A Possible Crosstalk Between GRP78 and mTOR: New Therapeutic Target in Disease Management
Adria Hasan1, Safia Irfan2, Ejazul Haque2,3, Mohd Kamil2,4 and Snober S Mir 1*
1Department of Bioengineering, Faculty of Engineering, Integral University, India
2Department of Biosciences, Faculty of Science, Integral University, India
3Department of Immunology and Medical Genetics, University of Split, Croatia
4epartment of Microbiology, Bezmialem Vakif University, Turkey
Submission: December 10, 2018; Published: March 19, 2019
*Corresponding author: Snober S Mir, Department of Bioengineering, Faculty of Engineering, Integral University, Kursi Road, Lucknow, 226026, India
How to cite this article: Adria Hasan, Safia Irfan, Ejazul Haque, Mohd Kamil, Snober S Mir. A Possible Crosstalk Between GRP78 and mTOR: New Therapeutic Target in Disease Management. Adv Biotech & Micro. 2019; 13(2): 555856. DOI: 10.19080/AIBM.2019.13.5558856
Abstract
The Endoplasmic Reticulum (ER) is the fundamental organelle which plays an imperative role in numerous cellular activities including protein synthesis, folding, and post-translational modifications. ER stress is caused by multiple pathological and physiological conditions like the accumulation of misfolded proteins. ER stress plays a dual role by either promoting cell survival or triggering cell death which is dependent on the imbalance between ER protein folding load and capacity. ER stress due to the buildup of misfolded proteins in ER lumen causes the Unfolded Protein Response (UPR) that recruit the expression of various chaperones and proteins, like Glucose-Regulated Protein 78 (GRP78), calnexin and calreticulin. GRP78 is an ER chaperone which plays a vital role in protein folding and misfolding. Protein homeostasis in the cells is also maintained by another protein namely mTOR (mammalian target of rapamycin), a serine/threonine kinase. For the cells to function correctly, a balance is to be maintained between protein synthesis, maturation, and degradation. mTOR plays a role in protein synthesis while the regulation of protein folding and repair/or degradation of misfolded proteins is maintained by the ER, and, more specifically the ER chaperones. Any dysregulation in either one or both of these pathways will lead to different diseases including cancer. In this review, we discuss a possible connection between ER chaperone GRP78 and mTOR and also highlight the significance of their functional interaction in different diseases.
Keywords: Endoplasmic Reticulum; mTOR; UPR; Crosstalk
Abbrevations: ER: Endoplasmic Reticulum; UPR: Unfolded Protein Response; GRP78: Glucose-Regulated Protein 78; ERAD: ER-Associated Degradation;
Introduction
The ER is an intracellular organelle present adjacent to the nucleus found in the eukaryotic cells. ER is a tube-like structure that forms a series of flattened sacs inside the cytoplasm of the cells. ER plays a crucial role in normal cellular functioning, by processing of posttranslational modification and folding of secretory and membrane proteins. The ability of ER to properly fold nascent proteins depends on chaperone proteins [1]. Chaperones play an important role in the proper folding of proteins, and any dysfunction in these chaperones may lead to protein folding disorders in the human body. Maintaining protein homeostasis within the cell is vital for the cells to function and survive. However, under conditions of cellular stress, proteostatic mechanisms must be activated to recycle, refold, or initiate degradation of misfolded or unfolded proteins [2]. Thus, in this mini-review, we will discuss the importance of chaperones, specifically the 78 kD glucose-regulated protein GRP78 (also known as BiP and HSP5a), and its possible relation with mTOR. mTOR has a significant role in the maintenance of protein homeostasis.
Protein homeostasis can be called as a fragile balance between protein synthesis, correcting misfolded proteins and degradation of damaged proteins [3]. Molecular chaperones are responsible for overseeing the process of protein folding, refolding of misfolded proteins and degradation of damaged proteins. Any change in the function of these chaperones can lead to disturbance in protein homeostasis which may further cause several diseases including neurological disorders and cancer [4]. mTOR kinase and ER stress pathway also trigger UPR which controls many cellular processes like translation, apoptosis, autophagy, metabolism, and inflammation [5].
Discussion
GRP78 is a major ER chaperone of HSP-70 family that plays a pivotal role in normal ER functioning and its increased expression also works as an indicator of ER stress. GRP78 is commonly localized inside the ER lumen however it has been reported that GRP78 can be detected in the mitochondria, cytosol, nucleus, or even the cell surface, depending on specific cell type and conditions [6,7]. Therefore, different locations prime GRP78 to activate different molecular signaling events [7]. GRP78 also helps in many cellular processes, like translocation of the newly synthesized polypeptides across the ER membrane, help in protein folding and assembly of proteins, regulating calcium homeostasis, targeting misfolded/unfolded proteins for ER-Associated Degradation (ERAD) pathway as well as serving as an ER stress sensor [8, 9]. Moreover, GRP78 is known as a master regulator of ER stress because of its antiapoptotic properties and its ability to control the UPR signaling [9]. UPR is usually activated after ER stress in the cells and regulates many cellular processes [10].
The UPR is a well-conserved pathway from yeast to mammalian cells. Misfolded proteins and their accumulation generate protein aggregates and depletion in ATP levels which in turn leads to ER stress and UPR activation [11]. To manage ER stress, UPR increases the ER folding capacity through transcriptional upregulation of ER folding, lipid biosynthesis, and ERAD (Endoplasmic-reticulum-associated protein degradation) along with a decrease in folding load through selective degradation of mRNA and translational repression. GRP78 manages the UPR by regulating three ER transmembrane proteins: transcription factor 6 (ATF-6), inositol-requiring enzyme 1 (IRE1), and protein kinase R-like endoplasmic reticulum kinase (PERK) [12]. GRP78 also has a role in autophagic protein quality control wherein it participates in the destruction of misfolded proteins in the cytosol [11].
Apart from the regulation of autophagy, GRP78 may also control activated phosphatidylinositol 3-kinase (PI3K)/AKT pro-survival pathways [13]. Activation of the PI3K/AKT pathway eventually leads to the upregulation of mTOR (Figure 1).
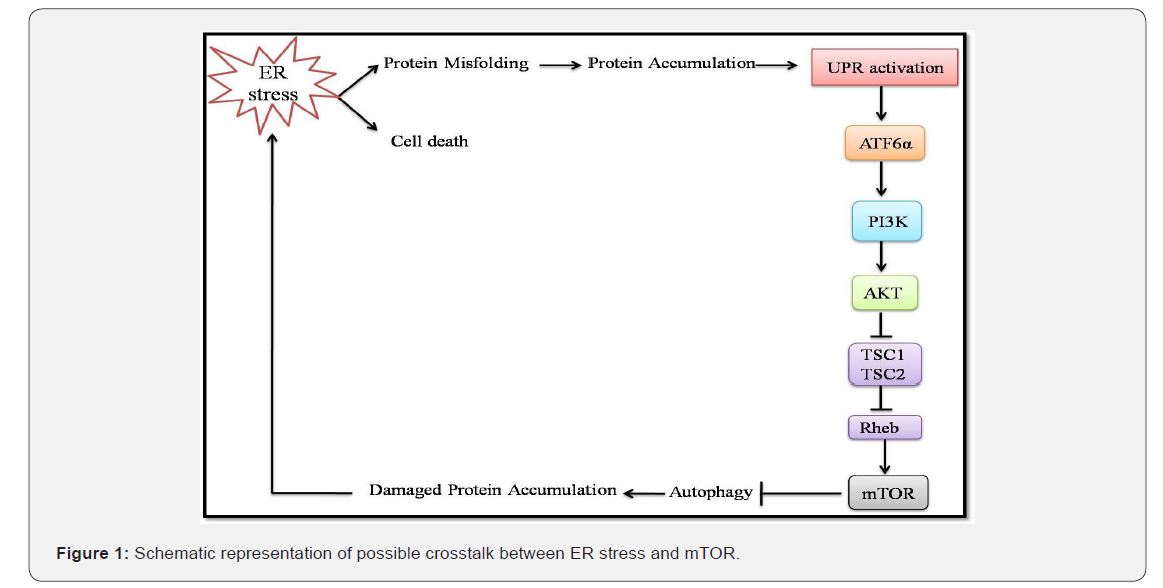
Although both of these signaling pathways, i.e., UPR (via GRP78) and mTOR have attracted extensive attention, the crosstalk between the two pathways has emerged recently. mTOR complex 1 (mTORC1) functions both upstream and downstream of ER stress signals, which can either upregulate or downregulate the anabolic output of mTORC1. Upon prolonged ER stress, mTORC1 aids in apoptosis by suppressing the Akt through feedback inhibition. Similarly, constant ER stress inhibits activation of Akt by mTOR complex 2 (mTORC2) [5]. Functioning of ER stress upstream of mTORC1 can add different molecular players in controlling this crosstalk. Inhibiting UPR leads to the activation of PI3k-Akt-mTORC1 [14, 15] signaling pathway via ATF6α through a still unknown mechanism [16]. As mTORC1 and UPR are interdependent, there are several common targets for these two. UPR and mTORC1 work synergistically and regulates angiogenesis [17], insulin resistance [18], hepatic lipid synthesis [19], and NF-κB signaling [20,21]. Alternatively, the antagonistic effect of these two signals when they work together is observed in apoptosis, autophagy, translation and ribosome biogenesis [22-25].
Although not much is known about the relation between ER stress and mTORC2, it was observed that ER stress when induced pharmacologically for long hours, led to GSK3β catalyzed phosphorylation of rictor, a major component of mTORC2, which also suppresses activation of Akt [26]. Till now there is no substantial evidence which suggests whether mTORC2 plays any role either in activation or downregulation of Akt. Although one study demonstrates that a portion of mTORC2 is present on the ER membrane, thus making it a possibility that mTORC2 may phosphorylate Akt on ER membrane [27]. Thus, we would like to hypothesize mTOR’s association with specific membranes may control the mTOR complexes, as demonstrated by mTORC1, which couples the sensing of amino acids in the lysosomal lumen with its activation on the surface of lysosomes [28].
Interdependence of UPR and mTORC1 have been observed in some pathologies, one such example being Tuberous sclerosis (TSC) [29]. TSC, a multisystem disorder is caused by activation of ER stress pathways and mTORC1 [30,15]. An epileptic seizure is the most common symptom of TSC which is usually caused by cerebral cortical tubers but now is also thought to be associated with mTORC1-dependent ER and oxidative stress through the ATF4-CHOP pathway [15]. Another disease characterized by an interaction between mTORC1 and ER stress is diabetic nephropathy [31]. As mTOR is important in maintaining the overall cell viability, it is suggested that low doses of rapamycin along with phenylbutyric acid (PBA), an ER-stress-alleviating ‘chemical chaperone’, for inhibition of ER stress, can be used to treat nephropathies [31]. However, the combination of mTOR inhibitors and PBA requires preclinical studies for the treatment of TSC or diabetic nephropathy. TSC cells are usually sensitive to ER-stress-induced cell death. Thus, activation of ER stress along with the activation of PI3K-Akt–mTORC1 pathway could kill tumors selectively. For this, Mcl-1, a member of the Bcl-2 family, may provide a link between ER stress- mTORC1 based therapy. Further the inhibition of 4E-BP, downstream effectors of mTOR, controlled translation of Mcl-1 plays a key role in the treatment of mTORC1-hyperactive cancers [22]. Therefore, understanding of the mTOR and GRP78 axis may help in opening of new avenues in the treatment of different diseases.
Conclusion
Even though some of the interplays between ER stress and mTOR has been uncovered, several recent studies have reported new links between the two. Thus, this field of combined effect of mTOR and ER stress is significantly intriguing and needs lot of scientific progress for its understanding.
Acknowledgment
The authors are thankful to Hon’ble Vice Chancellor, Integral University, Lucknow for necessary infrastructural support. For the financial assistance, we are grateful to the Science & Engineering Research Board (SERB-DST) for the research grant (YSS/2015/001902).
References
- Malhotra JD, Kaufman RJ (2007) The endoplasmic reticulum and the unfolded protein response. Semi in Cell Dev Bio 18(6): 716-731.
- Hartl FU, Hayer Hartl M (2002) Molecular chaperones in the cytosol: from nascent chain to folded protein. Science 295(5561): 1852-1858.
- Goldberg AL (2003) Protein degradation and protection against misfolded or damaged proteins. Nature 426(6968): 895-899.
- Balch WE, Morimoto RI, Dillin A, Kelly JW (2008) Adapting proteostasis for disease intervention. Science 319(16): 916-919.
- Appenzeller-Herzog C, Michael N Hall MN (2012) Bidirectional crosstalk between endoplasmic reticulum stress and mTOR signaling. Trends Cell Biol 22(5): 274-282.
- Zhang Y, Liu R, Ni M, Gill P, Lee AS (2010) Cell surface relocalization of the endoplasmic reticulum chaperone and unfolded protein response regulator GRP78/BiP. J BiolChem 285(20): 15065-15075.
- Shin BK, Wang H, Yim AM, Le Naour F, Brichory F et al. (2003) Global profiling of the cell surface proteome of cancer cells uncovers an abundance of proteins with chaperone function. J Biol Chem 78(9): 7607-7616.
- Li J, Lee AS (2006) Stress induction of GRP78/BiP and its role in cancer. Curr Mol Med 6(1): 45-54.
- Lee AS (2005) The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods 35(4): 373- 381.
- Wang M, Wey S, Zhang Y, Ye R, Lee AS (2009) Role of the Unfolded Protein Response Regulator GRP78/BiP in Development, Cancer, and Neurological Disorders. Antioxid Redox Signal 11(9): 2307-2316.
- Casas C (2017) GRP78 at the Centre of the Stage in Cancer and Neuroprotection. Front Neurosci 11: 177.
- Gardner BM, PincusD, Gotthardt K, Gallagher CM, Walter P (2013) Endoplasmic reticulum stress sensing in the unfolded protein response. Cold Spring Harb Perspect Biol 5(3): a013169.
- Axe EL, Walker SA, Manifava M, Chandra P, Roderick H, et al. (2008) Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J Cell Biol 182(4): 685-701.
- Kato H, Nakajima S, Saito Y, Takahashi S, Katoh R, et al. (2012) mTORC1 serves ER stress-triggered apoptosis via selective activation of the IRE1–JNK pathway. Cell Death Differ. 19(2): 310-320.
- Di Nardo A, Kramvis I, Cho N, Sadowski A, Meikle L, et al. (2009) Tuberous sclerosis complex activity is required to control neuronal stress responses in an mTOR dependent manner. J Neurosci 29(18): 5926-5937.
- Yamazaki H, Hiramatsu N, Hayakawa K, Tagawa Y, Okamura M, et al. (2009) Activation of the Akt–NF-kappaB pathway by subtilase cytotoxin through the ATF6 branch of the unfolded protein response. J Immunol 183(2): 1480-1487.
- Thomas GV, Tran C, Mellinghoff IK, Welsbie DS, Chan E, et al. (2006) Hypoxia-inducible factor determines sensitivity to inhibitors of mTOR in kidney cancer. Nat Med 12(1): 122-127.
- Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, et al. (2004) Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 431(7005): 200-205.
- Peterson TR, Sengupta SS, Harris TE, Carmack AE, Kang SA, et al. (2011) mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell 146(3): 408-420.
- Dan HC, Cooper MJ, Cogswell PC, Duncan JA, Ting JP, et al. (2008) Aktdependent regulation of NF-kB is controlled by mTOR and Raptor in association with IKK. Genes Dev 22(11): 1490-1500.
- Hotamisligil GS (2010) Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140(6): 900-917.
- Hsieh AC, Costa M, Zollo O, Davis C, Feldman ME, et al. (2010) Genetic dissection of the oncogenic mTOR pathway reveals druggable addiction to translational control via 4EBP–eIF4E. Cancer Cell 17(3): 249-261.
- Zoncu R, Efeyan A, Sabatini DM (2011) mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 12(1): 21-35.
- Rutkowski DT, Hegde RS (2010) Regulation of basal cellular physiology by the homeostatic unfolded protein response. J Cell Biol 189(5): 783- 794.
- Mayer C, Zhao J, Yuan X, Grummt I (2004) mTOR-dependent activation of the transcription factor TIF-IA links rRNA synthesis to nutrient availability. Genes Dev 18(4): 423-434.
- Chen CH, Shaikenov T, Peterson TR, Aimbetov R, Bissenbaev AK, et al. (2011) ER stress inhibits mTORC2 and Akt signaling through GSK- 3beta-mediated phosphorylation of rictor. Sci Signal 4(161): ra10.
- Boulbés DR, Shaiken T, Sarbassov dos D (2011) Endoplasmic reticulum is a main localization site of mTORC2. Biochem Biophys Res Commun 413(1): 46-52.
- Zoncu R, Bar-Peled L, Efeyan A, Wang S, Sancak Y, et al. (2011) mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science 334(6056): 678–683.
- Crino PB, Nathanson KL, Henske EP (2006) The tuberous sclerosis complex. N Engl J Med 355(13): 1345-1356.
- Ozcan U, Ozcan L, Yilmaz E, Düvel K, Sahin M, et al. (2008) Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis. Mol Cell 29(5): 541–551.
- Inoki K, Mori H, Wang J, Suzuki T, Hong S, et al. (2011) mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J Clin Invest 121(6): 2181-2196.






























