Purification of Extracellular Collagenase from Pseudomonas sp: Remarkable Collagenolytic Activity
Manisha Gautam* and Wamik Azmi
Department of Biotechnology, Himachal Pradesh University, India
Submission: May 30, 2017; Published: July 20, 2017
*Corresponding author: Manisha Gautam, Department of Biotechnology, Himachal Pradesh University, Summer Hill, Shimla (HP) 171005, India, Tel: +91-177-2831948; Fax: +91-177-2832154; Email: manisha.gautam21@gmail.com/wamikazmi@rediftrnail.com
How to cite this article: Manisha G, Wamik A.Purification of Extracellular Collagenase from Pseudomonas sp: Remarkable Collagenolytic Activity. Adv Biotech & Micro. 2017; 4(2): 555633. DOI: 10.19080/AIBM.2017.04.555633
Abstract
A collagenase from Pseudomonas sp. was purified upto 13-fold with a yield of 3.6% using hydrophobic interaction chromatography. Molecular weight of 34kDa collagenase was identified using sodium dodecyl sulfate-polyacrylamide gel electrophoresis method. Gelatin and collagen substrates were used in the characterization of purified collagenase. The optimum temperature and pH were 45 °C, pH 7.5 for gelatin and 50 °C, pH 8.5 for collagen respectively. The collagenase activity was highly inhibited by Hg2+, Pb2+, EDTA, DTT at concentration of 1mM and significant negative effect of Fe2+, Cu2+ and Co2+ was observed at similar concentration. However, Ca2+ (1mM) and PEG (1mM) increased the enzyme activity. The Pseudomonas based collagenase have the ability to hydrolyse protein substrates. The Km and Vmax values for the purified collagenase were found to be 1.65mg/mL and 2.12U/mL with collagen substrate while for gelatin substrate values were 6.60mg/mL and 6.58U/mL, respectively.
Keywords: Collagen; Pseudomonas; Collagenase; Gelatine; Microorganism
Graphical Abstract (Figure 1)
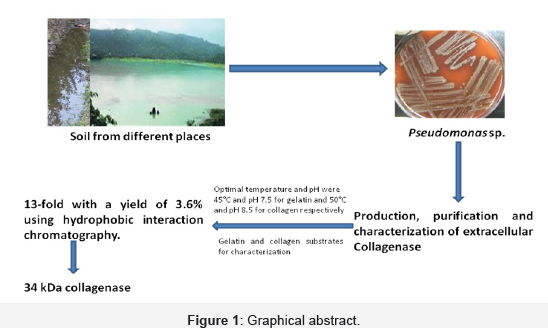
Highlights
A. Screening of soil/sewage samples for the hyper producer strain with maximum collagenase activity
B. Strain was identified as Pseudomonas sp.
C. Determination of protein content and collagenase activity
D. Production, purification and characterization of collagenase enzyme from Pseudomonas sp.
Introduction
Abundantly found extracellular matrix protein (collagen) plays critical roles in the formation of tissues and organs [1]. Col- lagenases are responsible for the degradation of helical regions of native collagen into small fragments due to its proteolytic activity. However, the tissue collagenases are only responsible for the first step of the catabolism of collagen [2]. The most extensive research has been carried out on the bacterial collagenases due to their broad substrate specificities and abilities to degrade native and denatured collagens [3]. Bacterial collagenases hydrolyze both water-insoluble and water-soluble collagens [4]. Collagenas- es hold the industrial applications due to its proteolytic enzymes activity. Collagen is partly responsible for toughness of red meats and have been utilized as tenderizers in the food industry [5,6]. They are also used in leather industry due to its applications in fur and hide tanning to ensure a uniform dying to leathers [7,8]. However, the most common uses of this enzyme is appears to be in medicine e. g. used to treat burns and ulcers [9,10], to eliminate scar tissue [11] and also in transplantation of specific organs [12,13]. The importance of Pseudomonas family in human disease is well known, especially in nosocomial infections and in patients being treated with corticosteroids, radiation therapy, antineoplastic drugs and prolonged antimicrobial therapy. Pseudomonas sp. produces a variety of extracellular substances that may play a role in the disease process particularly the proteolytic enzymes [14,15]. Some of these enzymes attack coiled polypeptide chains but are inactive against collagen molecules. Hence, the true col- lagenolytic activity can only be analyzed using the collagen as substrate. In the present study, we have described the purification and characterization of the collagenase from the extracellular cul-ture filtrate of the Pseudomonas sp.
Materials and Methods
Microorganism
A total 27 soil/sewage samples were collected from local area of fish market and slaughterhouse of Bilaspur and Shimla districts, Himachal Pradesh (India). They were screened for the hyper producer strain with maximum collagenase activity and one of them found suitable for study. The strain was identified as Pseudomonas sp. Collagenase from Pseudomonas sp. was found to be extracellular in nature. Hence, cell free supernatant obtained from 24h old culture broth was used as crude enzyme. Collagenase was produced with a medium having pH 7.0, sucrose 1.0, tryptone 1.0, yeast extract 0.25, meat extract 0.2 and gelatin 0.3 %, w/v respectively. The production medium was inoculated with 4%(v/v) inoculum of Pseudomonas sp. and incubated at 37 °C for 24h at 150rpm. The culture broth was centrifuged at 15000g for 15min, and supernatant obtained was used as crude enzymes. Protein content and collagenase activity was determined in crude enzyme and it was purified by applying different protein precipitation methods and chromatography techniques.
Enzyme purification
The supernatant of culture was precipitated with saturated ammonium sulphate (40-60% w/v). The precipitate was separated by centrifugation and dissolved in 0.1M Tris-HCl buffer (containing 30mM calcium chloride, pH 7.5). The enzyme solution was dialyzed against same reaction buffer for 12h and stored at 4 °C. The sample was then subjected for hydrophobic ion chromatography on octyl sepharose column for the separation of the protein mixtures. The column was equilibrated with binding buffer [0.1M Tris-HCl buffer containing 1.5M (NH4)2SO4 (pH 7.5)] at until the baseline was stable (minimum of 2 column volumes). Protein fractions were eluted with decreasing salt gradient concentration of (NH4)2SO4 (1.5; 1.25; 1.00; 0.75, 0.50 and 0.25M) in Tris-HCl buffer, pH 7.5 at a rate of 5mL/min. The fractions having collagenase activity were pooled together and concentrated. Finally, the pooled collagenase activity fractions were stored and used to establish its purity.
Assay of collagenase activity
Collagenase activity of Pseudomonas sp. was assayed using different substrates including gelatin and collagen. Initially, it was measured using gelatin substrate [16]. The standard reaction mixture [ 0.3mL of gelatine, 0.2mL of Tris-HCl buffer (pH 7.5) and 50mM CaCl2] was incubated with 100μL cell free supernatant enzyme at 37 °C for 30min. Trichloroacetic acid (0.6mL) was added to stop the reaction and released amount of free amino groups was measured using ninhydrin method [17]. Collagenase activity was also measured using collagen substrates as described by Endo et al. [18]. The reaction mixture [0.3mg collagen (from bovine a chilles tendon) in 500μL, 0.1M Tris-HCl buffer and 50mM CaCl2 (pH 7.5)] was incubated with 100μL enzyme at 37 °C for 30min. The released amount of free amino groups was also measured using ninhydrin method [17]. The amino acid composition of denatured collagen is similar to gelatine. Collagenase has the ability to hydrolyze gelatine and was used as substrate for initial screening of collagenase producing micro-organisms.
Protein determination
Protein was analysed and estimated using Bradford's method (1976) [19].
Molecular weight determination
The polyacrylamide gel electrophoresis was done to analyze the molecular weight and purity of the protein isolated from hydrophobic interaction chromatography under denaturating conditions (SDS-PAGE) with 10% polyacrylamide gels [20,21].The molecular weight of the protein marker ranged from 11kDa to 245kDa and middle range molecular weight SDS-protein marker (Genei Pvt. Ltd., Banglore) was used for subunit molecular mass analysis of collagenase of Pseudomonas sp. Enzymes activity in situ was determined using zymogram and method used by Tya- gi, 1993. Protein samples (0.165mg/mL) were mixed with buffer free ß-mercaptoethanol and applied to 10% native-PAGE, which had been copolymerized with 0.25% (w/v) gelatin (Sigma). The gel was incubated overnight at room temperature in a reaction buffer [100mM Tris-HCl, 30mM CaCl2 and pH 7.5]. After incubation the gel was stained with Coomassie Brilliant Blue R-250. The clear zones appearing on the gel indicated the location of proteins exhibiting proteolytic or collagenolytic activities.
Characterization of purified collagenase (Pseudomo- nas sp.) using gelatin and collagen substrates and com-parative study with commercial collagenase (C. histo- lyticum)
The various reaction parameters (buffer pH, role of calcium ions, molarity of calcium ions, molarity of buffer, reaction temperature, incubation time, substrate specificity, substrate concentration, temperature stability of enzymes and role of metal ions and inhibitors) were analyzed to find out the optimum reaction conditions for maximum activity in purified collagenase of Pseu- domonas sp. The purified collagenase was compared with commercial collagenase using gelatin and acid hydrolysed collagen (0.2%, w/v) as substrates for assay. The collagenase activity was measured using 50µL of purified and commercial collagenase having s 0.165mg/mL protein.
Effect of buffer pH
Collagenase activity of the purified enzyme was measured using Tris-HCl buffer (100mM), the pH of the buffer was varied from 7.0-9.0 in order to find out the most suitable pH for the enzyme. The collagenase activity in purified and commercial collagenase was determined in each buffer pH. The reaction mixture was incubated with both substrates (0.2%, w/v; gelatin or collagen) at 45 0C for 30min.
Role of calcium ions in buffer
The calcium chloride was reported to be important for the stability of the collagenase. In order to establish the role of calcium ions on the collagenase activity, calcium chloride was added at 30mM concentration in 100mM Tris-HCl buffer (pH 7.0-9.0).
Effect of buffer molarity
The molarity of the Tris-HCl buffer was varied (25-200mM) for assay of purified and commercial collagenase. The reaction was performed with collagen (buffer pH 8.5) and 0.2% gelatin (buffer pH 7.5) at 45 °C for 30min.
Effect of reaction temperature and incubation time
The effect of temperature on collagenase activity of the purified enzyme in the presence of 80mM CaCl2 in Tris-HCl buffer of pH 7.5 (100mM) was measured with gelatin at different incubation temperatures (25-60 °C). However, Tris-HCl buffer (100mM, pH 8.5) with 70mM calcium chloride was used for collagen substrate. The reaction mixture containing purified and commercial enzyme was incubated with both substrates for 60min and collagenase activity was determined at regular interval of 5min.
Substrate specificity
Collagenase activity on various protein substrates (0.2%, w/v) such as gelatin, casein, bovine serum albumin and hydrolysed collagen were assayed by mixing 0.50|iL of enzyme and 300μL of substrate (casein, gelatin and fibrin) and 250μL Tris-HCl buffer. The reaction mixture was incubated for 30min at 45 °C and collagenase activity was measured.
Determination of kinetic properties
The apparent Michaelis-Menten constant (Km) and maximum velocity number (Vmax) were calculated using different concentrations (1-10 mg/mL) of collagen and gelatin. Enzymes were incubated at 45 0C for 20min. Lineweaver-Burk plot between 1/V and 1/[S] was used for determination of Km and Vmax values.
Effect of metal ions, inhibitors and additives on collagenase activity
The effects of various metal ions and inhibitors (Co2+, Na+, K+, Ca2+, Mg2+, Cu2+, Mg2+, Zn2+, Pb2+ , Ag2+, Fe2+, Urea, SDS, PEG, DTT, EDTA and PMSF were tested on purified and commercial enzyme activity, which were used in 1mM concentration for reaction mixture. The residual activity was determined as a percentage of activity in control sample without the addition of metal ions.
Results and Discussion
Purification of collagenase
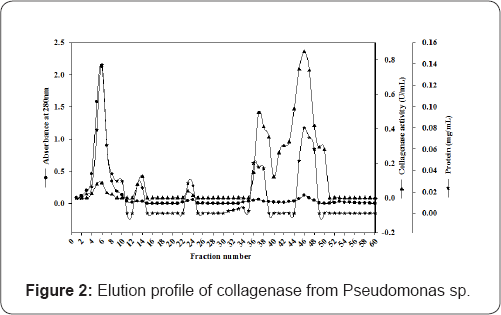
The Purification of collagenase from Pseudomonas sp. has been given in Table 1 and Figure 2. Enzyme was purified upto 13-fold with a yield of 3.6 %. We have used the octyl sepharose coloumn chromatography for purification while Kristjansson and Sakurai co-workers used phenyl-substituted and more hydrophobic butyl substituted agarose column respectively [22,23].
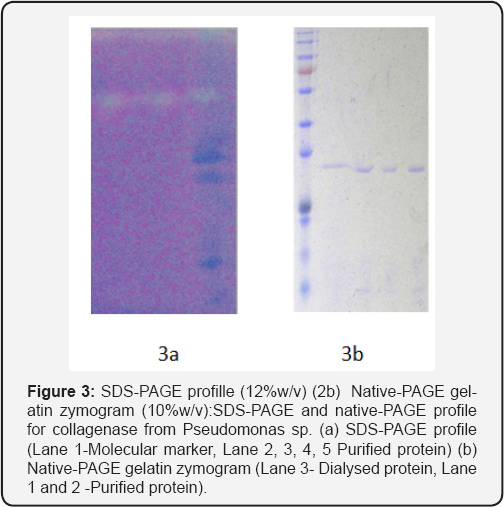
The active fractions (with high specific activity) were pooled together and assessed for purity by electrophoresis. Molecular weight was estimated by electrophoresis under denaturating (SDS-PAGE) and non-denaturing (native-PAGE) conditions [20]. The analysis of the gel run under denaturing conditions after hydrophobic ion chromatography, revealed single band of molecular weight approximately 34kDa (Figure 3). The purified fractions were also subjected to gelatin zymogram and showed clear bands formed due to the digestion of gelatin used in the gel during polymerization (Figure 3).
Characterization of purified collagenase from Pseudomonas sp.
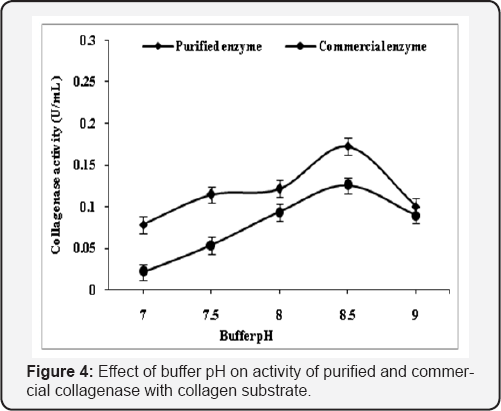
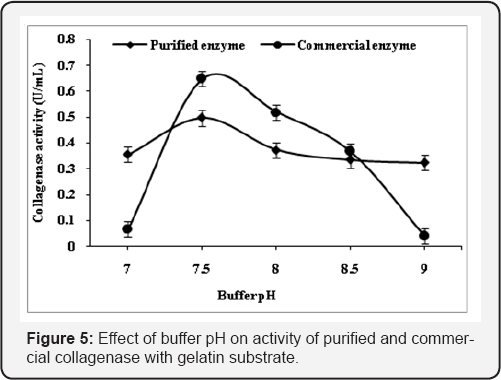
The various reaction parameters were analyzed to explore the optimum reaction conditions for maximum activity of purified collagenase of Pseudomonas sp. and its comparison with commercial collagenase by using gelatin and collagen (0.2%, w/v). Comparative effect of effect of buffer pH, role of Ca2+, buffer molarity, incubation temperature, incubation time, substrate specificity, kinetic properties and effect of metal ions on purified and commercial collagenase were studied and found that Tris-HCl buffer (0.1M) with pH 8.5 was found optimum for the activity of purified and commercial enzyme with collagen substrate while gelatin substrate showed maximum activity at pH 7.5. Activity altered with change in pH and this may be due to the fact that active site losses its affinity towards substrate (Figure 4 & 5). The purified collagenase showed more activity than commercial at optimized pH with gelatin and collegen substrate.
Role of calcium chloride in buffer
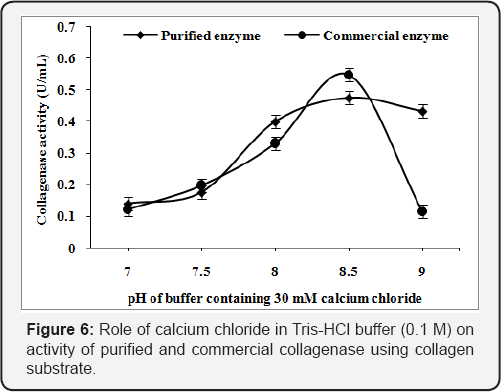
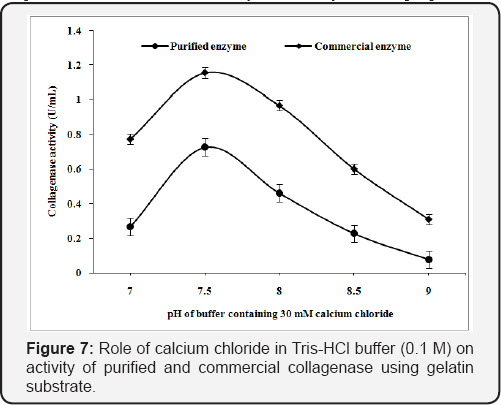
The Tris-HCl buffer (7.0-9.0 pH) were supplemented with calcium chloride (30mM) to analyze the role of Ca2+ in the buffer for collagenase activity from Pseudomonas sp. The purified and commercial collagenase has showed 0.475 U/mL and 0.547 U/mL as maximum collagenase activity, in Tris-HCl buffer at pH 8.5 using collagen substrate (Figure 6) while gelatin substrate showed 0.727U/mL and 1.155U/mL respectively (Figure 7). Calcium ion helps in the enhancement of enzyme activity in both [24].
Effect of buffer molarity
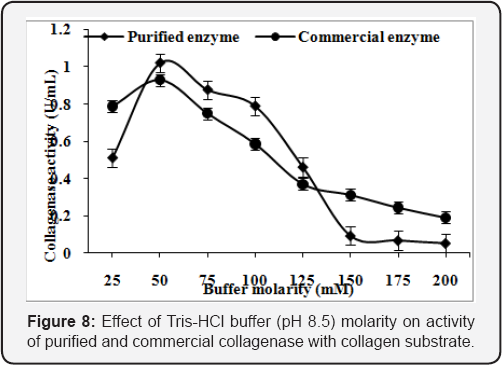
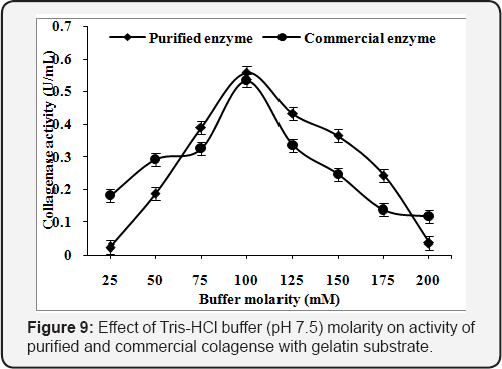
Purified enzyme of Pseudomonas sp. and commercial collagenase exhibited the maximum enzyme activity of 0.l73U/mL and 0.l26U/mL, respectively, at 50mM concentration of Tris-HCl buffer (pH 8.5) with collagen as substrate (Figure 8). However, the incubation of purified enzyme of Pseudomonas sp. and commercial collagenase with gelatin showed maximum enzyme activity (0.497U/mL and 0.648U/mL, respectively) at lOOmM concentration of Tris-HCl buffer of pH 7.5 (Figure 9).
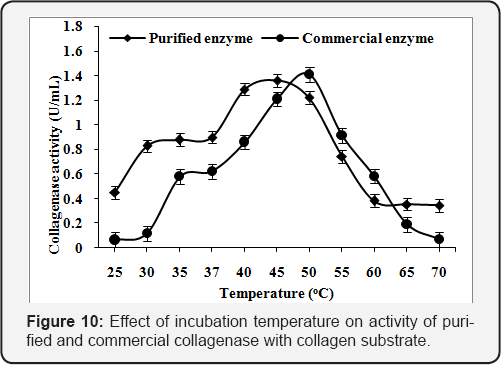
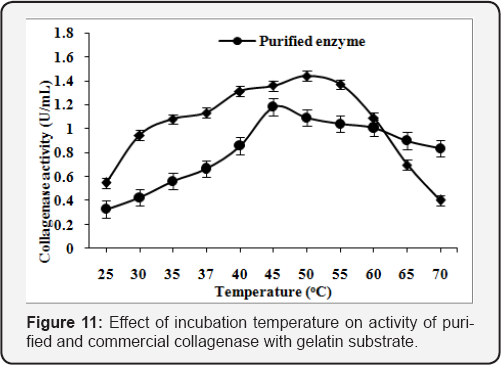
Effect of reaction temperature
The assay of purified and commercial collagenase was carried out at various incubation temperatures (25-70 oC). The maximum enzyme activity was found l.36U/mL at 45 "C and l.4lU/mL at 50 "C using collagen substrate in 50mM Tris-HCl buffer, pH 8.5 for purified and commercial collagenase (Figure 10). The enzyme activity was found to increase with the increase in reaction temperature upto 45 "C. The maximum enzyme activity was found l.l8U/mLat 45 "C and l.44U/mL at 50 "C using gelatin substrate. The activity was reduced with further increase in the reaction temperature (Figure 11).
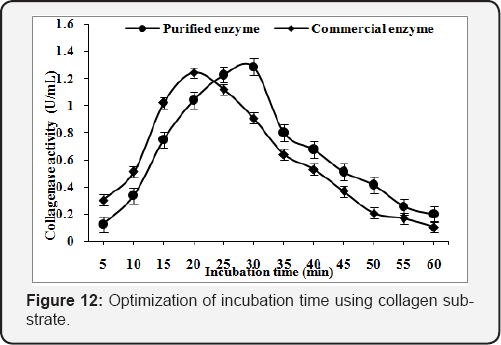
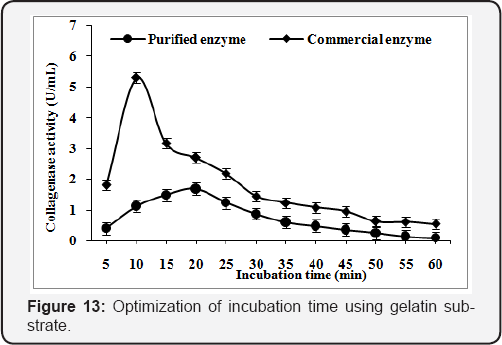
Effect of incubation time
In order to obtain the optimum incubation time for purified and commercial collagenase, the reaction mixture was incubated at 45°C & 50°C, for 5 to 60min respectively. The optimum reaction time was found 30min (1.29U/mL) & 20min (1.24U/mL) for purified and commercial collagenase with collagen substrate (Figure 12) while time of reaction reduce to 20 & 10min for purified and commercial collagenase with gelatin substrate. The maximum enzyme activity obtained was 1.68U/mL and 5.30U/mL, respectively (Figure 13).
Substrate specificity
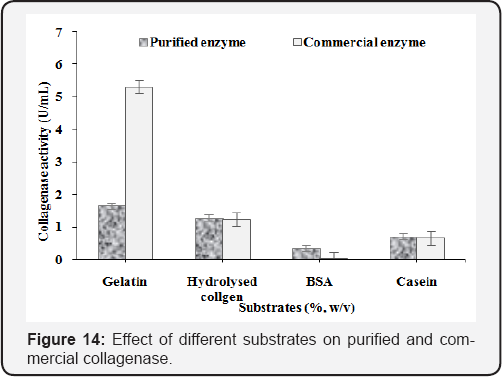
Four substrates such as gelatin, collagen, BSA and casein were used and maximum collagenase activity was found 1.69U/mL and 5.30U/mL using gelatin substrate for purified and commercial enzyme (Figure 14). Moreover, good results were obtained when hydrolysed collagen was used as substrates for purified (1.29U/ mL) and commercial (1.25U/mL) collagenase. The results showed that collagenase of Clostridium origin did not digest BSA but purified collagenase hydrolysed effectively BSA as well as collagen. Purified enzyme has both collagenolytic and proteolytic activities. Gelatin and collegen substrate was found specific for collagenase activity.
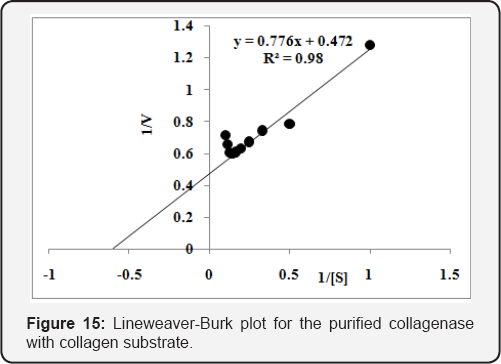
Kinetic properties of purified and commercial collagenase
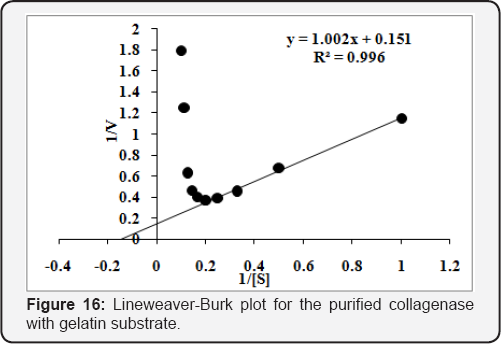
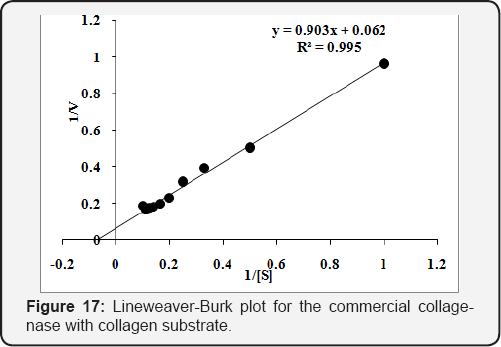
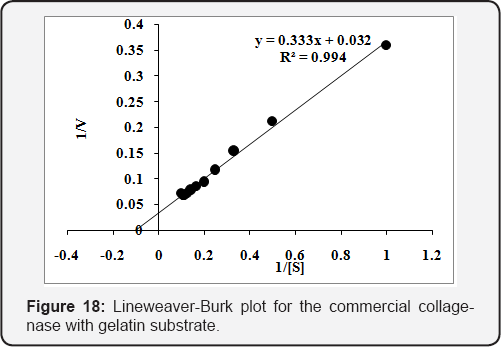
The Km & Vmax was determined using different concentrations [0.1 to 1.0 % (w/v)] of collagen and gelatin substrates. The purified enzyme was incubated for 20min at 45 oC to calculate Km & Vmax . The Km & Vmax values calculated from Lineweaver-Burk plot for the purified collagenase and were found 1.65mg/mL and 2.12U/mL, respectively, using collagen substrate (Figure 15). While 6.60mg/mL & 6.58U/mL, respectively, with gelatin substrate (Figure 16). The values of Km & Vmax were also calculated for commercial collaegenase using both substrates and were found 14.4mg/mL & 14.95U/mL with collagen (Figure 17) and 10.17mg/mL &30.48 U/mL with gelatin respectively, (Figure 18).
Effect of metal ions, inhibitors and additives on collagenase activity
Generally, metal ions act as cofactors for many enzymes. In order to establish the role of metal ion as a cofactor for purified col- lagnease various metal ions and inhibitors (K+, Ca2+, Fe2+, Mg2+,Zn2+, Co2+, Cu2+, Na+, Ag2+, Hg2+, Pb2+, Urea, SDS, PEG, DTT, EDTA and PMSF at concentration of 1mM in the reaction mixture. The enzyme activity was strongly inhibited by Hg2+, Pb2+, EDTA, DTT and significant negative effect was also observed by Fe2+, Cu2+ and Co2+ (Table 2).
Acknowledgement
We would like to thank Department of Biotechnology, Him-achal Pradesh University for providing necessary facility.
References
- Hay ED (1984) Cell-matrix interaction in the embryo: cell shape, cell surface, cells Skeltons, and their role in differentiation. In: Trelstad RL and Alan R Liss (Eds.), The role of extracellular matrix in development. New York, USA, pp. 1-31.
- Harris ED, Cartwright EC (1977) Mammalian collagenases, chapter6,in Proteases in Mammalian Cells and Tissue, Barrett AJ (Ed), North Holland, Netherlands, pp. 249-284.
- Peterhofsky B (1982) Bacterial collagenase. Methods Enzymol 82: 453-471.
- Mookhtiar K, Steinbrink SD, Van Wart HE (1985) Mode of hydrolysis of collagen-like peptidase by class I and class II Clostridium histolyticum collagenases: Evidence for both indopeptidase and tripeptidyl- carboxypeptidase activities. Biochemistry 24: 6527-6533.
- Foegeding EA, Larick DK (1986) Tenderization of beef with bacterial collagenase. Meat Sci 18: 201-214.
- Cronlund AL, Woychik JH (1987) Solubilization of collagen in restructured beef with collagenases and a-Amylase. J Food Sci 52: 857-860.
- Goshev IA, Gousterova E, Vasileva-Tonkova, Nedkov P (2005) Characterization of the enzyme complexes produced by two newly isolated thermophylic actinomycete strains during growth on collagen- rich materials. Proc Biochem 40: 1627-1631.
- Kanth SV, Venba R, Madhan B, Chandrababu NK, Sadulla S (2008) Studies on the influence of bacterial Collagenase in leather dyeing. Dyes and Pigments 76(2): 338-347.
- Agren MS, Taplin CJ, Woessner JF, Eagistein WH, Mertz PM (1992) Collagenase in wound healing: Effect of wound age and type. J Invest Dermatol 99: 709-714.
- Pullen R, Popp R, Volkers P, Fusgen I (2002) Prospective randomized double-blind study of the wound-debriding effects of collagenase and fibrinolysin/deoxyribonuclease in pressure ulcers. Age Ageing 31: 126-130.
- Shmoilov AM, Rudenskaya GN, Isev VA, Baydakov AV, Zhantiev RD (2006) A comparative study of Collagenase complex and new homogeneous Collagenase preparations for scar treatment. J Drug Delivery Sci Technol 16: 285-292.
- Klock G, Kowalsk MB, Hering BJ, Eiden ME, Weidemann A, et al. (1996) Fractions from commercial Collagenase preparations: Use in enzymic isolation of the islets of Langerhans from porcine pancreas. Cell Transplant 5: 543-551.
- Kin T, Zhai X, Murdoch TB, Salam A, Shapiro J, et al. (2007) Enhancing the success of human islet isolation through optimization and characterization of pancreas dissociation enzyme. Amer J Transplant 7: 1233-1241.
- Finland M, Jones WF, Barnes MW (1959) Occurrence of serious bacterial infections since the introduction of antibacterial agents. J Amer Med Ass 170: 2188-2197.
- Iococca VF, Sibinga MS, Barbero GJ (1963) Respiratory tract bacteriology in cystic fibrosis. Amer J Dis Child 106: 315-324.
- Nagano H, Kim A (2000) Purification of Collagenase and specificity of its related enzyme from Bacillus subtilis FS-2. Biosci Biotechnol Biochem 63(1): 181-183.
- Rosen H (1957) A modified ninhydrin colorimetric analysis for amino acids. Arch Biochem Biophy 67: 10-15.
- Endo A, Murakawa S, Shimizu H, Shiraishi Y (1987) Purification and Properties of Collegenase from a Streptomyces Sp. J Bacteriol 102: 163-170.
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein- dye binding. Anal Biochem 72: 248.
- Laemmli UK (1970) Cleavage of structural protein during the assembly of the head of bacteriophage T4. Nature 227: 680-685.
- Bollag DM, Edelstein SJ (1996) Protein Methods. In: John Wiley A and Sons (Eds.), John Wiley A and Sons Inc. Publications, (2nd edn), USA, pp. 96-139.
- Kristjansson MM, Cudmundsdotti S, Fox JW, Bjarnason JB (1995) Characterization of a collagenolytic serine proteinase from the Atlantic cod (Gadus morhua). Comp Biochem Physiol 110B: 707-717.
- Sakurai Y, Inoue H, Nishii W, Takahashi T, Iino Y, et al. (2009) Purification and Characterization of a Major Collagenase from Streptomyces parvulus. Biosci Biotechnol Biochem 73: 21-28.
- Kim SK, Park PJ, Kim JB, Shahidi F (2002) Purification and characterization of a collagenolytic protease from the filefish, Novoden modestrus. J Biochem Molecular Biol 335: 165-171.






























