Detection Of Campylobacter in Poultry: Antibacterial Profile Study
H Laidouci-Al Amir*, F Mouffok, M Berkane, R Zouar, A Amrouche and S Daouadji
Institut Pasteur d’Algérie, Route du petit Staouéli, Dely Brahim. Alger, Algérie
Submission: December 12, 2021; Published: August 31, 2023
*Corresponding author: H Laidouci-Al Amir, Institut Pasteur d’Algérie, Route du petit Staouéli, Dely Brahim. Alger, Algérie
How to cite this article: H Laidouci-Al Amir, F Mouffok, M Berkane, R Zouar, A Amrouche, S Daouadji . Detection Of Campylobacter in Poultry: Antibacterial Profile Study. Arch Anim Poult Sci. 2023; 2(3):555589. DOI: 10.19080/AAPS.2023.04.555589
Summary
Campylobacter is one of the most important causes of gastro-enteritis worldwide. Many studies have been performed on this topic. In the present study the ISO 10272 norm has been used to look for this bacterium in food. A total of 346 food samples were tested and 62 strains of Campylobacter were isolated. it was observed that:
• Butzler medium gave the best results for isolating Campylobacter.
• The modified enrichment broth was very important for detecting this bacterium.
• Antimicrobial resistance of this bacterium was observed for some antibiotics used in the treatment of Campylobacteriosis.
In Algeria statistics about the implication of Campylobacter in infectious diseases are not well known.
To evaluate the contamination’s origin and frequency to improve the disease’s treatment, Campylobacter should be systematically looked for.
Keywords: Campylobacter; Food; Antibiotic; Bacterium; Gastroenteritis; Nalidixic acid; Hippurate test; Dromigny; Chicken carcasses; Metronidazole
Introduction
Our food constitutes a potential source of food poisoning of bacterial origin because it is often a vehicle of contamination by microorganisms such as Campylobacter. It was long believed that Campylobacter were primarily pathogens for animals, but the development of isolation techniques has led to an increase in the detection of infections with these bacteria in humans. Since then, Campylobacter are the main cause of bacterial gastroenteritis in the world [1]. Given the great risk associated with food, many studies have contributed to the development of increasingly efficient techniques for the detection of this bacterial genus in food, and more particularly in poultry [2]. Another problem with Campylobacter is the emergence of new resistance to antibiotics which is increasing year by year. To do this, a surveillance system has been installed in some countries to deal with it. Campylobacteriosis is zoonosis, a disease transmitted to humans from animals or products thereof [1]. More than 50% of Campylobacter have one or more plasmids carrying factors of resistance to antibiotics. In particular, there has been an increase in strains resistant to nalidixic acid and the emergence of strains resistant to enrofloxacin [2,3]. In order to study Campylobacter, we analyzed various poultry samples to find out its frequency and to study its antibiotic resistance profile.
Material and Methods
For the detection of Campylobacter in poultry we followed the ISO10272 standard [4].
346 poultry samples were analyzed. These samples, from chicken, are broken down as follows:
• 121 necks
• 71 gizzards
• 64 livers
• 50 hearts
• 29 liver-hearts
• 11 wings
The culture media used are: Butzler (noted B) (virion, 5211.BULK), Columbia blood with filter (C), Campylobacter growth supplement (CG) (Oxoid, SR 0232E) and Skirrow (S) (Oxoid, SR0069E). These media are used in a minimum of two depending on the availability of reagents. Enrichment was performed for each sample using Preston Broth supplemented with 10% sheep blood and the additive Butzler. The incubation temperature was 37°C modified from the standard which provides for 42°C. Incubation was carried out in jars containing atmosphere generators micro aerobic (5% O2, 10% CO2, 85% N2).
Preparation of samples
25g of the poultry sample were mixed with 225 ml of Preston broth with passage through the stomacher for 2 minutes. This constitutes the basic suspension (Direct).
Enrichments
The suspension thus prepared was seeded on agar culture media and noted Direct (D). These and the “direct” Preston broths were incubated in a micro aerobic atmosphere at 37°C for at least 48 hours to obtain the 1st enrichment. After incubation, the 1st enrichment was also inoculated on agar media. A second enrichment was prepared from the 1st enrichment on Preston broth. Incubation is carried out in microaerobiosis at 37°C for 48 to 72hours. A possible second enrichment was prepared under the same conditions.
Identification
The identification involved the following steps:
• Preliminary tests: macroscopic appearance, microscopic appearance, catalase and oxidase test will confirm the genus Campylobacter.
• Biochemical tests: urea, TSI, nitrate reductase, hippurate test, cotrimoxazole and cephazoline discs, ApiCampy will be used for species identification.
Antibiogram
15 antibiotics were tested. The antibiogram was performed according to the standards of the Antibiogram Committee of the French Society of Microbiology (CA SFM) [5].
Results & Discussion
Identification of Campylobacter
Of the 346 samples analyzed, 62 Campylobacter strains were isolated, for an isolation percentage of nearly 18%. On the basis of the following results the strains isolated were all reported to the genus Campylobacter:
• macroscopic appearance: smooth and transparent colonies under the reflection of daylight with a regular edge.
• microscopic appearance:
fresh state: observation in the fresh state under an optical microscope shows that Campylobacter has characteristic mobility in “midges flight”.
Gram stain: after Gram stain, observation under an immersion light microscope (magnification 10X100) shows Gram-negative bacilli, curved in S or spiral. The coccoid form of Campylobacter is a form of degeneration. oxidase: oxidase is positive. catalase: catalase is positive. The other biochemical tests (hydrolysis of hippurate, temperature test, etc.) and the use of the API-Campy gallery led to the conclusion that:
• 53 strains belonged to the species Campylobacter jejuni
• 01 strain belonged to Campylobacter fetus
• 01 strain belonged to the species Campylobacter coli
• 07 strains were not identified because the subcultures remained negative.
Study of Positive Cases
Depending on the nature of the sample
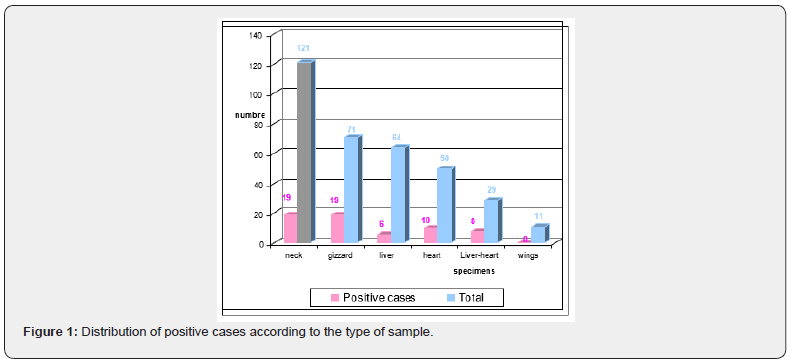
Figure 1 represents the rate of positive cases compared to the isolation rate for each type of sample:
* 20% of heart samples and nearly 10 and 28% of liver and liver-heart samples, respectively, are positive. A 1985 study conducted by Dromigny et al. [6], showed that 40% of samples of turkey heart and 20% of samples of turkey liver studied showed contamination with Campylobacter.
* The gizzard and the neck show respectively nearly 28 and 16% of positive cases. The positivity rate for neck swabs was much higher in two studies conducted by Simmon and Gibbs [7] and Jørensen et al. [8] reaching 80 and 84% respectively. According to Berrang et al. [9], half of the chicken carcasses tested before and after putting them in boiling water to reduce the number of Campylobacter had Campylobacter contamination in their respiratory tract. Similarly, Rosenquist et al. [10] concluded that during evisceration of chickens, contamination of carcasses with Campylobacter was highest in the neck.
Statistical analysis of the data [χ² test] yielded the following results:
• The comparison of the offal positivity rates and the calculation of χ² show that the difference between percentages is only due to the sampling and not the nature of the samples (p <0.05).
• the percentage of neck positivity and the percentage of positivity of all offal, suggest that the difference is not significant between these two samples.
Distribution According to the isolation environment
Figure 2 represents the percentage of strains isolated from each medium
ω 84% of the strains were isolated on Butzler medium
ω 61% of the strains were isolated on C.G.
ω 25% of the strains were isolated on Columbia medium with filter
ω 11% of the strains were isolated on Skirrow medium.
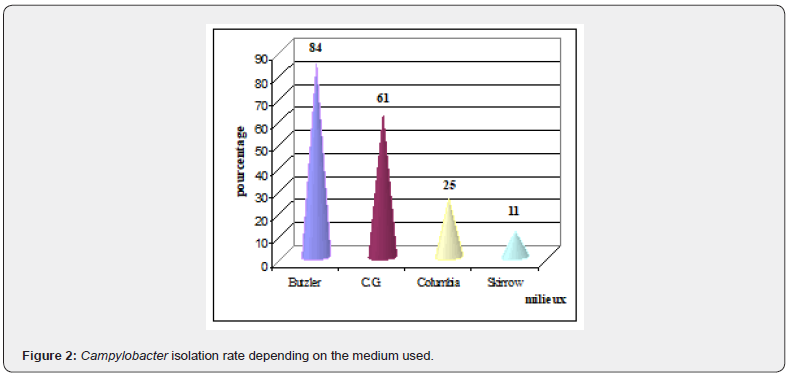
By comparing these different percentages (χ² test), it is noted that the difference observed between the different percentages is due to the media. These results show that Butzler’s medium is the most suitable for the isolation of Campylobacter, followed by CG medium. CG medium gave better results than Columbia and Skirrow media. This can be explained by the fact that the CG medium contains an additive (mixture of sodium pyruvate, ferrous sulfate and sodium meta bisulfite) which promotes the growth of Campylobacter. It is followed by Columbia medium which is a medium without additives or antibiotics, therefore facilitates the development of the most sensitive strains. Research in this direction continues around the world where several scientists are studying various culture media and different protocols to try to find those that best allow the isolation of Campylobacter from food samples [11,12].
Breakdown by stage of enrichment
Figure 3 illustrates the positivity rate of samples by stage of enrichment. Almost 47% of the strains are derived from the basic suspension (direct). This can be explained by a strong contamination of food with Campylobacter. 33% of the strains are isolated from the first enrichment, 13% from the second and finally almost 7% from the third, so nearly 53% of the strains were only obtained after at least one enrichment. These results show the importance of enrichments in the presence of selective substances, by reducing the competitive microflora, thus promoting the growth of Campylobacter.
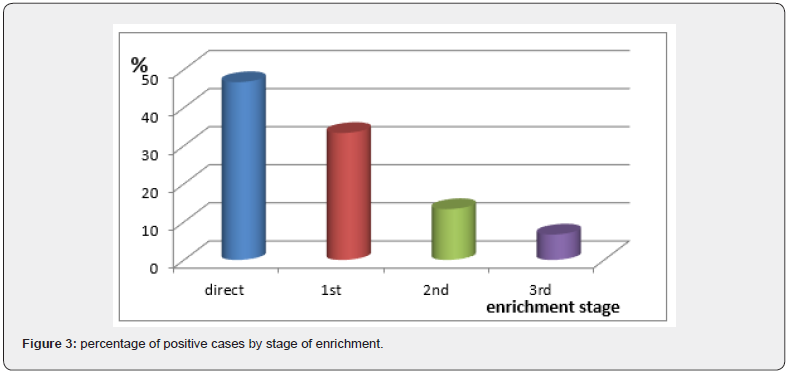
The strains isolated at the level of the third enrichment come from samples stored at + 10 °C for more than 48 hours. Davis and Connert [13] noted that Campylobacter survival was higher at + 4 °C than at + 10 °C Following high rates of contamination of chicken carcasses by Campylobacter, several researchers are trying to study the causes, frequencies in order to reduce this contamination. Some have studied several factors that may influence the level of Campylobacter in chicken carcasses such as ventilation and storage temperature [14], the age of the poultry, its transport and seasonality [15]. As water is a potential source of contamination by Campylobacter, some researchers [16,17] have added the drinking water of poultry with organic acids or with glycerol and they have noted an absence of Campylobacter in this water as well. a decrease in this bacterium in chickens without affecting their development. These organic acids have also been tested by Berrang et al. [18] but this time placed in the cloaca of the chickens to try to decrease the level of Campylobacter on their skin after evisceration. Corry et al. [19] noted that immersing chicken carcasses in hot water for a few moments decreased the number of Campylobacter on them.
Antibiotic resistance
When performing the antibiogram, 15 antibiotics from various families on Columbia blood medium were tested.
The different results are shown in Figure 4:
.• Quinolones The highest rate of resistance was observed for ciprofloxacin (cip), nalidixic acid (NA) and pefloxacin (pef). According to a WHO report [20], to investigate the relationship between the veterinary use of fluoroquinolones and the appearance of resistance to these antibiotics in animal and human strains, a study showed that before the introduction of the enrofloxacin in animal feed in 1987, no resistance to quinolones was observed. However, from that date onward resistance arose and quickly gathered pace. Many studies shown the high rate of Campylobacter fluoroquinolone resistance worldwide Nguyen et al. [21]; Sproston et al. [22].
• Tetracycline 83% of Campylobacter strains exhibited resistance to tetracycline (TE). However and according to the study by Kassa et al. [23] in Ethiopia, only 1.5–5.9% of Campylobacter strains were resistant to tetracycline.
• Metronidazole 83% of Campylobacter strains also exhibited resistance to metronidazole (MTR). According to the Stanley and Jones study in 1998 [24], already 80 to 100% of Campylobacter strains were resistant to this antibiotic.
• ß lactams The percentage resistance of Campylobacter to amoxicillin (AMX) and ampicillin (AMP) is 42%. This is similar to the results of Mégraud and Prouzet [3] where 40 to 50% of Campylobacter strains were resistant to amoxicillin (AMX) and ampicillin (AMP). The superior efficacy of amoxicillin-clavulanic acid (AMC) (only 27% resistance rate) is explained by the antibacterial action specific to clavulanic acid in addition to its action as a β-lactamase inhibitor.
• Macrolides The rate of resistance to erythromycin (30%) is comparable with that obtained by Rodrigo et al in 2007 [25].
• Aminoglycosides and furans We noted no resistance to the aminoglycosides Streptomycin (S) and gentamicin (GM) while for tobramycin 7% resistance was observed. For furans (F) no resistance was noted. According to Mégraud and Bultel [26], gentamicin remains the only antibiotic for which no resistance of Campylobacter has been recorded in the world. However Rodrigo et al. [25] found a certain rate of resistance to gentamicin (5.4%) and another higher for streptomycin (30%).
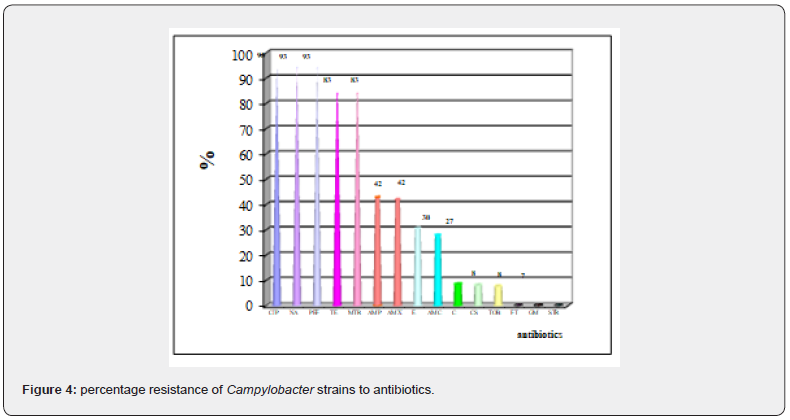
- Others For colistin and chloramphenicol, resistance rates were 8% for both. No resistance to chloramphenicol was observed by Kassa et al. [23]. These results show that there is worrying resistance to antibiotics, especially those used in human medicine.
Conclusion
Campylobacter is the most common cause of bacterial intestinal infections, so it presents a microbiological food safety problem. Our study confirms these data, and at the end of it we can conclude that:
i. Different parts of the chicken (offal, neck) were contaminated. This contamination is arguably cross-contamination during evisceration. This demonstrates the value of applying strict hygiene rules already upstream in the food chain.
ii. Midfielder Butzler is the most efficient.
iii. A worrying resistance to antibiotics has been noted especially for quinolones, tetracycline, metronidazole and to a lesser extent β-lactams.
In Algeria, the statistics concerning the implication of Campylobacter in the infections are not well known. It would be desirable to institute systematic surveillance and research for this germ at different levels, on the one hand to know its prevalence and on the other hand to monitor the evolution of its resistance to antibiotics. The best prevention is still good domestic food handling, respecting hygiene rules and ensuring that the food is properly cooked.
References
- Costa D, Iraola G (2019) Pathogenomics of Emerging Campylobacter Species. Clinical Microbiology Reviews 32(4): e00072-18.
- Mc Dermott PF, Simala GJL, Taylor DE (2009) antimicrobial resistance in Helicobacter and Campylobacter, in D. Mayers, antimicrobial drug resistance. Vol. 2 Humana Press, pp. 847-863.
- Mégraud F et V. Prouzet-Mauléon (2004) Evolution de la résistance des Campylobacter aux antibiotiques en France. BEH n° 32-33.
- AFNOR (1996) Méthode horizontale pour la recherche de Campylobacter thermo tolé Association Française de Normalisation.
- Soussy CJ (2010) Comité de l’antibiogramme de la société française de microbiologie. Edition Janvier.
- Dromigny E, Louve JL, Vachine I (1985) Campylobacter in turkeys at the slaughterhouse: frequencies and epidemiological markers. Revue Méd Vét 136(7): 541-546.
- Simmons NA, Gibbs FJ (1979) Campylobacter spp. In oven-ready poultry. J infect 1(2): 159-162.
- Jorgensen F, Bailey R, Williams S, Henderson P, Wareing DRA, et al. (2002) Prevalence and numbers of Salmonella and Campylobacter spp. on raw milk, whole chickens in relation to sampling methods. Int J Food Microbiol 76(1-2): 151-164.
- Berrang ME, Meinersmann RJ, Buhr RJ, Reimer NA, Philips RW, et al. (2003) Presence of Campylobacter in the respiratory tract of broiler carcasses before and after commercial. scalding. Poult Sci 82(12): 1995-1999.
- Rosenquist H, Sommer HM, Nielsen NL, Christensen BB (2006) The effect of slaughter operations on the contamination of chicken carcasses with thermotolerant Campylobacter. Int J Food Microbiol 108(2): 226-232.
- Beuchat LR (1985) Efficacy of media and methods for detecting and enumerating Campylobacter jejuni in refrigerated chicken meat. Appl environ microbiol 50(4): 934-939.
- Josefsen MH, Lubeck PS, Aalbæk B, Hoorfar J (2003) Preston and Park–Sanders protocols adapted for semi-quantitative isolation of thermotolerant Campylobacter from chicken rinse. Int J Food Microbiol 80(2): 177-183.
- Davis MA, Conner DE (2007) Antimicrobial Effects of Pseudomonas aeruginosa on Survivability and Recovery of Campylobacter jejuni on poultry products. Poult Sci 86(4): 760-764.
- Cole K, Donoghue AM, Blore PJ, Holliman JS, Cox NA, et al. (2004) Effects of aeration and storage temperature on Campylobacter concentrations in poultry semen. Poult Sci 83(10): 1734-1738.
- McCrea BA, Tonooka KH, VanWorth C, Boggs CL (2006) Prevalence of Campylobacter and Salmonella species on farm, after transport, and at processing in specialty market poultry. Poult Sci 85(1): 136-143.
- Chaveerach P, Keuzenkamp DA, Lipman LJA, Van Knapen F (2004) Effect of Organic Acids in Drinking Water for Young Broilers on Campylobacter infection, volatile fatty acid production, gut microflora and histological cell changes. Poult Sci 83: 330-334.
- Hilmarsson H, Thormar H, Thráinsson JH, Gunnarsson E (2006) Effect of Glycerol Monocaprate (Monocaprin) on Broiler Chickens : An Attempt at Reducing Intestinal. Campylobacter Infection. Poult Sci 85(4): 588-592.
- Berrang ME, Smith DP, Jr Hinton A (2006) Organic Acids Placed into the Cloaca to Reduce Campylobacter Contamination of broiler skin during defeathering. J Appl Poult Res 15(2): 287-291.
- Corry JEL, James SJ, Purnell G, Barbedo CSP, Chochois Y, et al. (2006) Surface pasteurisation of chicken carcasses using hot water. J Food Eng 79(3): 913-919.
- WHO (2001) WHO global strategy for containment of antimicrobial resistance.
- Sproston EL, Wimalarathna HML, Sheppard SK (2018) Trends in fluoroquinolone resistance in Campylobacter. MINI REVIEW. Microbial Genomics 4(8): e000198.
- Nguyen TNM, Hotzel H, Njeru J, Mwituria J, El-Adawy H, et al. (2016) Antimicrobial resistance of Campylobacter isolates from small scale and backyard chicken in Kenya. Gut Pathog 8(1): 39.
- Kassa T, Gebre SS, Astrat D (2007) Antimicrobial susceptibility patterns of thermotolerant Campylobacter trains isolated from food animals in Ethiopia. Vet Mic 119: 82-87.
- Stanley KN, Jones K (1998) High frequency of metronidazole resistance among strains of Campylobacter jejuni isolated from birds. The society for applied Microbiology, Letters in Applied Microbiology 27(5): 247-250.
- Rodrigo S, Adesiyun A, Asgarali Z, Swanston W (2007) Antimicrobial resistance of Campylobacter spp. isolated from broilers in small poultry processing operations in Trinidad. Food Cont 18(4): 321-325. Mégraud F, Bultel C (2004) Appréciation des risques alimentaires liés aux Campylobacter. Application au couple poulet/C. jejuni. rapport AFSSA, p. 20-47.






























