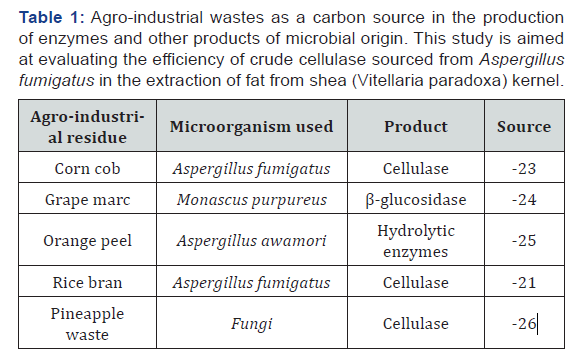
Cellulase is an important extracellular microbial enzyme, which hydrolyses cellulose an abundant biopolymer that constitutes most of the plant cell wall [16]. Microorganisms, mostly fungi which degrade cellulose for their growth while producing cellulase are used for industrial production of various enzymes which are utilized for industrial processes [17-20]. Agro waste such as sugarcane, pineapple peels, rice straw, wheat bran, rice bran and maize bran are used as substrate for microbial fermentation using fungi [21]. Fungal genera like Trichoderma sp and Aspergillus sp are taught to be the best cellulase producers and crude enzymes produced by these microorganisms are commercially available for agricultural use [22]. Cellulase in recent years has gained an increased attention due to its potential applications in various industrial processes including; extraction of oil/fat, bioconversion of hemicelluloses to sugars, ethanol and other useful substances, clarification of juices and wines, improving the nutritional quality of silage and green-feed, deinking processes of waste paper, pharmaceuticals, paper and pulp industry and agricultural-waste treatment processes [16] (Table1).
Materials and Methods
Biological Samples
Pure isolates of Aspergillus fumigatus stored on Agar slants was obtained from the Department of Microbiology, University of Nigeria Nsukka. Palm frond was sourced from Igogoro, Enugu- Ezike, Enugu state. Shelled Shea kernels were obtained from Wukari, Wukari local government Taraba state and identified by Mr R. Agbu of the Department of Biology Federal University Wukari.
Sample preparation
The palm fronds were sun-dried for fourteen days when a constant weight was recorded. The sun-dried palm fronds were pulverized using mortar and pestle after which it was filtered using a sieve to get fine powder. This was used as the sole carbon source in the fermentation process. Shelled kernels were cracked to get the kernels. The kernels were then sundried for several days until a constant weight was observed. It was then pulverized using mortar and pestle. The pulverized kernel was kept in a desiccator until it was needed for analysis.
Experimental design
A mineral broth was prepared using palm frond as the sole carbon source. The broth was inoculated with Aspergillus fumigatus suspension and allowed to ferment for days. A preliminary study of the fermentation culture was carried out to ascertain condition for optimal production of the enzyme. The crude cellulase produced was harvested on the 4th day which was the day of maximum cellulase activity. Fat was extracted by three different ways: enzyme-assisted, water-treated and solvent extraction. A quantity (50g) of the pulverized kernel was treated with 100ml of crude cellulase in a beaker to enhance hydrolysis of oil bearing oleosomes. The pH of the medium was adjusted to pH 5.5 using acetate buffer. It was then thoroughly mixed for one hour and incubated at 50 °C in a water bath for six hours. After six hours of incubation, the treated sample was dried in an oven at 50 °C till a constant weight was observed. The control which was treated with water in place of the enzyme was simultaneously set up with the enzyme-treated sample following the same procedure as in the enzyme-treated sample. Extraction was carried out using Soxhlet extractor at 70 °C. N-hexane was used as the extraction solvent. The extracted fats were then subjected to physicochemical analysis.
Production of crude cellulase
Pure isolates of Aspergillus fumigatus from Agar slants were sub-cultured on a Potato Dextrose Agar (PDA) for five days in an incubator at 37 °C to obtain biomass sufficient for submerge fermentation. Palm frond broth (100ml) was constituted by mixing 1g of magnesium sulphate heptahydrate (MgSO4.7H2O), 1g of potassium dihydrogen phosphate (KH2PO4), 0.5g of sodium chloride (NaCl), 0.5g of potassium chloride (KCl), 1g of sodium nitrate (Na- 2NO3) and 10g of pulverized palm frond. The volume was made up to 100ml in an Erlenmeyer flask with distilled water. The same procedure as in above was followed to make the broth in five 250 ml Erlenmeyer flasks. The palm frond broths were sterilized in an autoclave at 120 °C for 15 min. and allowed to cool. They were then inoculated with the Aspergillus fumigatus suspension (10 ml) which was prepared by harvesting the spores of the fungi using distilled water. The culture was incubated at 40 °C. Cell free supernatant from the culture obtained by filtration was subjected to protein concentration determination and cellulase assay daily in order to determine the day of peak cellulase activity. The cultures were harvested on the day of maximum cellulase activity. The cell free supernatant was used as a source of enzyme hereafter referred to as crude enzyme (cellulase) [22-26].
Determination of the protein concentration of the crude enzyme
The protein concentration of the crude enzyme was determined by the method of Lowry, et al. [27]. An aliquot of solution E (5 ml) (freshly prepared alkaline solution) was added to 0.5 ml of crude enzyme solution in a test tube. The solution was mixed thoroughly and allowed to stand for 10 min. After which 0.5ml of solution C (Folin- Ciocalteau phenol reagent) was added. The solution was mixed thoroughly and allowed to stand for 30min. Absorbance of the solution was then read at 750 nm using a spectrophotometer. The protein concentration was obtained using a calibration curve (plot of Absorbance versus protein concentration) made using Bovine Serum Albumin (BSA) as standard. The analysis was done in triplicate.
Enzyme assay
The crude enzyme activity was assayed by the method of Mandels, et al. [28]. A filter paper strip (1×3cm) was inserted into a test tube containing 1ml 0.05M sodium acetate buffer pH 5.5. An aliquot of the crude enzyme solution (0.5ml) was then added and mixed thoroughly. The mixture was incubated at 50 °C for 60 min. in a water bath, after which the reaction was terminated by adding 1ml of 3,5-dinitrosalicylic acid. The solution was boiled for 10min. and allowed to cool. It was then diluted with 1ml sodium potassium tartrate (75g/ 125ml in distilled water). Absorbance of the solution was read at 540nm against a blank using a spectrophotometer. The analysis was done in triplicate.
The cellulase activity was calculated using the formula:

Where,
0.37= Number of units contained in the estimated amount of enzyme which releases 2.0mg of glucose in the FPU reaction.
1Unit ml-1 = 1μmol min-1 ml-1.
Extraction of oil
The IUPAC method (1979) was employed in the extraction of the oil. The oil was extracted with a Soxhlet apparatus using analytical grade hexane (n-hexane) as the extraction solvent. The enzyme- treated, water-treated and untreated pulverized shea kernel (50g) were separately packed into the thimble of the Soxhlet apparatus and fitted to a round bottom flask containing 250ml n-hexane. The apparatus was then mounted on a heating mantle set at 70 °C for six hours for the extraction of the oil. At the completion of the extraction process, the oil was recovered from the mixture by evaporating the residual extraction solvent in an oven at 50 °C to a constant weight. The extracted oil was then stored in a bottle and kept in the refrigerator for further analysis.
Percentage yield of the oil was calculated using the formula:

Determination of specific gravity
A 50ml specific gravity bottle was first washed with detergent and rinsed with water. The bottle was dried in the oven at 50 °C, allowed to cool and weighed empty. The bottle was then filled with distilled water and the weight of the bottle and water noted. After which the bottle was kept in the oven to dry. The bottle was cooled and filled with shea butter sample. The weight was also noted. The analysis was made in triplicate.
The specific gravity of the oil was calculated using the formula:
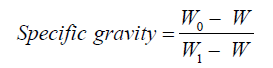
Where,
W = Weight of empty bottle (g), W0 = Weight of the bottle and oil content (g), W1 = Weight of bottle and water content (g).
Determination of acid value
Determination of acid value was done by the method of AOAC [29]. Shea butter (5g) was weighed into a 250ml conical flask. This was followed by the addition of 25ml of 0.5N absolute ethanol alcohol solution (prepared by mixing ethanol and diethyl ether in the ratio of 1:1 v/v) and then 3drops of phenolphthalein was added. The solution was heated in a water bath at 65oC for 10 min. It was cooled after which it was titrated against 0.1N KOH until a pink colour appeared. The volume of the KOH was recorded. The analysis was carried out in triplicate.
The acid value was calculated using the formula:
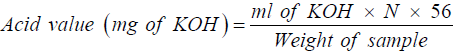
Where N = Normality of KOH
The percentage free fatty acid was calculated as:
% free fatty acid = Av × 0.503
Where Av= Acid value
Determination of saponification value
Approximately 2g of the melted shea fat was weighed into a 250ml conical flask. This was followed by the addition of 25ml of alcoholic potassium hydroxide solution (0.5N). The flask was attached to a reflux condenser and heated on a boiling water bath for 1 hour with occasional shaking. While the solution was still hot, 3 drops of phenolphthalein indicator was added. The excess potassium hydroxide was titrated against 0.5N hydrochloric acid. A colour less solution indicated the end point. The volume of the hydrochloric acid used at end point was noted and represented as S. A blank titration was also carried out and the Volume (ml) of the hydrochloric acid used at end point was noted and represented as B. [29]. The analysis was carried out in triplicates.
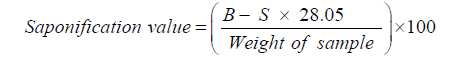
The saponification value was calculated using the formula:
Where, S = Volume of the hydrochloric acid used at end point for oil sample
B = Volume of the hydrochloric acid used at end point for blank
Determination of peroxide value
Shea butter sample (2g) was weighed into a 500ml conical flask after which 10ml of chloroform was added and stirred to dissolve the sample. Acetic acid (15ml) was then added. This was followed by the addition of 1ml of freshly prepared saturated potassium iodide solution. The flask was closed immediately and stirred for 1minute after which it was kept for exactly 5min. in the dark at room temperature. An aliquot of distilled water (75ml) was added and the solution was shaken vigorously. Few drops of starch solution were added as indicator. The liberated iodine was then titrated against 0.01N sodium thiosulphate solution. A blank test was done following the same procedure but omitting test sample [29]. The analysis was carried out in triplicate.
The peroxide value was calculated using the formula:
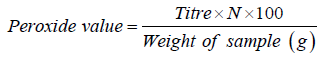
Where,
Titre = ml of sodium thiosulphate used (blank corrected)
N = Normality of sodium thiosulphate solution
Determination of iodine value
Iodine value was determined by the Hanus method [30]. The hanus iodine reagent was prepared by dissolving iodine (13.2g) in glacial acetic acid (1L) with the help of heat. The solution was cooled, and 3 ml of bromine added. The hanus iodine reagent was then kept in a brown bottle until the analysis was complete. Shea butter (2g) was weighed into a 500ml conical flask and 10ml of chloroform added. By use of a pipette 25ml of hanus iodine was added and left to stand in the dark for 30min. with occasional shaking. After which 15% potassium iodide was added and shaken thoroughly. Distilled water (100ml) was then used to rinse down any iodine on the stopper. The solution was titrated with 0.1N sodium thiosulphate until the yellow solution turned almost colorless. Three drops of starch indicator (1%) was added towards and the solution turned blue. The titration was continued until the blue colour turned colorless. A blank determination was done by following the same procedure as above but replacing the test sample with distilled water. The analysis was done in triplicate.
The iodine number was calculated using the formula:
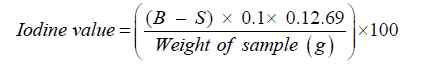
S = Volume (ml) of Na2S2O3 at end point for oil sample
B = Volume (ml) of Na2S2O3 at end point for blank
Statistical analysis
Data obtained from this study were analyzed using the statistical package for social scientists (SPSS) version 20 for windows. Analysis of variance (ANOVA) was used to compare means. Values were considered statistically significant at p < 0.05. Results were reported as mean ± SD of triplicate values.
Results
Production of crude cellulase
Protein concentration of the crude enzyme
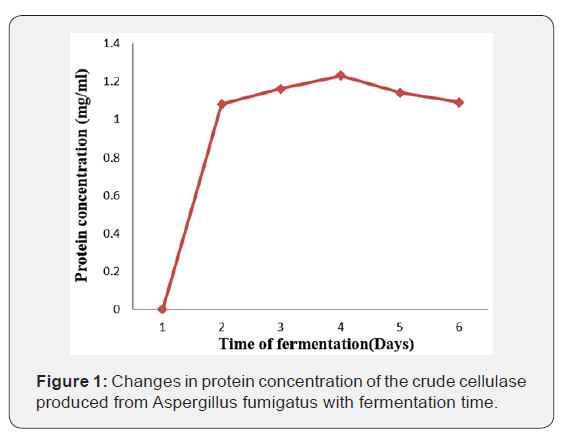
Figure 1 shows the changes in protein concentration of the crude cellulase produced from Aspergillus fumigatus with fermentation time. An increase in protein concentration of the crude cellulase was recorded on the 2nd day. Maximum protein concentration 1.23 mg/ml was obtained on the 4th day beyond which a steady drop in protein concentration was observed (Figure 1).
Cellulase activity of the crude enzyme

Figure 2 shows changes in cellulase activity of crude enzyme produced from Aspergillus fumigatus with fermentation time. There was no traceable cellulase activity at the time of inoculation. Cellulase activity increased to 0.21 U/ml on the second day and continued to rise until it reached its peak on the 4th day. A steady drop in activity was noticed from the 5th day (Figure 2).
Percentage oil yield of crude shea fat extracted by different methods
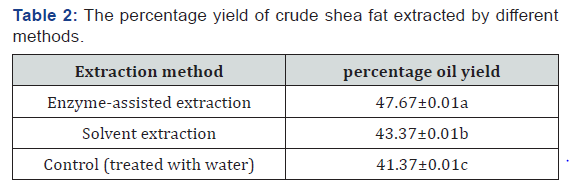
The percentage yield of the crude shea fat extracted by different methods is shown in Table 2 below. The result shows that fat extracted with the aid of enzyme had the highest yield when compared with fat extracted from water-treated kernels and solvent extracted fat (Table 2).
Physicochemical analysis of the extracted shea fat

Values are means of triplicate with ± standard deviation. Different superscript; a, b or c implies a significant difference between means at p < 0.05. Means with the same superscript are not significantly different p > 0.05.
Physical properties of the extracted shea fat: The physical properties of the crude fat extracted by different methods of extraction are shown in Table 3. There was no significant difference p > 0.05 in specific gravity between fats extracted by the different methods of extraction. The colour was yellow and was consistent in all the samples (Table 3).
Chemical properties of the extracted shea fat
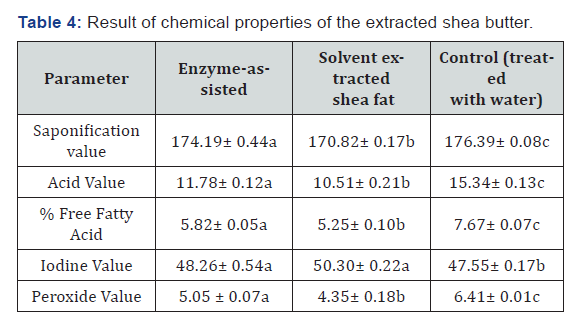
Values are means of triplicate with ± standard deviation. Different superscript; a, b or c implies a significant difference between means at p < 0.05. Means with the same superscript are not significantly different p > 0.05..
Table 4 shows the comparison of chemical properties of shea fat extracted by enzyme-assisted extraction, solvent extraction and control (treated with water). The result of the physicochemical studies show that there was a significant (p < 0.05) difference in all the chemical properties except for the iodine values of fat obtained by enzyme-assisted extraction and solvent extraction method which were found to have a non-significant (p > 0.05) difference (Table 4).
Discussion
This study considered the use of crude cellulase sourced locally to improve the yield of fat extracted from shea kernels. The enzyme was produced by submerged fermentation using Aspergillus fumigatus with palm frond as carbon source. The physicochemical analysis of the extracted oil was done to ascertain the quality of the oil. Crude cellulase was harvested on the 4th day of the fermentation period which was the day of maximum cellulase activity. The crude enzyme had a protein concentration of 1.23 mg/ml with an activity of 0.27 U/ml. This compares well with the work of Okoye et al. [23] who used Aspergillus fumigatus and Corn cob in the production of cellulase. The result also compares favorably with the study carried out by Khan and Singh [31] who reported a cellulase activity of 0.024 U/ml using corn cob, 0.02 U/ml using newspaper and 0.018 U/ml using saw dust used. Shobana and Maheswari [21] however reported higher cellulase activity with optimized conditions for fermentation and partial purification of the crude enzyme. They reported 0.96 U/ml, 0.54 U/ml and 0.51U/ ml for rice bran, wheat bran and coconut coir pith respectively. It can be deduced from this studies that the source of enzyme and the optimization of enzyme production procedures for example purification of the enzyme could be a reason for the variation in the quality of enzyme produced. Cellulase produced from Aspergillus fumigatus using palm frond can however be considered in the production of cellulase for use in industrial processes such as enzyme-assisted extraction of oil.
Fat extracted by the method of enzyme-assisted extraction had the highest yield; 47.67 % achieving a 4% increase in comparison with fat extracted by solvent extraction and a 6% increase when compared with the control (treated with water). This compares favorably with the work of Otu et al. [15] who reported a 4% increase from 40 to 44% using crude pectinase. The result also compares well with the works of Maranz et al. [32]; Ofosu et al. [33]; Ononogbu [34] who reported values ranging from 33 to 55% for shea butter extracted by the method of solvent extraction. Ikya et al. [35] reported lower values 34.1% for shea butter extracted by traditional method of extraction. Tano-Debrah and Ohta [36] and Otu et al. [15] however reported higher yields; 72-74% using different combinations of purified enzyme. The higher yield of oil with enzyme-treatment may be attributed to enzymatic hydrolysis of the cellulose cell wall of the oil-bearing seed by cellulase [7].
Purification of the enzyme, enzyme combinations and optimization of extraction conditions will likely increase the percentage yield of oil recovery by this method [15,36,37]. The specific gravity of the oil extracted with the aid of enzyme is comparable to 0.86 reported by Munir, et al. [38]. Values (0.9674) reported by Njoku, et al. [39] were however found to be slightly higher than those reported in this study. The specific gravity of the oil in this study varies slightly from that of oils from other seeds. Asuquo, et al. [1] reported 0.92 for rubber seed oil while Njoku and Ugwuanyi [40], reported 0.9 for dika fat. Statistical analysis of the values obtained for extracted fats shows that there was no significant difference p > 0.05 in the specific gravity of fat extracted by enzyme-assisted, water-treated and solvent extraction. The colour of the extracted fat was yellow and was consistent in the all samples. This agrees with the report of Okezie [41] & Omujal [42]. The result of the colour alongside the specific gravity indicates that the fat extracted in this study is unadulterated shea fat.
The acid value of the enzyme-assisted extracted fat compares well with 6.97-15.35 reported by Abdulai, et al. [43]. It also falls within the range of values: 2.30-12.59 reported by Omujal [42], Okezie [41] however reported a lower value (1.96). The result of the free fatty acid compares well with 7.72 reported by Out, et al. [15] but higher than 0.77 reported by Njoku, et al. [39]. Acid values of fat extracted by enzyme-assisted, water-treated and solvent extraction were found to be significantly different p < 0.05. Variation in these values may have resulted from the extraction procedures [44]. Higher acid values obtained for water-treated and enzyme-treated extracted fat may be attributed to hydrolysis due to water and enzyme activity. Shea fat with acid value as reported in this study can be used in the production of soap and can also be refined for use in the food industry [38].
The saponification value of the enzyme-assisted extracted fat compares well with 145-192 reported by Omujal [42] and 168- 176 by Okezie [41] but slightly lower in comparison with 186.64 reported by Njoku, et al. [39]. The saponification value of the extracted fats showed a significant difference p < 0.05 between the fats extracted by the different methods. Variation in the saponification value may be attributed to the hydrolysis of fat induced by water and enzyme activity. The saponification value of the extracted fat compares well with that of Dennettia tripatala fruit (Pepper fruit) oil (159.33±1-20) which was reported by Nwinuka & Nwiloh [45] to be suitable for soap making. The fat extracted by the method employed in this study is therefore suitable for soap making, for making of shampoos and lather shaving creams [46].
The peroxide value obtained in this study compares well with 5.33 reported by Otu et al. [15] & 6.35-9.49 reported by Abdulai, et al. [43] Olaniyan & Oje, et al. [4] however reported higher values 22.1 - 44.9. Low peroxide values reported in this study is an indication of low levels of oxidative rancidity and the presence of antioxidants. The fats extracted in this study showed a significant difference p < 0.05 in the peroxide values of fat extracted by enzyme-assisted, water-treated and solvent extraction. Variations in the peroxide values of the fats may be attributed to extraction procedures [44].
Higher values recorded for enzyme and water-treated extracted fats may be attributed to hydrolysis of the fats due to water and enzyme activities. The iodine value obtained for enzyme-assisted extracted shea fat is comparable to 53.20 and 49.43 obtained by Adamu, et al. [47] & Bukar, et al. [48] respectively. The iodine value of shea butter was found to be lower when compared with that of bread fruit (164.97), melon seed oil (126.90), soybeans oil (123.0), coconut oil (134.51) [49]. There was no significant difference p > 0.05 between fats extracted by enzyme-assisted and water-treated extraction. Fats with Iodine value within the range obtained in this study that is, less than 100 are termed as non-drying [50] and could be used in the manufacture of lubricants and hydraulic brake fluids [49].
Conclusion
This study has shown the possible use of crude cellulase produced from Aspergillus fumigatus and palm frond to improve the percentage yield of oil extraction from shea kernels. Treatment with crude enzyme (cellulase) gave a higher oil yield when compared with the fat extracted without the aid of enzyme. This method of oil extraction compares well with conventional methods of oil extraction. Although there were variations in some of the physicochemical properties, values were either slightly above or slightly below, indicating that the use of crude enzyme to assist extraction of oil does not have very significant effects on the quality of the oil. The quality of crude enzyme-assisted extracted fat can be increased by optimizing the extraction conditions.
References
- Asuquo JE, Anusiem ACI, Etim EE (2012) Extraction and Characterization of Rubber Seed Oil. International Journal of Modern Chemistry 1(3): 109-115.
- Dawodu AA (2009) Physio-chemical studies on oil extraction process from some Nigerian grown plant seeds. Electronic Journal of Environmental, Agriculture and Food Chemistry 8(2): 102-110.
- Alander J (2004) Shea butter - A multi-functional ingredient for food and cosmetics. Lipid Technology 16: 202-205.
- Olaniyan AM, Oje K (2007) Quality characteristics of shea butter recovered from shea kernel through dry extraction process. Journal of Food Science and Technology 44: 404-407.
- Mohagir AM, Bup ND, Abi CF, Kamga R, Kapseu C (2015) Optimization of Kernels Preparation Conditions Involved in the Press Extraction of Shea (Vitellaria paradoxa Gaertner F.) Butter. American Journal of Food Science and Technology 3(4): 103-110.
- Akpabio UD, Akpakpan AE, Mathew IE, Akpan AU (2011) Extraction and characterization of oil from avocado pear (Persea Americana) and native pear (Dacryodes etulis) fruits. World Journal of Applied Science and Technology 3(2): 27-34.
- Silvamany H, Jahim JM (2015) Enhancement of palm oil extraction using cell wall degrading enzyme formulation. Malaysian Journal of Analytical Sciences 19: 77-87.
- Moreau R, Johnston D, Powell M, Hicks K (2004) A comparison of commercial enzymes for the aqueous enzymatic extraction of corn oil from corn germ. Journal of the American Oil Chemists’ Society 81(11): 1071-1075.
- Abdulkarim SM, Lai OM, Muhammad SKS, Long K, Ghazali HM (2006) Use of enzymes to enhance oil recovery during aqueous extraction of Moringa oleifera seed oil. Journal of Food Lipids 13(2): 113-130.
- Lamsal B, Murphy P, Johnson L (2006) Flaking and extrusion as mechanical treatments for enzyme-assisted aqueous extraction of oil from soybeans. Journal of the American Oil Chemists’ Society 83(11): 973-979.
- Latif S, Diosady LL, Anwar F (2008) Enzyme-assisted aqueous extraction of oil and 36 protein from canola(Brassica napus L.) seeds. European Journal of Lipid Science and Technology 110(10): 887-892.
- Womeni HM, Ndjouenkeu R, Kapseu C, Mbiapo FT, Parmentier M (2008) Aqueous enzymatic oil extraction from Irvingia gabonensis seed kernels. European Journal of Lipid Science and Technology 110(3): 232-238.
- Kumar D, Jain VK, Shanker G, Srivastava A (2003) Utilization of fruit waste for citric acid production by solid state fermentation. Process Biochemistry 38(12): 1725-1729.
- Xie M (2004) Aqueous enzymatic extraction of wheat germ oil. M.Sc. thesis, Faculty of the Graduate College of the Oklahoma State University, Oklahoma, USA.
- Otu SA, Dzogbefia VP, Kpikpi EN, Essuman EK (2015) Comparative effect of crude and commercial enzyme in shea fat extraction. Journal of Biotechnology and Biochemistry 1(3): 18-27.
- Naseeb S, Sohail M, Ahmad A, Khan SA (2015) Production of xylanases and cellulases by Aspergillus fumigatus MS16 using crude lignocellulosic substrates. Pakistan Journal of Biotechnology 47(2): 779-784.
- Iftikhar T, Zia MA, Niaz M, Ashraf I, Abbas SQ (2010) Mutation Induced Enhanced Biosynthesis of Lipases by Rhizopus oligosporus var. microsporus. Pakistan Journal of Botany 42(2): 1235-1249.
- Malik S, Iftikhar T, Haq IU, Khattak MI (2013) Process optimization for amyloglucosidase by a mutant strain of Aspergillus niger in stirred fermenter. Pakistan Journal of Botany 45(2): 663-666.
- Abdullah R, Shaheen N, Iqtedar M, Naz S, Iftikhar T (2014) Optimization of cultural conditions for the production of alpha amylase by Aspergillus niger (BTM-26) in solid state fermentation. Pakistan Journal of Botany 46(3): 1071-1078.
- Iftikhar T, Niaz M, Shahid N, Haider MZ, DE Nayab R (2014) Morphomolecular identification of a novel Aspergillus spp. and its cultural optimization for lipases production. Pakistan Journal of Botany 46(6): 2297-2304.
- Shobana P, Maheswari NU (2013) Production of cellulase from Aspergillus fumigatus under submerged and solid state fermentation using agricultural waste. International Journal of Advances in Pharmacy, Biology and Chemistry 2(4): 595-599.
- Kazuhisa MR (1997) Biological system for alternative sustainable energy production. Agricultural Services Bulletin 128: 451-456.
- Okoye IG, Ezugwu AL, Udenwobele DI, Eze SOO, Anyawu CU (2013) Production and partial characterization of cellulases from Aspergillus fumigatus using two distinct parts of corn cob as carbon sources. Nigerian Journal of Biotechnology 26: 50-59.
- Daroit DJ, Silveira ST, Hertz PF, Brandelli A (2007) Production of extracellular β -glucosidase by Monascus purpureus on different growth substrates. Process Biochemistry 42(5): 904-908.
- Díaz AB, De Ory I, Caro I, Blandino A (2012) Enhance hydrolytic enzymes production by Aspergillus awamori on supplemented grape pomace. Food and Bioproducts Processing 90(1): 72-78.
- Omojasola PF, Jilani OP, Ibiyemi SA (2008) Cellulase Production by some Fungi Cultured on Pineapple Waste. Nature and Science 6(2): 64-79.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with Folin phenol reagent. J Biol Chem 193(1): 265-275.
- Mandels M, Andreotti R, Roche C (1976) Measurement of saccharifying cellulase. Biotechnol Bioeng Symp 6: 21-33.
- AOAC (1997) Association of Official Analytical Chemists. Official Methods of Analysis. 17th (ed). Washington, USA.
- Hanus method (1996) Association of Official Analytical Chemists (AOAC) 920. 158 (ISO 3961: 1996 Animal and vegetable Fat and oil – determination of Iodine Value).
- Khan JA, Singh SK (2011) Production of cellulase using cheap substrates by solid state Fermentation. International Journal of Plant, Animal and Environmental Sciences 1(3): 179-187.
- Maranz S, Kpikpi W, Wiesman Z, Saint Sauveur A, Chapagain B (2004) Nutritional values and indigenous preferences for shea fruits (Vitellaria paradoxa) in African agroforestry parklands. Economic Botany 58(4): 588-600.
- Ofosu MA (2009) Anaerobic digestion of shea waste for energy generation. PhD Thesis submitted to the University of Cape Coast, Cape Coast.
- Ononogbu IC (2002) Lipid in human existence. A P Express, Nsukka. p.1- 80.
- Ikya kJ, Umenger LN, Iorbee A (2013) Effect of the extraction method on the yield and quality characteristics of oil from shea nut. Journal of Food Resource Science 2(1): 1-12.
- Tano Debrah K, Ohta Y (1995) Enzyme-assisted aqueous extraction of shea fat: A rural approach. Journal of American Oil Chemists’ Society 72(2): 251-256.
- Hernandez N, Rodriguez-Alegria ME, Gonzalez F, Lopez-Munguia CA (2000) Enzymatic treatment of rice bran to improve processing. Journal of American Oil Chemists Society 77(2): 177-180.
- Munir SM, Musa U, Abdulrahman Z, Mohammed I A, Aliyu AM, Salihu Y (2012) Extraction and characterization of Nigerian shea butter oil. Journal of Science Technology Mathematics and Education 8(2): 66-73.
- Njoku OU, Eneh FU, Ononogbu IC, Adikwu MU (2000) Compositional and toxicological studies on shea butter. Journal of Nutraceuticals Functional and Medical Foods 2(3): 33-39.
- Njoku OU, Ugwuanyi JO (1997) Nutritional and toxicological properties of dika fat (Ivingia gabonensis). Journal of Herbs, Spices and Medicinal Plants 4(4): 53 - 58.
- Okezie SM (2007) Production of cocoa butter substitutes/equivalents from palm oil, shea butter and dika fat using Aspergillus niger lipase. MSc thesis, Department of Biochemistry, University of Nigeria, Nsukka.
- Omujal F (2009) Post-Harvest Handling Practices and Physico- Chemical Characteristics of Shea (Vitellariaparadoxa) Fruit in Uganda MSc Thesis, Makerere University, Uganda.
- Abdulai A, Akwasi A, Iddrisu A (2015) Effect of soil variation on quality of shea butter in selected areas of the northern region of Ghana. Journal of Agricultural Biotechnology and Sustainable Development 7(5): 43-50.
- Akingbala JO, Adebisi ET, Baccus-Taylor, GSH, Falade KO, Lambert IA (2007) Effect of nut roasting temperature, extraction, process and packaging material on the storage properties of shea butter. West Indian Journal of Engineering 30: 32-36.
- Nwinuka NM, Nwiloh BI (2009) Physico-chemical properties and fatty acid composition of Dennetta tripetala fruit oil (peper fruit). Nigerian Journal of Biochemistry and Molecular Biology 24(1): 42 - 46.
- Oderinde RA, Ajayi IA, Adewuyi A (2009) Characterization of seed and seeds oil of Hura crepitans and the kinetics of degradation of the oil during heating. Electronic Journal of Environmental, Agricultural Food Chemistry, 8(3): 201- 208.
- Adamu HM, Ushie OA, Nansel E (2013) Antimicrobial activity of oil from Butyrospermum parkii Seed (Shea Butter). International Journal of Modern Biology and Medicine 3(2): 50-59.
- Bukar N, Gungula DT, Kapsiya J (2015) Effects of Shea (Vitellaria paradoxa C F Gaertn) nuts storage environments on the quality of shea butter in dry savannah environment of Adamawa state, Nigeria. International Journal of Plant and Soil Science 4(1): 54-60.
- Eze OOS (2012) Physico-chemical properties of oil from some selected underutilized oil seeds available for biodiesel preparation. African Journal of Biotechnology 11(42): 10003-10007.
- Asuquo JE, Anusiem ACI, Etim EE (2010) Extraction and characterization of shea butter oil. World Journal of Applied Science and Technology 2(2): 282-288






























