Biotransformation of Selenite to Red Elemental Selenium
Shafeeq-ur-Rehman1, Ayesha Alam2, Mushtaq A Saleem3, Imran Sajid1, Muhammad Faisal1, Artifa Tahir4, Haleema Saadia5, Faiza Anum4 and Shahid Raza2*
1Department of Biotechnology, University of South Asia, Pakistan
2Department of ORIC, Lahore Garrison University, Pakistan
3Department of Life Sciences, University of central, Pakistan
4Department of Environmental sciences, Lahore College for Women University, Pakistan
5Departmemt of Microbiology, Baluchistan University of Information Technology and management sciences, Pakistan
Submission: September 14, 2018; Published: January 03, 2019
*Corresponding author: Shahid Raza, Department of ORIC, Lahore Garrison University, Lahore, Pakistan
How to cite this article: Shafeeq-ur-Rehman, Ayesha A, Mushtaq A S, Imran S, Md Faisal. et al. Biotransformation of Selenite to Red Elemental Selenium. Adv Biotech & Micro. 2019; 12(1): 555830. DOI: 10.19080/AIBM.2019.12.555830
Abstract
Selenium is an element of great interest due to its nutritional importance for humans and animals. Selenium undergoes biotic transformation like oxidation, dissimulator and assimilatory reduction, alkylation and de-alkylation, also due to which “cocktail” of various selenium specie is present in environment, posing a big challenge to Se specie analysis. In current study, we isolated bacterial strain, Bacillus licheniformis, Exiguobacterium sp., Bacillus subtilis, that convert (SeO3)-2 (toxic) to elemental (reduced and less toxic) selenium. Different physical parameters were checked experimentally to increase Se reduction like pH, temperature, selenite concentration, incubation time, aeration. Se reduction increased with increased pH, Maximum reduction, 31 to 90% was check at pH 9 and different strain have different ranges of temperature, optimum temperature 37 °C and 44 °C was noted for selenium reduction. Microbes can reduce selenium in aerobic as well as anaerobic condition and reduction decrease with the increase in incubation time.
Keywords: Selenium; oxidation; Bacillus licheniformis; Reduction; Strain; Temperature; pH; Microbes
Introduction
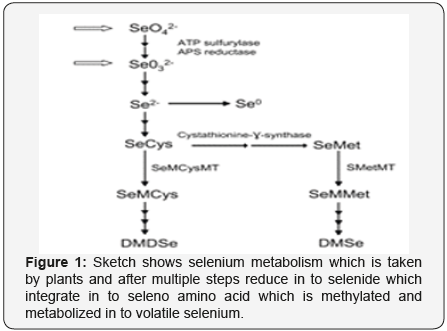
Selenium (Se) is an important nutritional element for humans as well as animals because it is required at low concentrations in the body. However, a minor increase in concentration of selenium can become toxic for the body [1]. Atomic weight and atomic number of selenium are 78.94 and 34 respectively. In the periodic table, selenium is placed between tellurium (a metal) and sulfur (a nonmetal) in Group VIA. In context of physical and chemical properties selenium shares properties of both metals and nonmetals. When selenium binds with metals or hydrogen, it acquires -2 oxidation state. But it acquires +4 or +6 oxidation states in oxygenated compounds. Hence gives rise to a series of selenium containing compounds. [2,3] (Figure 1&2).

Selenium exists in varying oxidation states i.e. (0), (+6), (+4), and (-2). Oxidation state dictates toxicity, solubility, movement in nature and bioavailability of selenium. In turn oxidation states of selenium themselves depends on solution pH, redox conditions and microbial activity. Elemental selenium Se (0) is very stable and highly water insoluble. It exists in several allotropic forms. Inorganic selenium in water dissolved form is exists either as (+4) selenite (SeO2) or (+6) selenate (SeO2). In (-2) oxidation state selenium can exist in two forms; hydrogen selenide (H2Se) or metallic selenides. H2Se is a toxic gas at room temperature and in aqueous solutions it is thermodynamically unstable. Most insoluble forms of selenium are heavy metal selenides. In reducing, moderately oxidized and oxidizing environments selenium exists in Se (-2), Se (-4) and Se (-6) oxidation states respectively [4].
It is well established that selenium metalloid has toxicological, biological, environmental, and industrial importance. Toxic effects caused by selenium and its compounds are of great interest because domestic animals get selenium poisoning when they feed on seleniferous plants [5]. Low concentration of selenium is required by humans as well as animals to remain healthy. However, selenium deficiencies can cause disorders in both humans and animals [5-7].
Suitable concentration of selenium and vitamin E with other antioxidants is very beneficial for the health of an organisms Se has been found in different concentration in beverages and food across globe [5-8]. Level of Se consumed in animal diet is directly reflected products obtained from those animals [9]. It’s not only case with animals but even plants are affected by Se as well. Se is predominantly present in the form of Selinate or selenite in soil, once plant are grown in soil having significantly high levels of Se they start to take up Se from soil and it is mainly transformed in Se-Met in Cereal grains [10]. There are many factors which directly or in directly affect the Se absorption by plant such as redox state or pH of the soil, moisture content, sulphate concentration. not only types of other organic and inorganic compounds affect the absorbance of Se from soil but oxidation state, nature of draining water and climate conditions also play significant role [9,11,12]. Se is predominantly in form of Selenite with very low solubility if soil has acidic pH. Whereas if alkaline is oxidized to selenite which makes it more soluble and ultimately more available to plants for absorbance [13-15]. Selenium has different concentration levels in different regions across globe. Such as in Finland, New eland and some parts of China soil content of Se is very low b0.05ppm. It is also reported that such low level has adverse effects not only on livestock, but human health is also adversely affected. On other hand regions with high Se soil levels N5 ppm such as Canada, Ireland west USA and some part of Europe are reported high Se level in food as well as compared to the food from countries with low Se solid levels [10,11,16,17].
To increase dietary intake of selenium, its concentration in crops has been increased in UK through application of selenium containing fertilizers [18,19]. Bio-fortification has been proved more direct strategy to enrich selenium in our diet [20,21]. Selenium is used in xerography, photographic exposure meters, rectifiers and solar cells [22]. Amorphous Selenium Nanoparticles (SNs) has properties of X-ray-sensing, semi conduction and photo electricity. These nanoparticles also exhibit ability of good absorption and biological activity because of interaction between the C-N, NH, C = O, COO_ groups of proteins and nanoparticles [23]. We find solution in plants for both sides of selenium problems [24]. Crops might accumulate selenium in health-beneficial chemical forms [8,25]. Wheat (Triticum aestivum) grain grown in seleniferous soils may accumulate selenomethionine (SeMet) and become main source for dietary selenium [8] (Figures 3a, 3b,4a, 4b).
Broccoli (Brassica oleracea var italica) has the ability to accumulate high level of Se-methyl selenocysteine (SeMCys) and SeMet when grown on seleniferous soil [26]. Other plant foods, such as Brazil nut (Bertholletia excelsa) and garlic (Allium sativum), have been marketed as dietary selenium supplements due to enriched selenium [25]. Apart from this, secondary accumulators such as Indian mustard (Brassica juncea) and selenium-hyper accumulating plant species such as Astragalus bisulcatus have attracted great interest because of their ability to volatilize and accumulate selenium for phytoremediation of selenium-contaminated soils [27]. Volatilization of Selenium changes highly toxic selenite and selenate into volatile dimethyl diselenide (DMDSe), and dimethyl selenide (DMSe) which are 500 to 700 times less toxic [28].
It not only provides low-cost approach to lessen selenium contamination in environment, but it is also highly efficient and environment friendly approach for that purpose [27,29]. Sulfur metabolic pathway appears to change the inorganic forms of selenium into volatile selenium in plants [30].
Aims and Objective
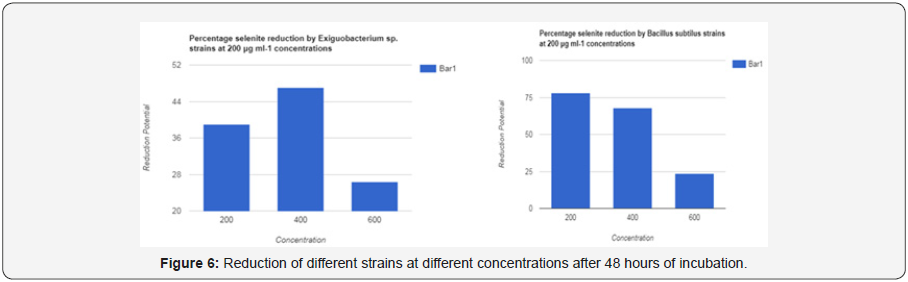
The aim of this study is to isolate the bacterial strains that have the ability to convert SeO-23 which is too much toxic, in to elemental selenium which is less toxic, in a cost-effective way and give us a huge source of potentially and economically valuable microorganisms for the production of elemental selenium and helping us towards use of selenium for human health(Figures 5a,5b & 6a,6b).
Materials and Methods
Materials
All media and solution were autoclaved and prepared in autoclaved distilled water for 15 min at 121 oC at 15Ib/inch2. All the chemicals were used are of analytical grade. I was provided with the strains that were previously isolated and well characterized biochemically and genetically.
Cyclohexane
Commercially prepared Cyclohexane used.
Methods
Bacterial Selenite Reduction Experiments
Present study described the reduction of selenite to elemental selenium by bacterial strains already isolated.
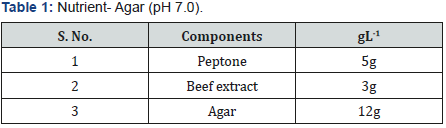
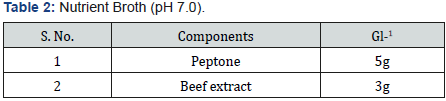
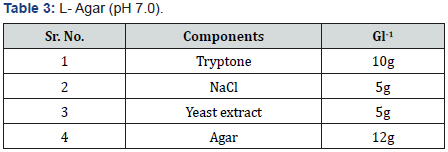
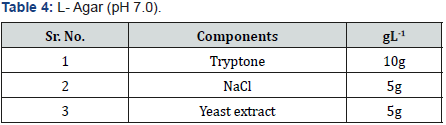
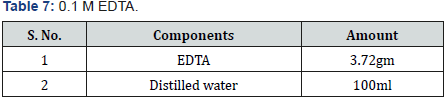
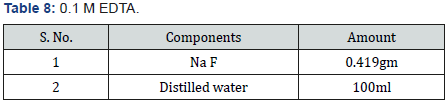
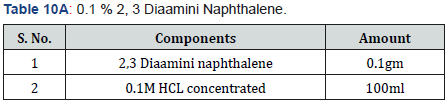
Selenite Content Determination [31], under aerobic conditions nutrient broth were used to determine the selenium content. Nutrient broth was prepared for measuring the selenite by different microbial cultures. In autoclaved nutrient broth sodium selenite with concentration 500μg-1was added. 10 μg of bacterial culture which was suspended in autoclave water was mixed with 5ml of nutrient broth which was supplemented with sodium selenite and then incubate at 37 oC for 2 days.1 ml of 48 hr old culture was taken in centrifuged tube and centrifuged at 14000 rpm for 5 min for measure the selenium content. Mix 10ml of 0.1MHCL (Table 1), 0.5ml of 0.1MEDTA (Table 2), 0.5ml of 0.1MNaF (Table 3), and 0.5ml of 0.1M disodium oxalate (Tables 4&5) with 250-μl sample and then 2.5ml of 0.1% 2,3-diaminonaphthalene (Table 4) in 0.1MHCL was added. After mixing tube was incubated at 40 oC for 30 min and then cooled at room temperature. Tube was shaking vigorously and selenium 2,3-diaminonaphthalene complex was comes out with 6ml of cyclohexane. Organic phase absorbance was determined at 377nm, but zero absorbance was made for cyclohexane (Table 6).

A = remaining % selenite concentration
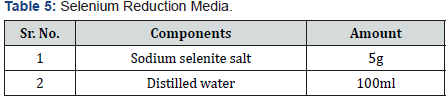
Dissolve 5g sodium selenite salt in 100ml autoclaved distilled water.

Chemicals for the estimation of se (iv) reduction.
Percentage reduction was calculating percentage of selenite that has been converted to selenium precipitates in the medium.
Percentage reduction =100-A
Standard curve for selenite
Standard curve was made by making solutions of known concentrations of sodium selenite in nutrient broth. Known concentrations were 100 μg ml-1, 200 μg ml-1, 300 μg ml-1, 400 μg ml-1, 500 μg ml-1, 600 μg ml-1, 700 μg ml-1, 800 μg ml-1, 900 μg ml-1 and 1000 μg ml-1. Selenite content was estimated for each concentration by using modified Brown and Watkinson [31] method as previously described.
Effect of pH on selenite reduction
Four test tubes were taken to check effect of pH on selenite reduction. Nutrient broth was prepared in test tubes. These tubes were then prepared for each strain and supplemented with 200microgramer milliliter of sodium selenite. 3, 5, 7 and 9 pH were adjusted in these test tubes and labeled. Incubated for 48 hours at 37degree centigrade after inoculation. Optical densities were first measured then plotted on calibration curve which helped measuring selenite concentration. Separate tube containing inoculated nutrient broth supplemented with 200 microgram per milliliter was taken as negative control, which after 48 hours, was estimated for selenite content (Tables 7&8).
Effect of temperatures on Selenite reduction
Different temperatures affect growth of thermophiles which reduce sodium selenite. To check the effect of temperature, three different temperature ranges were selected. Tubes were selected in which nutrient broth was prepared, with 200 microgram per milliliter sodium selenite supplementation. PH was adjusted according to optimum selenite reduction for each strain. From prepared tubes one was prepared as negative control and other were prepared for three temperatures for each strain (32 °C, 37 °C and 42 °C) and labeled accordingly. Inoculation was given by same method. These test tubes were then incubated at 32 °C, 37 °C and 42 °C for 2 days according to their labeling. Selenite content in supernatant after incubation was measured which helped in checking selenite reduction at each temperature for each strain.
Aeration effect on selenite reduction
Effect of aeration on selenite reduction was studies as strains were cultivated as still compared to nutrient broth supplemented with 300 microgram per milliliter sodium selenite at 37 °C for 48’hours, containing shaking cultures. Like in previous experiments negative control was also prepared.
Effect of selenite concentrations on selenite reduction
In this experiment, three different sodium selenite concentration was taken and supplemented in test tubes containing 5ml nutrient broth for each strain with 200microgram per milliliter, 400 microgram per milliliter, 600 microgram per milliliter. Negative control was also prepared for each concentration. PH and temperature were adjusted accord to maximum selenite reduction. Suspension were made in distilled autoclaved water. Cultures were given for 48 hours and selenite content was determined after this (after 48 hours) (Tables 9&10A,10B).

Effect of incubation time on reduction of selenite
In this experiment, to determine effect of incubation time, tubes containing nutrient broth were supplemented with 400 microgram per milliliter of sodium selenite, with adjusted pH and temperature that show maximum selenium reduction to which inoculum was given by making suspension in distilled autoclaved water. Then those tubes were incubated for 168 hours. Selenite reduction potential was checked first after 24 hours, then after 48 hours and then 96 hours.
Effect of metals on selenite reduction
Metals affect selenite reduction specially those metals which are toxic and present with selenium in environment. These metals may be cadmium, arsenic, chromium etc. So, this experiment was conducted in such a way to introduce selenite with metals to study their influence. Cultures were prepared with 400 microgram per milliliter of sodium selenite and 50 microgram per milliliter chromium and copper salts were added. After inoculation tubes were incubated for 48 hours at 37 °C. After incubation time selenite was estimated.
Effect of supernatant on Selenite reduction
First nutrient broth was prepared in autoclaved flask. 200 micro gram per milliliter sodium selenite concentration was added in broth. Then inoculum was added. Incubated at 37 °C for 24 hours. After incubation, it was centrifuged at 14000 rpm for 10 minutes. Supernatant was taken, and pallet was discarded. Again, fresh nutrient broth was prepared, 200 microgram per milliliter sodium selenite was added then this mixture was dispensed in test tubes and labeled. Then 2.5 microliter supernatant was added in fresh selenium reduction media. OD was taken at 377 nm wavelength after 30 min, 120 min, 480 min, and 720 min.
Results
Selenite reduction was checked by different environmental factors i.e., temperature, pH, aeration, various concentrations of selenite, various incubation time, comparison of selenium with other metals and supernatant.
Effect of pH on Selenite reduction
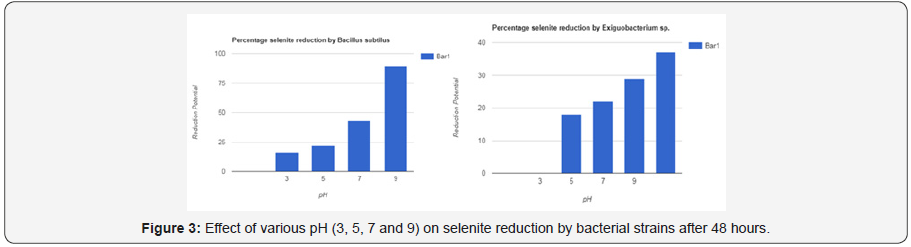
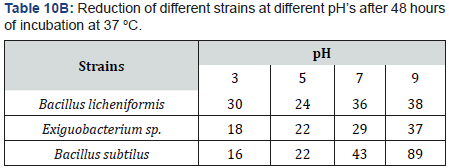
Experiments for studying the effect of pH on selenite reduction and its result proves that pH first affect growth of bacterial strains which further controls reaction. At pH 3, 5, 7 and 9, different bacterial growth was checked. At lowest pH (pH3), reduction of strains were seen to be very low. Maximum reduction resulted at pH5, pH7, and pH9.
At pH7, average percentage of selenite reduction observed was 30 to 72%. At pH9, the percentage was 31to 90%. PH5 showed average 20 to 45% percentage of selenite reduction. PH3 showed 15 to 33% reduction on average. So increased or alkaline pH causes bacteria to reduce selenium efficiently. Reduction is not efficient at low r acidic ph. Also, the precipitation of red color indicates the better selenite reduction.
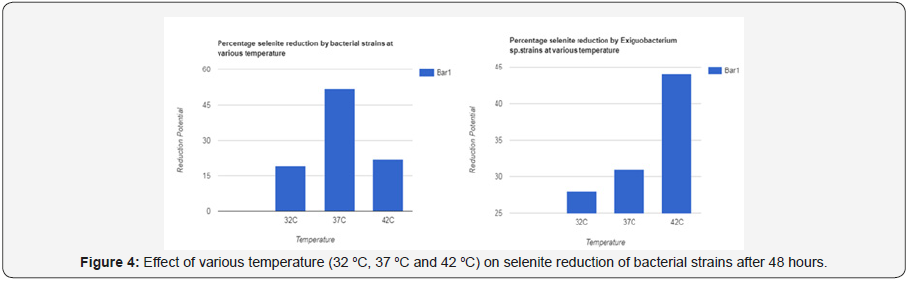
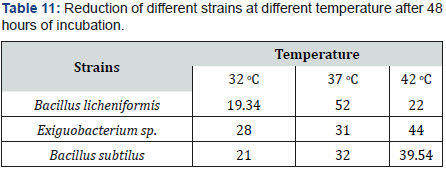
Effect of various temperatures on selenite reduction
As temperature affects bacterial growth, this experiment showed results that temperature affected bacterial growth which further affected selenite reduction. Each strain showed different reduction level at different temperature I.e. 30 °C, 37 °C, 44 °C. According to Result maximum reduction was observed by majority strains at 37 °C and 44 °C. But optimum temperature is 37 °C. Strains were thermophile in nature so they were adapted to every temperature (Table 11&12).
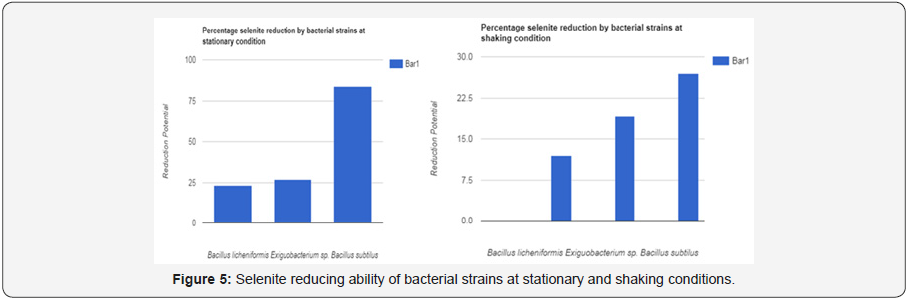
Aeration effect on selenite reduction
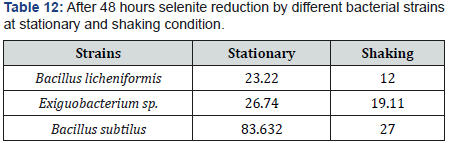
As Se (IV) can be reduced by bacteria in any aerobic or nonaerobic conditions. additional condition is that selenite reduction potential was more in stationary condition than in shaking condition. In former or stationary condition, percentage of reduction was 23 to 83%. In later or shaking condition, percentage was 12 to 27 %. Selenite reduction needs closed system, so reaction flask became anoxic by bacterial metabolism. The conclusion was made that better selenite reduction was occurred by facultative anaerobes facing any condition may be aerobic or anaerobic.
Effect of selenite concentrations on selenite reduction
Reduction was observed for all concentrations (200 microgram per milliliter, 400 microgram per milliliter, 600 micrograms per milliliter). Experiment showed results that low concentration showed high reduction. In tubes, supplemented with low as 200 microgram per milliliter, the reduction was observed to be high (39to 82%) on average for 48 hours. 20 to 30 % reduction of selenite was shown by tubes containing 600 microgram per milliliter concentration. The relation between concentration and reduction of selenite was inversely proportional. Less concentration showed high growth of strain, which showed high reduction. Taking very high concentration to study will make environment toxic when disposed of. High concentration also showed less red coloration which means low reduction (Tables 13&14).
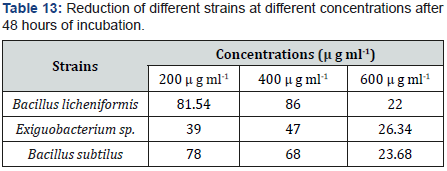
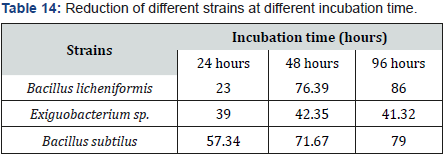
Effect of various incubation time (24, 48 and 96 hours) on selenite reduction
In experiment, cultures supplemented with 400 microgram per milliliter selenite, were incubated for 2 days. Reduction rate was proceeded constantly but with the time it generally increased. Incubation is directly proportional to temperature and also depends on time duration and quantity of inoculum. Increased temperature caused increased reduction with red precipitation but with decreased time, decreased the selenite reduction. Maximum reduction was resulted by all incubation times (24, 48 and 96 hours) but excellent reduction in all was observed at 48 and 96 hours of incubation. After 24 hours 21 to 67 % bacillus subtilus showed 67 % Reduction. After 48 hours 84.36% bacillus licheniformis showed 37 to 78 % reduction. 68 to 87% reduction was occurred after 96 hours. After 96 hours, left selenite content in tube each inoculated by exiquo bacterium sp. 52.4 %.
Effect of different metals on selenite reduction
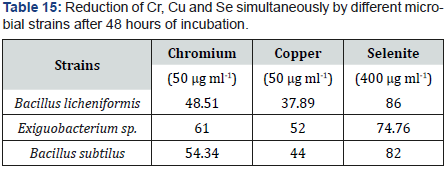
As presence of toxic metals increase selenite reduction. Result of this experiment showed that bacillus licheniformis efficiently work in presence of copper and chromine and reduce maximum selenite as compared to other metals. Chromium had 41 to 62 % reduction potential on average. Copper has low range I.e. 38 to 52 %. Selenite at these conditions had 44 to 86 % range (Table 15).
Effect of supernatant on selenite reduction
Observed result of experiment was that maximum reduction was shown by protein enzymes when time interval was increased. Considered time was 30 min, 120 min, 480 min and 720 min. After 30 min reduction showed by strains was low. While reduction was maximum at 120 min, 480 min and 720 min. Even increased time in interval of 480 min and 720 min showed red color indicating maximum reduction.
Discussion
Se is outstanding element for its photoelectric, semiconductor, free radical scavenging, anti-oxidative and anti-cancer properties [23]. The generation of Se can be accomplished through chemical and biological strategies. Chemical detoxification of metals is demonstrated to be exceptionally costly and frequently brings about auxiliary impacts on the environment. The production of Se0 by using microorganisms has been recommended as a conceivable green strategy. Selenium (Se) is an important nutritional element for humans as well as animals because it is required at low concentrations in the body. However, a minor increase in concentration of selenium can become toxic for the body [1].
Selenium occurs in the environment as a trace element. Places where selenium is in high concentration nutritional toxicity has been observed in livestock. On the other hand, places where selenium is in low concentration nutritional deficiencies have been observed in livestock. These diseases can affect economy adversely. Hence researches are being carried out around the globe to better understand the biogeochemical cycling of selenium in the environment. The information gathered through these studies will help us counter the diseases caused in animals due to selenium abundance or deficiency [32].
In this research work various environmental factors were taken into account to have an impact on not only growth abilities of the selenium reducing cells but also on their ability to utilize and reduce various forms of selenium. For example, effect of pH was studied on the selenium reducing abilities of experimental strains. Since it has already been reported that pH and temperature are important factors in biotransformation of selenate and selenite to elemental selenium and is known as dissimulator selenate also known as selenite reduction. So, this reduction pattern of selenite was studied with respect to different environmental factors such as temperature, pH, and aeration. Concentration of selenite its incubation time and we even compared it with other metals and supernatant. In case of variable pH different pattern of selenium reduction was seen among different bacterial strains. For example, bacterial growth at different pH was checked at pH 3, 5, 7 and 9. At pH 3 all of the strains shown very low reduction while at pH 5, 7, 9 there was maximum reduction. With the increase of pH there was more reduction. That’s why at pH 9 there are maximum reduction (Table 16).

Just like pH even temperature had varying effects on different strains behaviors. Optimum temperature range for reduction of selenium for different species had been reported to be 25 to 30 C. whereas most of the strains in our work showed reduction at temperatures ranging from 37 to 42 C. it indicates that there might be some adaptation at the site of isolation towards temperature because at high temperature fluid are more prone to mixing with soil.
Lot of metals which are present in the environment, inhibit the process of selenite reduction. Chromium being one of the major risks to environment was studied for its relation with reduction of selenite. Exiguobacterium sp. shows maximum reduction in chromium and copper stress i.e., 67 % and 56 %. Bacillus licheniformis shows maximum reduction in selenite stress. On the whole selenium had highest reduction as compared to other metals.
At very high levels of heavy metal contamination (e.g., > 1 % by weight, most bacterial life is. However, at lower concentrations (approximately 1 to 10mM individual metals), microbial activity is temporarily impaired, but acquisition and expression of heavy metal resistance genes can occur, and these genes are often plasmid or transposon encoded. In order to check the selenite reduction in protein after 30 min all the strains show less reduction and less red color produced, very slight color appearance if less colored produced then less OD. After 120 min, 480 min and 720 min all the strains show maximum reduction.
In our experiments. strains showed better reductions when they were grown under still conditions as compared to shaking conditions because more precipitates were seen in the bottom of flask under stationary conditions as compared to shaking. On average there was 23 to 83 % selenite reduction potential in stationary condition. Whereas on average there was 12 to 27 % selenite reduction potential in shaking on average there was 23 to 83 % selenite reduction potential in stationary condition. Whereas on average there was 12 to 27 % selenite reduction potential in shaking
Reduction of selenium oxyanions by microbes generates red elemental selenium which can be either amorphous in nature or crystalline. [20] In recent years it had be reported by that Se particles generated by some of the bacterial strains are very unique in their shape [33]. And they are different as compared to the chemically synthesized crystals. It was also reported that these unique selenium crystals had different absorbance properties toward spectrum of light such as UV, which indicates that they differ at atomic structure levels.
In this research work effect of selenite concentration was also determined towards reduction abilities of bacteria. At the concentration of around 400 ug/ml reduction was found to be around 17-90% on average whereas on higher concentration of selenium such as 600ug/ml reduction rate was significantly reduced to 20-40%. It is reported that if selenite concentrations are increase above 19mM it decrease reduction potent ion of some pseudomonas strains. So, it can be assumed that high concentration levels of selenite themselves have toxic effects towards reducing bacteria. Lot of metals which are present in the environment, inhibit the process of selenite reduction. Chromium being one of the major risks to environment was studied for its relation with reduction of selenite. Exiguobacterium sp. shows maximum reduction in chromium and copper stress i.e., 67 % and 56 %. Bacillus licheniformis shows maximum reduction in selenite rich medium. On the whole selenium had highest reduction as compared to other metals.
Conclusion
At very high levels of heavy metal contamination (e.g., > 1 % by weight, most bacterial life is. However, at lower concentrations (approximately 1 to 10mM individual metals), microbial activity is temporarily impaired, but acquisition and expression of heavy metal resistance genes can occur, and these genes are often plasmid or transposon encoded. In order to check the selenite reduction in protein after 30 min all the strains show less reduction and less red color produced, very slight color appearance if less colored produced then less OD. After 120 min, 480 min and 720 min all the strains show maximum reduction.
In conclusion we found that microorganisms have the ability to convert SeO-23 to selenium and this study give us a huge source of potentially and economically valuable microorganisms for the production of red elemental selenium and helping us towards application of selenium for human health.
References
- Drury SA (2007) Selinus O, Alloway B, Centeno Ja, Finkelman Rb, Fuge R, Lindh U & Smedley P(eds) 2005 Essentials of Medical Geology. Impacts of the Natural Environment on Public Health. xiv+ 812 pp. Amsterdam: Elsevier. Price£ 59.99 (hard covers). ISBN 0 12 636341 2. Geological Magazine, 144(5): 890-891.
- Lakin HW (1972) “Selenium accumulation in soils and its absorption by plants and animals.” Geological Society of America Special Papers 140: 45-54.
- Haygarth PM (1994) Global importance and global cycling of selenium. Selenium in the Environment. 1-27.
- Long R H, Benson SM, Tokunaga TK,Yee A (1990) Selenium immobilization in a pond sediment at Kesterson Reservoir. Journal of environmental quality, 19(2): 302-311.
- Fishbein L (1983) Environmental selenium and its significance. Fundam appl toxicol 3(5): 411-419.
- Cooper W C, Westbury RA (1974) Selenium, eds. RA Zingaro and WC Cooper.
- Schrauzer GN (1976) Selenium and cancer: a review. Bioinorganic Chemistry 5(3): 275-281.
- Whanger PD (2002) Selenocompounds in plants and animals and their biological significance. J Am Coll Nutr 21(3): 223-232.
- Barclay MN, MacPherson A, Dixon J (1995) Selenium content of a range of UK foods. Journal of food composition and analysis 8(4): 307-318.
- Sathe S K, Mason AC, Rodibaugh R, Weaver CM (1992) Chemical form of selenium in soybean (Glycine max L.) lectin. Journal of agricultural and food chemistry 40(11): 2084-2091.
- Aro A, Alfthan G, Varo P (1995) Effects of supplementation of fertilizers on human selenium status in Finland. Analyst 120(3): 841-843.
- Combs G F (2001) Selenium in global food systems. Br J Nutr 85(5): 517-547.
- Goyer RA, TW Clarkson (1996) “Toxic effects of metals.” Casarett & Doull’s Toxicology. The Basic Science of Poisons, 5th Ed, Klaassen, CD [Ed]. McGraw-Hill Health Professions Division.
- Alcock NW (2002) Pruebas de laboratorio para valorar el estado nutricional. Nutrición en Salud y Enfermedad. Shils ME, Olson JA, Shike M, Ross CA, (eds). McGraw-Hill Interamericana, México, 1057-1071.
- Yang R, Liu Y, Zhou Z (2017) Selenium and Selenoproteins, from Structure, Function to Food Resource and Nutrition. Food Science and Technology Research 23(3): 363-373.
- Seko Y, Imura N (1997) Active oxygen generation as a possible mechanism of selenium toxicity. Biomed environ sci BES, 10(2-3): 333-339.
- Turner R J, Weiner JH, Taylor DE (1998) Selenium metabolism in Escherichia coli. Biometals 11(3): 223-227.
- Adams ML, Lombi E, Zhao FJ, McGrath SP (2002) Evidence of low selenium concentrations in UK bread‐making wheat grain. Journal of the Science of Food and Agriculture 82(10): 1160-1165.
- Rayman MP (2002) The argument for increasing selenium intake. Proc nutr Soc 61(2): 203-215.
- Losi ME, Frankenberger Jr WT (1997) Bioremediation of selenium in soil and water. Soil Science 162(10): 692-702.
- Gupta UC, Gupta SC (2002) Quality of animal and human life as affected by selenium management of soils and crops. Communications in Soil Science and Plant Analysis 33(15-18): 2537-2555.
- Zhang X, Shi B, Spallholz JE (1993) The selenium content of selected meats, seafoods, and vegetables from Lubbock, Texas. Biological trace element research 39(2-3): 161-169.
- Zhang J, Zhang SY, Xu JJ, Chen HY (2004) A new method for the synthesis of selenium nanoparticles and the application to construction of H2O2 biosensor. Chinese Chemical Letters 15(11): 1345-1348.
- Pilon-Smits EA, LeDuc DL (2009) Phytoremediation of selenium using transgenic plants. Curr Opin Biotechnol 20(2): 207-212.
- Dumont E, Vanhaecke F, Cornelis R (2006) Selenium speciation from food source to metabolites: a critical review. Anal Bioanal Chem 385(7): 1304-1323.
- Cai X J, Block E, Uden PC, Zhang X, Quimby BD, Sullivan JJ (1995) Allium chemistry: identification of selenoamino acids in ordinary and selenium-enriched garlic, onion, and broccoli using gas chromatography with atomic emission detection. Journal of Agricultural and Food Chemistry 43(7): 1754-1757.
- Bañuelos G, Leduc DL, Pilon-Smits EA, Terry N (2007) Transgenic Indian mustard overexpressing selenocysteine lyase or selenocysteine methyltransferase exhibit enhanced potential for selenium phytoremediation under field conditions. Environ sci technol 41(2): 599-605.
- Wilber CG (1980) Toxicology of selenium: a review. Clin toxicol17(2): 171-230.
- Afkar E, Lisak J, Saltikov C, Basu P, Oremland RS, et al. (2003) The respiratory arsenate reductase from Bacillus selenitireducens strain MLS10. FEMS microbiol lett 226(1): 107-112.
- Sors TG, Ellis DR, Salt DE (2005) Selenium uptake, translocation, assimilation and metabolic fate in plants. Photosynth res 86(3): 373-389.
- Brown MW, Watkinson JH (1977) An automated fluorimetric method for the determination of nanogram quantities of selenium. Analytica Chimica Acta 89(1): 29-35.
- Girling CA (1984) Selenium in agriculture and the environment. Agriculture, ecosystems & environment 11(1): 37-65.
- Oremland RS, Kulp TR, Blum JS, Hoeft SE, Baesman S, Miller LG, Stolz JF (2005) A microbial arsenic cycle in a salt-saturated, extreme environment. Science 308(5726): 1305-1308.
- Simonoff M, Sergeant C, Garnier N, Moretto P, Llabador Y, et al. (1992) Antioxidant status (selenium, vitamins A and E) and aging. EXS 62: 368-397.






























