Aflatoxin B1 Production, Toxicity, Mechanism of Carcinogenicity, Risk Management, and Regulations
Chinaza Godswill Awuchi1,2*, Erick Nyakundi Ondari2, Hannington Twinomuhwezi1,3, Victory S. Igwe4 and Ikechukwu O Amagwula4
1Department of Biochemistry, Kampala International University, Uganda
2School of Natural and Applied Sciences, Kampala International University, Uganda
3Department of Chemistry, Kyambogo University, Uganda
4Department of Food Science and Technology, Federal University of Technology Owerri, Nigeria
Submission: June 02, 2021;Published: July 30, 2021
*Corresponding author: Chinaza Godswill Awuchi, Department of Biochemistry, Kampala International University, Bushenyi, Uganda.
How to cite this article: Chinaza G A, Erick N O, Hannington T, Victory S. I, Ikechukwu O A. Aflatoxin B1 Production, Toxicity, Mechanism of Carcinogenicity, Risk Management, and Regulations. Arch Anim Poult Sci. 2021; 1(4): 555568. DOI: 10.19080/AAPS.2021.01.555568
Abstract
Aflatoxin B1 is released by A. flavus and A. parasiticus. It is well known strong carcinogenic substance with median lethal dose (TD50) of 3.2 μg per kg a day in rat model. Mechanism of AFB1 carcinogenicity has been defined. The carcinogenicity of AFB1 differs from species with certain species, e.g. monkeys and rats, reportedly mostly susceptible compared to the other species. In animals and humans, aflatoxin B1 has been shown to be teratogenic, mutagenic, and immunosuppressant. The worldwide maximum tolerated aflatoxin B1 levels was reported by the FAO to be in within 1 to 20 μg per kg in food; 5 to 50 μg per kg in cattle feed. Aflatoxin B1 permeates via skin. Dermal exposures to AFB1 in specific conditions usually result in concerning health risk. Liver is organ mostly vulnerable to the toxicity of AFB1. AFB1 is a genotoxic hepatocarcinogen that has its exposures linked to hepatocellular carcinoma development, tumors of the liver, particularly when simultaneously occurred with hepatitis B viral infection. The hepatocellular carcinoma prevalence in people exposed to aflatoxins, has shown to increase with simultaneous occurrence of hepatitis B viral infection. Oral median lethal dose (LD50) of AFB1 is 0.3 to 17.9 mg per kg bw for many animals. Embryonic deaths and weakened development of embryo of Fabricius bursa in chicken by AFB1 was reported. Exposures to aflatoxin B1 is mostly taken care of with the measures directed at the prevention of crop contamination in field, handling in post-harvest, and also in storage, or through measures targeted at identifying and disinfecting contaminated foods and feeds, as well as the materials used in their preparation.
Keywords: Aflatoxin B1; Carcinogenicity of AFB1; Toxicity of AFB1; Management of AFB1
Introduction
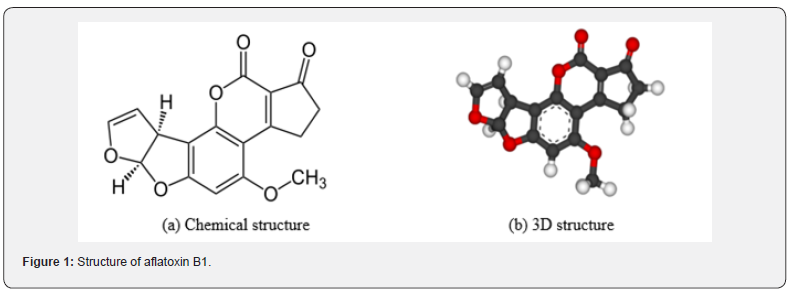
Aflatoxin B1 (AFB1), the most toxic of all aflatoxins, is released by Aspergillus parasiticus and Aspergillus flavus. AFB1 is strong carcinogen that has TD50 of 3.2 μg per kg per day in rat. The carcinogenicity of AFB1 differs from species with certain species, e.g. monkeys and rats, reportedly mostly susceptible compared to the other species [1]. “Aflatoxin B1 is common contaminant in many foods including peanuts, acha, corn, cottonseed meal, millet, and other grains; and animal feeds” [2 -4]. Aflatoxin B1 is most toxic of all aflatoxins and is greatly associated with hepatocellular carcinoma (HCC) in human [5]. AFB1 has been indicated as teratogenic, mutagenic, and immunosuppressant in animals [6]. Many sampling and analytical methods including high-performance liquid chromatography (HPLC), thin-layer chromatography (TLC), mass spectrometry, enzyme-linked immunosorbent assay (ELISA), etc., are used to test for AFB1 contamination in foods [7]. According to the FAO, the global maximum tolerated aflatoxin B1 levels was reported to be within 1 to 20 μg/kg in foods, and 5 to 50 μg/kg in dietary cattle feeds in 2003.
People are exposed to aflatoxins when they consume contaminated plant products (such as maize, peanuts, acha, millets, etc.) or when they consume meat, milk, or dairy product from the animal which was fed with infested feeds. Farmers and farm workers might can get exposure through breathing in the dust particles created in processing and handling of food and feed contaminated with aflatoxin. About 14 or more types of aflatoxins naturally occur, however four – aflatoxin B1, aflatoxin B2, aflatoxin G1, and aflatoxin G21 – are mostly harmful to animals and humans as all of them are detected in almost every staple agricultural crop; however, many human exposures come from infested grains and nuts, as well as their products [4, 8, 9]. In addition, aflatoxin M1 (AFM1), metabolite of aflatoxin B1, is detected in milk and dairy products in regions with significant aflatoxin exposures [8]. Consequently, humans can get exposed to aflatoxins via dairy products and milk, such as breast milk, particularly in regions in which grains of very poor quality are often fed to animals. Aflatoxin exposure is associated with increased risks of liver cancer.
The nationwide estimations of diet exposures to aflatoxin show variations between developing and developed nations. For the developed nations, the average dietary exposure to aflatoxin is commonly below 1 ng per kg bw a day, while estimations for many countries in Africa surpass 100 ng/kg body weight a day, though the estimation is usually obtained from few data [8]. Estimation of diet exposures to aflatoxin M1 has hardly gone beyond 1 ng per kg body weight a day in all nations, though around 6.5 to 8.8 ng per kg body weight a day for breastfed infants and children were reported [8]. Food and feed can get contaminated with mycotoxins in field, during harvest, and/or post-harvest [9], as well as in storage. The pre-harvest contamination with aflatoxins is often reported for peanuts, maize, tree nuts, and cottonseed. Post-harvest contamination is often seen in many other commodities, including rice, spices, and coffee [8]. Inappropriate storage in the conditions which encourage the proliferation of mould (humid and warm storage environments) can typically result in contamination levels much higher than the ones found in the field. You can reduce your exposure to aflatoxins, such as AFB1, through only purchasing main approved brands of grains, grain products, nuts, and nut butters, as well as through getting rid of nuts which appear mouldy, shriveled, and/or discolored. To reduce risk, agencies such as, FDA, NAFDAC, UNBS, etc., test food and feed which might have aflatoxin, including peanut butter, peanuts, and other grains and grain products. So far, no known human illness outbreak due to aflatoxin reported in advanced countries such as the US, but aflatoxin outbreak has been reported in few developing nations such as Kenya [8].
Production, Toxicity, Mechanism of Carcinogenicity, Risk Management, and Regulations of Aflatoxin B1
Source of exposures to AFB1
Aflatoxin B1 is mainly detected in aflatoxin-infested foods and feeds. Human and animals can get exposed to AFB1 nearly completely via diet. Occupational AFB1 exposures have been detected in poultry and swine production [10, 11]. While contamination with AFB1 commonly occur in most staple food, its release is highest in food stored under humid and hot climate [12]. Consequently, exposure to AFB1 is most common in Sub-Saharan Africa, Southeast Asia, and South America [12].
Aflatoxin B1 pathology
Aflatoxin B1 can pass via skin. Dermal exposures to AFB1 in certain conditions in the environment can result in severe health risk [12, 13]. Liver is most vulnerable to the toxicity of AFB1. In studies using animal models, the pathological lesions which are linked to AFB1 toxicity are vacuolation of hepatocytes, decrease in liver weight, and hepatic carcinoma [14]. Other liver lesions are fatty infiltration, hepatic cells enlargement, fibrosis, hemorrhage, necrosis, bile duct proliferation/hyperplasia, and regeneration of nodules [15].
Aspergillus flavus
A fungus of Trichocomaceae known as Aspergillus flavus is distributed globally. It thrives in soil and feed on dead animal and plant matters, although it spreads via air through “airborne conidia” [16]. Aspergillus flavus grows in branched and lengthy hyphae. It has the capability to survive on many foods such as peanuts, corn, millet, etc. Aspergillus flavus and its metabolic product have been known to be pathogenic to many animals and humans [16, 17]. While the toxicity of aflatoxin B1 (aflatoxins), products of the mould, are reviewed throughout this systematic review, A. flavus by itself has pathogenic effect causing “aspergillosis”, or by infection. “The infection mostly occurs in the lungs of those with compromised immune system but infection might also occur in skin or other organs” [17]. “Unlike many species of mold, Aspergillus flavus prefers dry and hot conditions; it has optimal growth at 99 °F (37 °C) which contributes to its pathogenic effects in humans” [16].
Aflatoxin B1 (AFB1) pathway for biosynthesis
AFB1 is obtained biochemically from polyketide synthase (PKS) and dedicated fatty acid synthase (FAS), jointly referred to as “norsolorinic acid synthase”. Biosynthesis of AFB1 starts with hexanoate synthesis by FAS that turn into starting unit for “iterative type I polyketide synthase” [18, 19]. “The PKS adds seven (7) malonyl-CoA extenders to the hexanoate, forming the C20 polyketide compound; PKS folds the polyketide in a special way to induce cyclization aimed to form anthraquinone norsolorinic acid; Then a reductase catalyzes reduction of the ketone on norsolorinic acid side-chain in order to yield averantin” [18, 19]. “Averantin is then converted to averufin through two different enzymes, an alcohol dehydrogenase and a hydroxylase” [18, 19]. This oxygenates and cyclizes side chain of averantin leading to formation of the “ketal in the averufin”. “From this point on the aflatoxin B1 biosynthetic pathway becomes much more complicated, with numerous major skeletal changes; majority of the enzymes involved have not been characterized. There might be many more unknown intermediates” [18]. “However, it is known that averufin is oxidized by AvfA, a P450-oxidase, in a Baeyer-Villiger oxidation, opening the ether rings and then upon rearrangement the versiconal acetate is formed.” “At this moment an esterase, EstA, catalyzes hydrolysis of the acetyl, leading to the formation of the primary alcohol in versiconal” [17-19]. “The acetal in versicolorin A forms from the cyclization of the versiconal side-chain, which is catalyzed by the VERB synthase, and then a desaturase (VerB) reduces versicolorin B, forming the dihydrobisfuran” [18, 19].
Additional 2 enzymes catalyze versicolorin A conversion to demethylsterigmatocystin: AflM, a reductase and AflN, an oxidase. “The two enzymes make use of both two NADPHs and molecular oxygen to dehydrate one of the –OH (hydroxyl) groups on the anthraquinone and also open the quinine with molecular oxygen” [18, 19]. “Upon formation of the aldehyde in the step of ring opening, it is oxidized to form carboxylic acid and afterwards a decarboxylation event takes place to close the ring leading to the formation of the six-member ether ring system found in demethylsterigmatocystin.” “Next two steps in the AFB1 biosynthetic pathway include the methylation by the S-adenosyl methionine (SAM) of the two –OH groups on xanthone part of demethysterigmatocystin by the two different methyltransferases, OmtA and OmtB” [18, 19]. This yield “O-methylsterigmatocystin”. “The final steps involve the oxidative cleavage of the aromatic ring as well as the loss of one carbon in the O-methylsterigmatocystin that is catalyzed by an oxidoreductase, OrdA” [18, 19]. At this ponit, final recyclization takes place, forming aflatoxin B1.
Mechanism of aflatoxin B1 carcinogenicity
Aflatoxin B1 (AFB1) has been known to be strong genotoxic hepatocarcinogen that has its exposures strongly linked to manifestation of liver tumors, hepatocellular carcinoma, mostly simultaneously occurring with hepatitis B viral infection [12]. The effects appear as mostly mediated by “mutations at guanine in codon 249 of the p53 gene, a tumor suppressing gene, and at many guanine residues in the 12th and 13th codons of the ras gene, a gene whose product controls cellular proliferation signaling” [20, 21]. First, AFB1 must undergo metabolization into aflatoxin B1-8,9-exo-epoxide (its active electriphilic form) by the action of the enzyme cytochrome p450 (CYPs) [12]. This reactive electriphilic form then undergoes intercalation between base residues of DNA, forming adducts with residues of guanine, mostly aflatoxin B1-N7-Gua. The adducts could reorganize or get removed all-together from the backbone, and form apurinic site. The alterations and adducts denote lesions which, on replication of DNA, result in insertion of un-matched base in opposite strands. About 44 percent of hepatocellular carcinomas in areas that have high exposures to aflatoxin have “GC → TA transversion at codon 249 of p53”, a typical mutation detected with AFB1 [12, 20, 21].
Hepatocellular carcinoma prevalence in people exposed to aflatoxins, intensifies when simultaneous hepatitis B viral infection [12]. A study suggested that whilst people that have aflatoxin biomarkers in urine were at higher risks than normal people for “hepatocellular carcinoma”; people with “hepatitis B” viral infection are at four times the risks; people with urinary aflatoxin bio-markers and hepatitis B viral infection at same time were at sixtyfold higher risk for hepatocellular carcinoma compared with normal people [12, 21].
Toxicity of aflatoxin B1
Numerous studies of aflatoxin B1 toxicity have been done on several species of animal.
Acute toxicity of AFBI
Oral median lethal dose (LD50) of AFB1 estimates range from 0.3 to 17.9 mg/kg bw for many animals [22]. For example, oral LD50 of AFB1 has been reported as 17.9 mg/kg bw and 7.2 mg per kg bw in female and male rat respectively. However, intraperitoneal LD50 of AFB1 in male rats has been estimated as 6.0 mg/kg bw [23]. The symptoms are low-grade fever, malaise, and anorexia [24].
Teratogenicity of AFBI
Teratogenicity of AFB1 in rabbit include enlargement of eye socket, degeneration of lenticular, microphthalmia, caudal vertebrae agenesis, cardiac defect, wrist drop, and decreased fetal weight, among others [25].
Immunotoxicity of AFBI
Research in fish found that AFB1 has significant immunosuppression including decreased bactericidal activities and decreased serum total globulin [26].
Subacute toxicity of AFBI
Aflatoxin B1 subacute toxicity in animal species indicated moderately to very severe liver impairment [26]; e.g., subacute toxicity of monkeys showed fatty change and portal inflammation.
Chronic toxicity of AFBI
Aflatoxin B1 chronic toxicity studies in chicken indicated decreased weight gain, decreased concentration of hepatic microsomal cytochrome P-450, and decrease in consumption of feed [26].
Subchronic toxicity of AFBI
Aflatoxin B1 subchronic toxicity studies in fish indicated fish has preneoplastic lesions, simultaneously with variations in gill, spleen, pancreas, and intestine [26].
Genotoxicity of AFBI
Human liver cell treatment with AFB1 in dose ranging from 3 to 5 μmol/l led to development of 8-hydroxyguanine lesions, aflatoxin B1-DNA adducts, and DNA damage [27].
Carcinogenicity of AFBI
Aflatoxin B1 carcinogenicity characterized with liver cell carcinoma development, has been found in rats [22, 23].
Embryotoxicity of AFBI
Embryonic deaths and weakened development of embryo of Fabricius bursa in chicken by AFB1 was reported [28].
Risk regulation and management of AFB1
Exposures to aflatoxin B1 are mostly managed with the measures directed towards the prevention of crop contamination in field, handling in post-harvest, and also in storage, or through measures targeted at identifying and disinfecting contaminated foods and feeds, as well as the materials used in their preparation. For example, bio-decontamination using a single strain of bacteria, Flavobacterium aurantiacum, has been applied in removing AFB1 from corn and peanut grains. Many nations have policies and regulatory actions on AFB1 in food and feed, and they often include recommended levels and maximum permissible limits of AFB1 for specific foods and feeds [4, 29]. Food safety regulation in the US sets maximum permissible limit of 20 μg per kg for AFB1, combined with other aflatoxins (AFB2, AFG1 and AFG2) in every food, except milk with maximum permissible limit at 0.5 μg per kg. High AFB1 level of 100 to 300 μg per kg can be tolerated for few feeds meant for animal consumption [29, 30]. In EU, the has set maximum permissible limits for AFB1 in spices, cereals, dried fruits, and nuts from 2-12 μg per kg, whereas maximum permissible limit for AFB1 in infant formula is 0.1 μg per kg [4, 31]. Maximum permissible limits for AFB1 in animal feed given by the European Union are 5–50 μg per kg; these permissible limits are lesser compared to ones in the United States [31].
The UN FAO/WHO “Joint Expert Committee on Food Additives (JECFA)”, known as the “FAO/WHO JECFA”, sets maximum permissible limits of AFB1 combined with other aflatoxins (AFB2, AFG1, and AFG2) at 15 μg per kg and 10 μg per kg for raw and processed peanuts respectively; whereas the tolerable limit for AFB1 alone is at 5 μg/kg feed meant dairy cattle consumption [32].
Conclusion
Aflatoxin B1 is released by A. flavus and A. parasiticus. The carcinogenicity of AFB1 differs from species with certain species, e.g. monkeys and rats, reportedly mostly susceptible compared to the other species. In animals and humans, aflatoxin B1 has been shown to be teratogenic, mutagenic, and immunosuppressant. The worldwide maximum tolerated aflatoxin B1 levels was reported by the FAO to be in within 1 to 20 μg per kg in food; 5 to 50 μg per kg in cattle feed. Aflatoxin B1 permeates via skin. Liver is organ mostly vulnerable to the toxicity of AFB1. Mechanism of AFB1 carcinogenicity has been defined. AFB1 is a genotoxic hepatocarcinogen that has its exposures linked to hepatocellular carcinoma development, tumors of the liver, particularly when simultaneously occurred with hepatitis B viral infection. The hepatocellular carcinoma prevalence in people exposed to aflatoxins, has shown to increase with simultaneous occurrence of hepatitis B viral infection. Oral median lethal dose (LD50) of AFB1 is 0.3 to 17.9 mg per kg bw for many animals. Embryonic deaths and weakened development of embryo of Fabricius bursa in chicken by AFB1 was reported. Exposures to aflatoxin B1 is mostly taken care of with the measures directed at the prevention of crop contamination in field, handling in post-harvest, and also in storage, or through measures targeted at identifying and disinfecting contaminated foods and feeds, as well as the materials used in their preparation.
Author Contributions
Conceptualization, CG Awuchi, EN Ondari; investigation, CG Awuchi, H Twinomuhwezi, IO Amagwula, VS Igwe, writing, CG Awuchi, EN Ondari, H Twinomuhwezi; Editing, CG Awuchi, EN Ondari, H Twinomuhwezi, IO Amagwula, VS Igwe, All authors have read and approved the version for publication
Funding
No funding was received for this research
References
- McLean M (1995) Cellular interactions and metabolism of aflatoxin: an update. Pharmacology & Therapeutics 65(2): 163-192.
- Galvano F, Ritieni A, Piva G, Pietri A (2005) Mycotoxins in the human food chain. In: Diaz D.E (Editor), The Mycotoxin Blue Book. (UK: 2005), Nottingham University Press, Nottingham, pp. 187-224.
- Azab Rania M, Tawakkol Wael M, Abdel Rahman M Hamad, Abou Elmagd Mohamed K and El Agrab Hassan M, et al. (2005) Detection and estimation of aflatoxin B1 in feeds and its biodegradation by bacteria and fungi. Egyptian Journal of Natural Toxins 2: 39-56.
- Awuchi CG, Owuamanam IC, Ogueke CC, Hannington T (2020) The Impacts of Mycotoxins on the Proximate Composition and Functional Properties of Grains. European Academic Research 8(2): 1024-1071.
- Kucukcakan B, Hayrulai-Musliu Z (2015) Challenging Role of Dietary Aflatoxin B1 Exposure and Hepatitis B Infection on Risk of Hepatocellular Carcinoma. Open Access Macedonian Journal of Medical Sciences 3(2): 363-369.
- Meissonnier GM, Pinton P, Laffitte J, Cossalter AM, Gong YY, et al. (2008) Immunotoxicity of aflatoxin B1: Impairment of the cell-mediated response to vaccine antigen and modulation of cytokine expression. Toxicology and Applied Pharmacology 231(2): 142-149.
- Wacoo Alex P, Wendiro Deborah, Vuzi Peter C, Hawumba Joseph F (2014) Methods for Detection of Aflatoxins in Agricultural Food Crops. Journal of Applied Chemistry 2014: 1-15.
- (2018) WHO Aflatoxins Food Safety Digest. Department of Food Safety and Zoonoses. REF. No.: WHO/NHM/FOS/RAM/18.1. February 2018. World Health Organization.
- Chinaza GA, Clifford IO, Chika CO, Victory SI (2019) Evaluation of Patulin Levels and impacts on the Physical Characteristics of Grains. International Journal of Advanced Academic Research 5(4): 10-25.
- Viegas, Susana, Veiga, Luísa, Figueredo, et al. (2013) Occupational Exposure to Aflatoxin B1in Swine Production and Possible Contamination Sources. Journal of Toxicology and Environmental Health Part A 76(15): 944-951.
- Viegas, Susana, Veiga, Luisa, Malta-Vacas, et al. (2012) Occupational Exposure to Aflatoxin (AFB1) in Poultry Production. Journal of Toxicology and Environmental Health Part A 75(22-23): 1330-1340.
- Kew MC (2013) Aflatoxins as a cause of hepatocellular carcinoma. Journal of Gastrointestinal and Liver Diseases 22(3): 305-310.
- Boonen, Jente, Malysheva, Svetlana V, Taevernier, et al. (2012) Human skin penetration of selected model mycotoxins. Toxicology 301(1-3): 21-32.
- Larsson P, Busk L, Tjälve H (1994) Hepatic and extrahepatic bioactivation and GSH conjugation of aflatoxin B1 in sheep. Carcinogenesis 15(5): 947-955.
- Patterson DSP (1977) Aflatoxin and related compounds: Introduction. In: Wyllie TD, Morehouse LG (Mycotoxic Fungi, Mycotoxins, Mycotoxicoses, Encyclopaedic Handbook. (1st Vol 1), Marcel Dekker Inc, New York, NY, USA, Pp. 131-135.
- Hedayati MT, Pasqualotto AC, Warn PA, Bowyer P, Denning DW (2007) Aspergillus flavus: human pathogen, allergen and mycotoxin producer. Microbiology 153(6): 1677-1692.
- CDC (2017) Definition of Aspergillosis | Aspergillosis | Types of Fungal Diseases | Fungal Diseases | CDC www.cdc.gov.
- Dewick PM (2009) Medicinal Natural Products: A Biosynthetic Approach (3rd ed.). Wiley. pp. 122–4. ISBN 978-0470742792.
- Yu J, Chang PK, Ehrlich KC (March 2004) Clustered pathway genes in aflatoxin biosynthesis. Appl Environ Microbiol 70(3): 1253-1262.
- Fernández Medarde, Alberto and Santos, Eugenio (2017) Ras in Cancer and Developmental Diseases. Genes & Cancer 2(3): 344-358.
- Semela, Maryann (2001) The chemistry and biology of aflatoxin B1: from mutational spectrometry to carcinogenesis. Carcinogenesis 22(4): 535-545.
- Agag BI (2004) Mycotoxins in foods and feeds 1-Aflatoxins. Ass Univ. Bull Environ. Res 7 (1): 173-205.
- Butler WH (1964) Acute Toxicity of Aflatoxin B1 in Rat. Br J Cancer 18(4): 756-762.
- Azziz Baumgartner, Eduardo, Lindblade, Kimberly, Gieseker, et al. (2005) Case-Control Study of an Acute Aflatoxicosis Outbreak, Kenya, 2004. Environmental Health Perspectives 113(12): 1779-1783.
- Wangikar PB, Dwivedi P, Sinha N, Sharma AK, Telang, AG (2005) Effects of aflatoxin B1 on embryo fetal development in rabbits. Food and Chemical Toxicology 43(4): 607-15.
- Sahoo PK, Mukherjee SC, Nayak SK, Dey S (2001) Acute and subchronic toxicity of aflatoxin B1 to rohu Labeo rohita (Hamilton). Indian J Exp Biol 39(5): 453-458.
- Gursoy Yuzugullu, Ozge, Yuzugullu, Haluk, Yilmaz, et al. (2011) Aflatoxin genotoxicity is associated with a defective DNA damage response bypassing p53 activation. Liver International 31(4): 561-571.
- Sur E, Celik I (2003) Effects of aflatoxin B1on the development of the bursa of Fabricius and blood lymphocyte acid phosphatase of the chicken. British Poultry Science 44(4): 558-566.
- Rustom, Ismail YS (1997) Aflatoxin in food and feed: Occurrence, legislation and inactivation by physical methods. Food Chemistry 59: 57-67.
- Park DL, Liang B (1993) Perspectives on aflatoxin control for human food and animal feed. Trends Food Sci Technol 4: 334-342.
- EEC (1991) EEC Council Directive 91/126/EEC Amending the annexes to Council Directive 74/63/EEC on undesirable substances and products in animal nutrition. Off. J. Eur. Commun., No. L 60.
- FAO/WHO (1992) FAO/WHO Standards Programme. Codex Alimentarius Commission Alinorm 93/12.






























