Effect of the Consciousness Energy Healing Treatment on the Characteristic Properties of Cefazolin Sodium
Gopal Nayak1, Mahendra Kumar Trivedi1, Alice Branton1, Dahryn Trivedi1 and Snehasis Jana2*
1Trivedi Global Inc, Henderson, USA
2Trivedi Science Research Laboratory Pvt Ltd, India
Submission: November 19, 2018; Published: February 21, 2019
*Corresponding author: Snehasis Jana, Trivedi Science Research Laboratory Pvt Ltd, Bhopal, India
How to cite this article: Gopal Nayak, Mahendra Kumar Trivedi, Alice Branton, Dahryn Trivedi, Snehasis Jana. Effect of the Consciousness Energy Healing Treatment on the Characteristic Properties of Cefazolin Sodium. Adv Biotech & Micro. 2019; 13(1): 555852. DOI: 10.19080/AIBM.2019.13.555852
Abstract
Cefazolin is a broad-spectrum first-generation cephalosporin antibiotic, used for the treatment of a number of bacterial infections. The aim of this research work was to evaluate the influence of the Trivedi Effect®-Consciousness Energy Healing Treatment on the physicochemical, and thermal properties of cefazolin sodium powder using modern analytical techniques. The test sample cefazolin sodium was divided into two parts. One part of the test sample was considered as a control sample (no Biofield Energy Treatment was provided), whereas the other part was treated with the Consciousness Energy Healing Treatment remotely by a renowned Biofield Energy Healer, Gopal Nayak and termed as a treated sample. The particle size values in the treated sample were significantly decreased by 3.95%(d10), 2.63%(d50), 13.02%(d90), and 21.7% {D (4,3)}; hence, the specific surface area was increased by 5% compared to the control sample. The decomposition temperature of the treated cefazolin was increased by 4.84 compared to the control sample. Similarly, the latent heat of evaporation of two peaks and decomposition was significantly increased by 56.81%, 225.38%, and 261.84% in the treated sample compared with the control sample. The total weight loss was decreased by 2.7%; however, the residual amount was significantly increased by 28.59% in the treated cefazolin compared with the control sample. A new form of cefazolin sodium might have generated after Trivedi Effect®-Consciousness Energy Healing Treatment which may offer better solubility, dissolution rate, bioavailability, and thermal stability compared to the control sample. The treated cefazolin sodium would be more efficacious pharmaceutical formulations against respiratory tract infections, cellulitis, urinary tract infections, joint infection, biliary tract infections, pneumonia, endocarditis, genital infections, blood infections, etc.
Keywords: Cefazolin sodium; The Trivedi Effect®; Consciousness energy healing treatment; Complementary and alternative medicine; Particle size; Surface area; DSC
Abbreviations: UTI: Urinary Tract Infections; CAM: Complementary and Alternative Medicine; PSA: Particle Size Analysis; PXRD: Powder X-Ray Diffraction; DSC: Differential Scanning Calorimetry; TGA: Thermo Gravimetric Analysis; DTG: Differential Thermo Gravimetric Analysis
Introduction
Cefazolin is a broad-spectrum first-generation cephalosporin antibiotic. It is used for the treatment of a number of both Gram-positive (i.e., Staphylococcus epidermidis, Streptococcus pyogenes, Staphylococcus aureus, Streptococcus agalactiae, Streptococcus pneumonia, and other strains of streptococci) and Gram-negative (i.e., Escherichia coli, Proteus mirabilis, etc.) bacterial infections [1,2]. Cefazolin inhibits the growth of bacteria by restricting the bacterial cell wall synthesis [3]. It is used for the treatment of diseases like Urinary Tract Infections (UTIs), respiratory tract infections, pneumonia, cellulitis, endocarditis, joint infection, blood infections, genital infections, biliary tract infections, and also prevent the streptococcal disease in the time of delivery and before surgery, etc. [1,2]. Some amount of cefazolin enters in the breast milk during the pregnancy and breastfeeding, so safety needs to follow in that period. Many side effects associated with the cefazolin medication are diarrhoea, vomiting, stomach pain, rash, blood dyscrasias, allergic reaction, hypoprothrombinemia, etc. [2,3]. The sodium salt form of cefazolin (cefazolin sodium) available in various dosage form, i.e., injectable, powder for injection, eye drop, etc. [4]. It is a white crystalline powder, insoluble in chloroform, acetone, dichloromethane, and ethyl acetate; slightly soluble in ethanol and methanol; and freely soluble in water and isopropanol; it has no fixed melting point but decompose at the temperature of ~193 °C [5].
The physicochemical properties of a pharmaceutical compound is very important for the dissolution, absorption, and bioavailability in the body [6]. All over the world pharmaceutical scientists working for the improvement of stability and bioavailability of pharmaceutical and nutraceutical compounds. Similarly, The Trivedi Effect®-Consciousness Energy Healing Treatment is one of the approaches which has the significant impact on the crystallite size, particle size, particle surface area, thermal behavior, and bioavailability of pharmaceutical/ nutraceutical compounds [7-9]. The Trivedi Effect® is a natural and only scientifically proven phenomenon in which a person can harness this inherently intelligent energy from the “Universe” and transmit it anywhere on the planet through the possible mediation of neutrinos [10]. A unique, infinite, and paradimensional electromagnetic field exists around every living organism, which originates from the continuous movements of the charged particles, ions, cells, blood/lymph flow, brain functions, heart, etc. in the body known as Biofield. The Biofield Energy Healer has a unique capability to harness energy from the “Universe” and can transmit anywhere in the world. This Biofield Energy Therapy has been reported with significantly beneficial outcomes against various disease conditions [11].
The National Institutes of Health/National Center for Complementary and Alternative Medicine (NIH/NCCAM) recommend and included the Energy therapy under Complementary and Alternative Medicine (CAM) category along with Yoga, homeopathy, Ayurveda medicine, Chinese herb and medicine, etc. that has been accepted by most of the U.S.A. population [12, 13]. The Trivedi Effect®-Consciousness Energy Healing Treatment has also shown significant impacts on the physicochemical and behavior of metals, organic compounds, ceramics [14-19], crops, cancer cell line, microbes [20-24]. Therefore, this experiment was designed to evaluate the impact of the Trivedi Effect®-Consciousness Energy Healing Treatment on the physicochemical, thermal, and behavioral properties of cefazolin sodium powder sample using Particle Size Analysis (PSA), Powder X-Ray Diffraction (PXRD), Differential Scanning Calorimetry (DSC), and Thermo Gravimetric Analysis (TGA)/ Differential Thermo Gravimetric analysis (DTG).
Materials and Methods
Chemicals and reagents
Cefazolin sodium (C14H13N8NaO4S3) powder was purchased from Tokyo Chemical Industry Co, Ltd, Japan and the additional chemicals were of analytical grade purchased in India.
Consciousness energy healing treatment strategies
The cefazolin sodium test sample was divided into two parts. One part of the test sample was received the Trivedi Effect®- Consciousness Energy Healing Treatment remotely under the standard laboratory conditions for 3 minutes by the renowned Biofield Energy Healer, Gopal Nayak, India, and known as the Biofield Energy Treated sample. However, the second part of cefazolin powder sample was considered as a control sample, which did not receive the Biofield Energy Treatment. Later, the control sample was treated with a “sham” healer; however, the sham healer did not have any knowledge about the Biofield Energy Treatment. After the treatment, the Biofield Energy Treated and control cefazolin sodium powder samples were kept in sealed condition and characterized using PSA, PXRD, DSC, and TGA/DTG analytical techniques.
Characterization
Particle size analysis (PSA)
The particle size analysis of cefazolin sodium powder samples was conducted on Malvern Rasterizer 2000, from the UK with a detection range between 0.01 μm to 3000 μm using wet method [7, 8]. The percent change in particle size (d) for cefazolin sodium powder sample at below 10% level (d10), 50% level (d50), 90% level (d90), and D (4,3) was calculated using the following equation 1
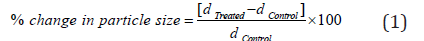
Where dControl and dTreated are the particle size (μm) for at below 10% level (d10), 50% level (d50), and 90% level (d90) of the control and Biofield Energy Treated samples, respectively.
The % change in surface area (S) was calculated using the following equation 2
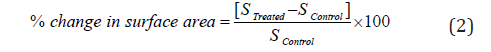
Where SControl and STreated are the surface area of the control and Biofield Energy Treated cefazolin sodium powder sample, respectively.
Powder X-ray diffraction (PXRD) analysis
The PXRD analysis of cefazolin sodium powder samples were performed with the help of Rigaku MiniFlex-II Desktop X-ray diffractometer (Japan) [25, 26]. The average size of individual crystallites are generally calculated using the Scherrer’s formula (3):

Where k is the equipment constant (0.94), G is the crystallite size in nm, λ is the radiation wavelength (0.154056 nm for Kα1 emission), β is the Full-Width at Half Maximum (FWHM), and θ is the Bragg angle [27].
Differential scanning calorimetry (DSC)
The DSC analysis of cefazolin sodium powder samples was performed with the help of DSC Q200, TA instruments. The sample of ~1-2 mg was loaded to the aluminium sample pan at a heating rate of 10 ºC/min from 30°C to 350°C [7, 8]. The % change in melting point (T) was calculated using the following equation 4:
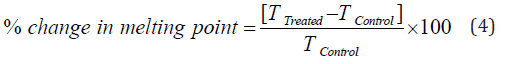
Where TControl and TTreated are the melting point of the control and Biofield Energy Treated samples, respectively.
The % change in the latent heat of fusion (ΔH) was calculated using the following equation 5:
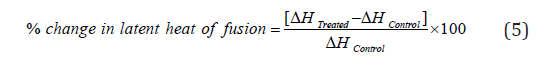
Where ΔHControl and ΔHTreated are the latent heat of fusion of the control and Biofield Energy Treated cefazolin sodium powder sample, respectively.
Thermal gravimetric analysis (TGA) / differential thermo gravimetric analysis (DTG)
TGA/DTG thermograms of cefazolin sodium powder samples were carried out with the help of TGA Q50 TA instruments. Sample of ~4-7 mg was loaded to the platinum crucible at a heating rate of 10oC/min from 25°C to 1000°C with the recent literature [7, 8]. The % change in weight loss (W) was calculated using the following equation 6:
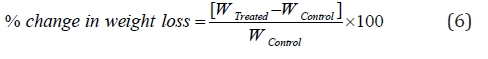
Where WControl and WTreated are the weight loss of the control and Biofield Energy Treated cefazolin sodium, respectively.
The % change in maximum thermal degradation temperature (Tmax) (M) was calculated using the following equation 7:
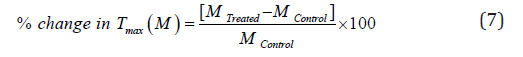
Where MControl and MTreated are the Tmax values of the control and Biofield Energy Treated cefazolin sodium, respectively.
Results and Discussion
Particle size analysis (PSA)
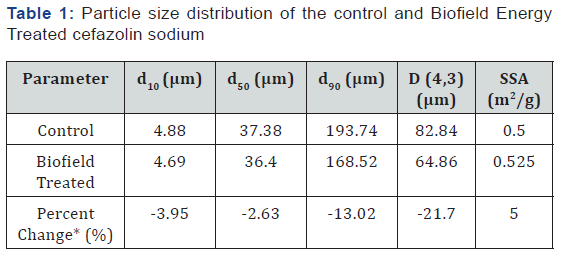
d10, d50, and d90: particle diameter corresponding to 10%, 50%, and 90% of the cumulative distribution, D(4,3): the average mass-volume diameter, and SSA: the specific surface area.
*denotes the percentage change in the Particle size distribution of the Biofield Energy Treated sample with respect to the control sample.
The PSD data of both the control and Biofield Energy Treated cefazolin are presented in Table 1. The particle size values of the control sample at d10, d50, d90, and D (4,3) were 4.88 μm, 37.38 μm, 193.74 μm, and 82.84 μm, respectively. Likewise, the particle sizes of the Biofield Energy Treated sample at d10, d50, d90, and D (4,3) were 4.69 μm, 36.4 μm, 168.52 μm, and 64.86 μm, respectively. The particle size values in the treated sample were significantly decreased at d10, d50, d90, and D (4,3) by 3.95%, 2.63%, 13.02%, and 21.7% compared to the control sample. The Specific Surface Area (SSA) of the Biofield Energy Treated sample (0.525 m2/g) was increased by 5% compared to the control sample (0.5 m2/g). It can be assumed that the Trivedi Effect® might have fractured the larger particle into smaller one, hence increased surface area. Due to the reduced particle size and increase surface area, the solubility, dissolution rate, and bioavailability would be higher the physiological system [6,28, 29]. Thus, the Consciousness Energy Healing Treated cefazolin sodium might offer better solubility, dissolution rate, and bioavailability compared with the untreated sample.
Powder X-ray diffraction (PXRD) analysis
Both the control and the Biofield Energy Treated cefazolin sodium samples did not show any clear, sharp, and intense peaks in the PXRD diffractograms (Figure 1). Therefore, it was difficult to compare PXRD of the Biofield Energy Treated cefazolin sodium with the control sample. It was concluded that both the samples were amorphous.

Differential scanning calorimetry (DSC) analysis
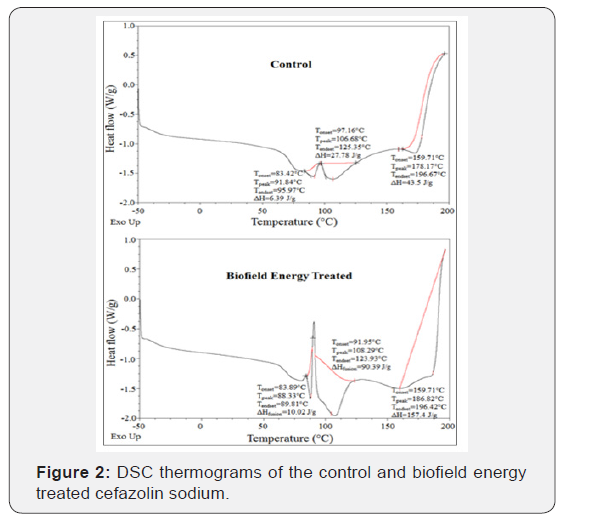
The DSC thermograms of the control and Biofield Energy Treated cefazolin sodium showed two endothermic and one exothermic peak (Figure 2). The evaporation temperatures of the Biofield Energy Treated cefazolin sodium was slightly altered by -3.82% and 1.51%, but the decomposition temperature was increased by 4.85% compared with the control sample (Table 2).

The latent heat of evaporation (ΔHevaporation) of the Biofield Energy Treated cefazolin sodium was significantly increased by 56.81% and 225.38% compared with the control sample (Table 2). Similarly, the latent heat of decomposition (ΔHdecomposition) of the Biofield Energy Treated sample was significantly increased by 261.84% compared with the control sample (Table 2). The disrupted the molecular chains and crystal structure of cefazolin [30], might be the root cause of altered thermal stability of the Biofield Energy Treated sample compared to the control sample.
thermo gravimetric analysis (DTG)
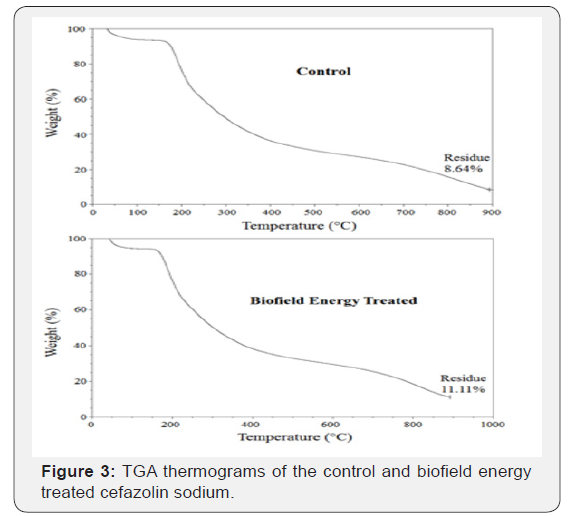
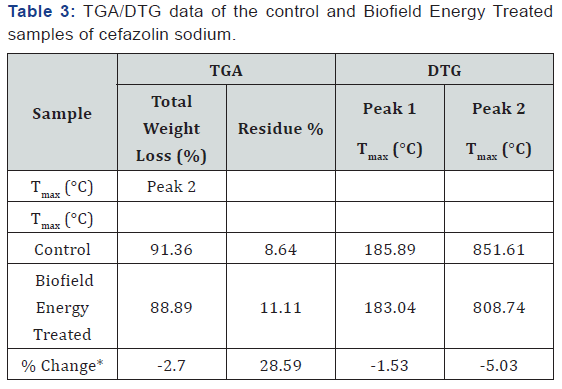
The TGA thermograms of the control and Biofield Energy Treated cefazolin sodium samples showed three steps of thermal degradation (Figure 3). The total weight loss in the Biofield Energy Treated sample was decreased by 2.7% compared with the control sample (Table 3). However, the residue amount was significantly increased by 28.59% in the Biofield Energy Treated cefazolin sample compared to the control sample (Table 3).

*denotes the percentage change of the Biofield Energy Treated sample with respect to the control sample
Tmax = the temperature at which maximum weight loss takes place in TG or peak temperature in DTG.
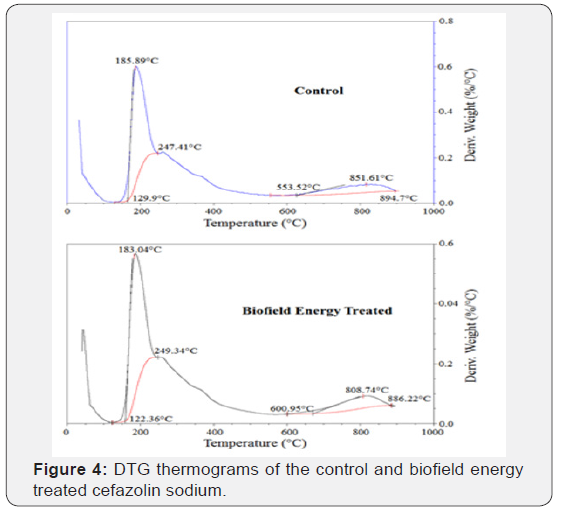
The control and Biofield Energy Treated cefazolin sodium exhibited two peaks in the DTG thermograms (Figure 4). The maximum thermal degradation temperature (Tmax) of the 1st and 2nd peaks of Biofield Energy Treated sample were slightly altered by -1.53% and -5.03% compared to the control sample (Table 3). Overall, thermal analysis by DSC and TGA/DTG of the samples revealed that the thermal stability of the Biofield Energy Treated cefazolin sodium was significantly improved compared with the control sample.
Conclusion
The Trivedi Effect®-Consciousness Energy Healing Treatment has significant effects on the physicochemical and thermal properties of cefazolin sodium. The particle size values in the Biofield Energy Treated sample were significantly decreased by 3.95%(d10), 2.63%(d50), 13.02%(d90), and 21.7% {D (4,3)}; hence, the specific surface area was increased by 5% compared to the control sample. The decomposition temperature of the Biofield Energy Treated cefazolin was increased by 4.84 compared to the control sample. Similarly, the ΔHevaporation of two peaks and ΔHdecomposition was significantly increased by 56.81%, 225.38%, and 261.84% in the Biofield Energy Treated sample compared with the control sample. The total weight loss was decreased by 2.7%; however, the residual amount was significantly increased by 28.59% in the Biofield Energy Treated cefazolin compared with the control sample. A new form of cefazolin sodium might have generated after Trivedi Effect®-Consciousness Energy Healing Treatment which may offer better solubility, dissolution rate, bioavailability, and thermal stability compared to the control sample.
The Biofield Energy Treated cefazolin sodium would be more efficacious pharmaceutical formulations against respiratory tract infections, cellulitis, urinary tract infections, joint infection, biliary tract infections, pneumonia, endocarditis, genital infections, blood infections, and also prevent group B streptococcal disease at the time of delivery and before surgery, etc.
Acknowledgement
The authors are grateful to Central Leather Research Institute, SIPRA Lab. Ltd., Trivedi Science, Trivedi Global, Inc., Trivedi Testimonials, and Trivedi Master Wellness for their assistance and support during this work.
References
- (2018) Cefazolin - cefazolin sodium injection, powder, for solution. Daily Med.
- Katzung, Trevor AJ (2015) Basic & Clinical Pharmacology. McGraw Hill Education, New York, USA, pp. 776-778.
- Cefazolin sodium - Drug Summary. pp. 1193.
- Stork CM (2006) Antibiotics, antifungals, and antivirals. McGraw-Hill, New York, USA, pp. 847.
- How TH, Loo WY, Yow KL, Lim LY, Chan EW, et al. (1998) Stability of cefazolin sodium eye drops. J Clin Pharm Ther 23(1): 41-47.
- Wang J, Qian Y, Zhang M, Wu J, Yang Z (2012) Cefazolin sodium pentahydrate crystal and its molecular assembly preparation method.
- Chereson R (2009) Bioavailability, bioequivalence, and drug selection. In: Makoid CM, Vuchetich PJ, et al. (Eds), Basic pharmacokinetics (1st edn), Pharmaceutical Press, UK.
- Branton A, Trivedi MK, Trivedi D, Nayak G (2018) Evaluation of the physicochemical and thermal properties of the biofield energy healing treated ofloxacin. J Pharm Pharmaceutics 5: 80-87.
- Nayak G, Trivedi MK, Branton A, Trivedi D, Jana S (2018) The energy of consciousness healing treatment: Impact on physicochemical and thermal properties of l-tryptophan. Journal of Food Science and Technology 5: 84-94.
- Branton A, Jana S (2017) The use of novel and unique biofield energy healing treatment for the improvement of poorly bioavailable compound, berberine in male Sprague Dawley rats. American Journal of Clinical and Experimental Medicine 5(4): 138-144.
- Trivedi MK, Mohan TRR (2016) Biofield energy signals, energy transmission and neutrinos. American Journal of Modern Physics 5(6): 172-176.
- Rubik B, Muehsam D, Hammerschlag R, Jain S (2015) Biofield science and healing: History, terminology, and concepts. Glob Adv Health Med 4: 8-14.
- Barnes PM, Bloom B, Nahin RL (2008) Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report 12: 1-23.
- Koithan M (2009) Introducing complementary and alternative therapies. J Nurse Pract 5(1): 18-20.
- Trivedi MK, Tallapragada RM (2008) A transcendental to changing metal powder characteristics. Metal Powder Report 63(9): 22-28.
- Dabhade VV, Tallapragada RMR, Trivedi MK (2009) Effect of external energy on the atomic, crystalline, and powder characteristics of antimony and bismuth powders. Bulletin of Materials Science 32(5): 471-479.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Panda P, et al. (2016) Determination of isotopic abundance of 13C/12C or 2H/1H and 18O/16O in biofield energy treated 1-chloro-3-nitrobenzene (3-CNB) using gas chromatography-mass spectrometry. Science Journal of Analytical Chemistry 4: 42-51.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Panda P, et al. (2016) Mass spectrometric analysis of isotopic abundance ratio in biofield energy treated thymol. World Journal of Applied Chemistry 1(1): 1-8.
- Trivedi MK, Nayak G, Patil S, Tallapragada RM, Latiyal O (2015) Studies of the atomic and crystalline characteristics of ceramic oxide nano powders after biofield treatment. Ind Eng Manage 4: 161.
- Trivedi MK, Tallapragada RM, Branton A, Trivedi D, Nayak G, et al. (2015) The potential impact of biofield energy treatment on the physical and thermal properties of silver oxide powder. International Journal of Biomedical Science and Engineering 3(5): 62-68.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Mondal SC, et al. (2015) Morphological characterization, quality, yield and DNA fingerprinting of biofield energy treated alphonso mango (Mangifera indica L). Journal of Food and Nutrition Sciences 3: 245-250.
- Nayak G, Altekar N, Nandini Altekar (2015) Effect of a biofield treatment on plant growth and adaptation. Journal of Environment and Health Science 1: 1-9.
- Trivedi MK, Patil S, Shettigar H, Gangwar M, Jana S (2015) In vitro evaluation of biofield treatment on cancer biomarkers involved in endometrial and prostate cancer cell lines. J Cancer Sci Ther 7: 253- 257.
- Trivedi MK, Branton A, Trivedi D, Shettigar H, Nayak G, et al. (2015) Assessment of antibiogram of multidrug-resistant isolates of Enterobacter aerogenes after biofield energy treatment. J Pharma Care Health Sys 2: 145.
- http://article.sciencepublishinggroup.com/html/10.11648.j.ajls.20150305.16.html
- Zhang T, Paluch K, Scalabrino G, Frankish N, Healy AM, et al. (2015) Molecular structure studies of (1S,2S)-2-benzyl-2,3-dihydro-2- (1Hinden-2-yl)-1H-inden-1-ol. J Mol Struct 1083: 286-299.
- Langford JI, Wilson AJC (1978) Scherrer after sixty years: A survey and some new results in the determination of crystallite size. J Appl Cryst 11(2): 102-113.
- Mosharrof M, Nystrom C (1995) The effect of particle size and shape on the surface specific dissolution rate of microsized practically insoluble drugs. International Journal of Pharmaceutics 122(1-2): 35-47.
- Buckton G, Beezer AE (1992) The relationship between particle size and solubility. International Journal of Pharmaceutics 82(3): R7-R10.
- Zhao Z, Xie M, Li Y, Chen A, Li G, et al. (2015) Formation of curcumin nanoparticles via solution-enhanced dispersion by supercritical CO2. Int J Nanomedicine 10: 3171-3181.






























