Cytotoxicity, Antimicrobial and Antioxidant Activities of Apocarotenoid Rich Leaf Essential Oil of Maesobotrya Barteri Var. Barteri (Baill.) Hutch
Patricia A Onocha1*, Michael G Ibok1, Olaoluwa O Olaoluwa1, Ganiyat K Oloyede1, Guido Flamini2
1Natural Products and Medicinal Chemistry Unit, Department of Chemistry, University of Ibadan, Nigeria
2Dipartimento di Famacia, Via Bonanno, Università di Pisa, 56126 Pisa, Italy
Submission: January 27, 2023; Published: February 17, 2023
*Corresponding author: Patricia A. Onocha, Natural Products and Medicinal Chemistry Unit, Department of Chemistry, University of Ibadan, Nigeria
How to cite this article: Patricia A Onocha, Michael G Ibok, Olaoluwa O Olaoluwa, Ganiyat K Oloyede, Guido Flamini. Cytotoxicity, Antimicrobial and Antioxidant Activities of Apocarotenoid Rich Leaf Essential Oil of Maesobotrya Barteri Var. Barteri (Baill.) Trends Tech Sci Res. 2023; 6(1): 555677. DOI: 10.19080/TTSR.2023.06.555677
Abstract
Background: Maesobotrya barteri var. barteri (Phyllanthaceae) has analgesic, antimicrobial and antitussive applications in ethno-medicine. Essential oils and their constituents from herbs have also been utilized in the management of a good number of ailments in ethno-medicine. The phytochemical profile along with the corresponding cytotoxic, antimicrobial and antioxidant activities of the leaf essential oil have been investigated in the current research. The essential oil was obtained by hydro-distillation using an all-glass Clevenger apparatus. Identification and characterization were done using Gas Chromatography-Mass Spectrometry (GC/MS) while cytotoxicity assay was carried out by brine shrimp lethality test. The antimicrobial property was assessed by broth dilution method and antioxidant activity was evaluated with 2, 2-diphenyl-1 picrylhydrazyl radical (DPPH*) method.
Results: The essential oil contained a total of twenty-six compounds constituting 91.50 % of the total oil composition dominated with apocarotenoids comprising 49.4%, of which (E)-geranyl acetone (21.9 %) is the most abundant. Some other apocarotenoid constituents of the essential oil include β -ionone (8.9 %), 4-dehydro-β-ionone (5.4 %) and (E)-β-ionone (5.0 %). Brine shrimp lethality test revealed that the essential oil was non-toxic. The essential oil exhibited significant inhibition against bacterial and fungal strains at concentrations ranging from 1000 μg/mL to 62.5 μg/mL. M. barteri leaf essential oil exhibited significant antioxidant activity (% inhibition 90.04-84.73μg/mL) comparable to standard Ascorbic acid ((93.05- 90.40μg/mL) and butylated hydroxy anisole (92.60 - 90.68μg/mL) used for the assay.
Conclusion: The chemical composition of leaf essential oil of M. barteri could be responsible for the significant pharmacological applications of the plant in ethno-medicine and the phytoconstituent of the leaves essential oil of M. barteri is reported for the first.
Keywords: Maesobotrya barteri; Cytotoxicity; Antioxidant; antimicrobial; essential oil; apocarotenoids; (E)-geranyl acetone
Abbreviations: GC/MS: Gas Chromatography/Mass Spectrometry; FRIN: Forestry Research Institute of Nigeria; DMSO: Dimethyl sulfoxide; IC50: The half-maximal inhibitory concentration; DPPH: 2,2-diphenyl-1-picrylhydrazyl radical; LRI: Linear Retention Index; TIC: Total ion concentration in percentage; GC: Gas Chromatography; ANOVA: Analysis of variance; NIST: National Institute of Standards and Technology; MBL: M. barteri leaf; BHA: Butyl hydroxyanisole; DMBA: 7,12-dimethylbenz(a)anthracene
Background
Essential oils and their constituents since ancient times have been known for their oral, topical, aromatherapy properties and their wide range of applications in consumer goods such as soaps, detergents, cosmetics, pharmaceuticals, confectionery, perfumes, soft drinks, beverages and insecticides are due to their therapeutic importance and lower toxicity profile [1]. Its phytoconstituents play an important role as an ideal natural source for antimicrobial, antioxidant, anticancer and chemo preventive agents [2-6].
Maesobotrya barteri var. bateri (Baill.) Hutch (Phyllanthaceae) is a high forest flowering shrub commonly found in the rainforest region of West Africa and the most widely distributed Maesobotrya species in Nigeria [7,8]. Its fruits are edible, succulent and other parts are used for the treatment of diarrhea, dysentery, urethral discharge, cough and pain reliever [9,10]. Phytochemicals such as tannins, saponins, cardiac glycosides, deoxy sugar and terpenes have been reported in its leaf, stem bark and root extracts [11,12]. Its pharmacological relevance includes analgesic, antimalarial [13] and antimicrobial [12] activities. β-amyrin and oleanolic acid isolated from the aerial part of M. barteri
have been reported to exhibit antibacterial activity [14]. However, there is little or no report on the essential oil composition of M. barteri leaf in the literature notwithstanding its medicinal and nutritional importance in ethnomedicine. This has prompted us to undertake this study and we herein report the GC/MS analyses, antioxidant, antimicrobial and cytotoxicity activities of M. barteri leaf essential oil.
Methods
Plant Collection and Preparation
Fresh leaves of Maesobotrya barteri were collected along an open area in Utit Uruan, Akwa Ibom - Nigeria, in October 2018. The fresh leaves were identified and authenticated at Forest Research Institute of Nigeria (FRIN), Oyo State, Nigeria where a voucher specimen with herbarium number FHI 112780 was deposited in the FRIN herbarium.
Chemicals and reagents
n-Hexance and methanol were purchased from BDH general purpose chemicals and distilled prior to use. Dimethylsulphoxide (May & Bayer), England and 2,2-diphenyl-1-picrylhydrazyl (DPPH), ascorbic acid, butylated hydroxyanisole (BHA), Gentamicin and Tioconazole were obtained from Sigma Chemical Co. (St. Louis, MO).
Equipment and Apparatus
All-glass Clevenger, UV-Visible spectrophotometer (Unico1200 & Perkin Elmer lambda 25 models) and GC-MS (Agilent 7809A gas chromatography hyphenated with an Agilent mass detector).
Isolation of Essential Oils
Fresh Leaf of M. barteri (200.01g) were placed in an all-glass Clevenger-type apparatus according to British Pharmacopoeia specifications [15] and hydro-distilled for 3hrs. Essential oil (MBL) was collected using n-hexane and dried over anhydrous sodium sulfate, filtered and stored in a refrigerator at 4oC prior to analysis. The yield of the oil was calculated on a dry basis.
Identification and Quantification of the Essential Oil Constituents
The leaf essential oil of M. barteri were subjected to GC-MS analysis on an Agilent 7809A gas chromatograph hyphenated with an Agilent mass detector having split/split less injector interfaced to mass selective detector operating at 70 eV. Other experimental parameters were as follows: The ion source temperature was set at 200ºC within a mass spectral range of m/z 50-700 at a scan rate of 1428 amu/sec. GC column was equipped with an HP-5MS column with a length of 30 m, having an internal diameter of 0.25mm and a film thickness of 0.25 μm. The oven temperature was programmed as follows: initial temperature of 80ºC for 2min, increased at 10ºC/min to the temperature of 240ºC for 6min. Helium was utilized as the carrier gas at a flow rate of 1 mL/ min. Injection volume, linear velocity and pressure were respectively adjusted at 1.0 μL, 362 cm/s and 56.2 KPa. The oven temperature was set at 60ºC, held for 1 min to 180ºC for 3 min at 10ºC /min, the final temperature was 280ºC for 2 min at 10ºC/ min. Both injector and detector temperatures were fixed at 250ºC. Identification of the essential oil components was based on their linear retention indices as determined according to homologous series of normal alkane as well as by comparison of their mass spectral fragmentation patterns (NIST data/base/chemstation data system) with the data previously reported in the literature [16].
Brine Shrimp Lethality Test
Brine shrimp lethality test of the oil was conducted as described below: About 40 mg of shrimp’s eggs were sprinkled on shallow rectangular plastic into a larger compartment of two unequal compartments (70/30) containing seawater. The shrimp’s eggs were incubated for 48hrs in dark at room temperature. The shrimps (nauplii) were harvested on the smaller compartment exposed to a light source. The essential oil was dissolved in DMSO, and different concentrations were prepared using the serial dilution method (1000, 100 and 10 μg/mL). Ten nauplii were introduced in vials containing different concentrations of the oil. DMSO was tested as solvent control while doxorubicin was used as a reference standard. After 24hrs, the vials were examined against a lighted background and the average number of larvae that survived in each vial was determined [17]. Each experiment was carried out in triplicate and the IC50 (concentrations at which 50 % of nauplii were killed) were calculated from inhibition versus concentration graphs using Microsoft Excel package.
Antimicrobial Activity on the Leaf Essential Oil of M. Barteri
All microorganisms were clinical isolates obtained from the Department of Pharmaceutical Microbiology, University of Ibadan, Nigeria. Leaf essential oil of M. barteri was tested against seven strains of microorganisms consisting of four bacteria; two Gram-negative; Escherichia coli and Klebsiella pneumonia, two Gram-positive (Staphylococcus aureus and Bacillus subtilis) and three fungi (Candida albicans, Penicillium notatum and Aspergillus niger). Pour broth dilutions and surface plate methods were employed for antibacterial and antifungal activities, respectively [18]. The oil sample was prepared such that 1mL of the oil was regarded as 100 % concentration; 0.5mL into 0.5mL of DMSO to give 50 % concentration and 25 % concentration was obtained using serial dilution. DMSO was tested as solvent control, Gentamicin (10 μg/mL) as bacteria control and Tioconazole (0.7 mg/mL) as fungi control. All experiments were carried out in duplicates and observed zones of inhibition of growth were measured and recorded in millimeters (mm).
Antioxidant Activity of M. Barteri Leaf Essential Oil
Free radical scavenging activities of the essential oil and standards were determined by their ability to react with 2,2-diphenyl-1-picrylhydrazyl radical (DPPH*) [15,19]. A methanol-DPPH* solution (0.1 mM) was prepared by dissolving 3.94 mg of DPPH* in 100 mL of methanol. The oil was dissolved in methanol to prepare five concentrations (1000, 500, 250, 125 and 62.5) μg/mL via serial dilution and was mixed with 2.0mL of DPPH* methanol solution (0.1 mM). The mixture was shaken robustly and left to incubate for 30 mins in the dark at room temperature. The absorbance at 517nm was recorded for sample using a GS UV-12, UV-Vis spectrophotometer. A control experiment was carried out applying the same procedure but without essential oil (DPPH* + Methanol) and the absorbance was recorded as Ac. The antioxidant activity of ascorbic acid and butylated hydroxy anisole (BHA) were used as standards for comparison. Each experiment was carried out in triplicates and the free radical scavenging activity of the essential oil was calculated as percentage inhibition (%I) using the formula:
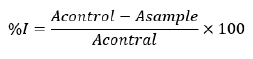
Data Analysis
All results were conveyed as means ± standard deviation. Analysis of variance was used to determine any significant difference between groups using the statistical analysis software package GraphPad 5.0. Values with p<0.05 were regarded as significant. All graphs/charts were drawn with GraphPad 5.0.
Results
Phytochemical Profile of the leaf essential oil of >M. barteri
The hydro-distillation of M. barteri leaves gave pale yellow oil (0.51%) with a floral scent. GC-MS qualitative and quantitative data on the component of Leaf essential oil of M. barteri are shown in Figure 1 and summarized in Table 1. Twenty-six (26) compounds were identified representing 91.5% of the oil constituent. Apocarotenoids were the main constituents identified in the leaf essential oil (49.4%) followed by non-terpene derivatives (38.5%). Minor quantifications were observed in oxygenated monoterpenes (2.7%) and monoterpene hydrocarbon (0.9%). The most abundant compound was found to be (E)-geranyl acetone (21.9%). Other apocarotenoids identified in leaf essential oil include (E)-α-ionone (8.9%), 3, 4-dehydro-β-ionone (5.4%), (E)-β-ionone (5.0%), (E)-β-damascenone (3.8%), β-cyclocitral (1.5%), β-cyclohomocitral (1.2%), Safranal (0.9%) and dehydro- ar-ionene (0.8%). Other minor constituents of the essential oil include oxygenated monoterpenes [neo-dihydrocarveol (1.8%) and geranial (0.9%)] and monoterpene hydrocarbon [dihydrocamphene (0.9%)]. Some non-terpenes and derivatives identified were hexanal (6.4%), 2-pentylfuran (5.3%), (E, E)-2,4-decadienal (3.6%), (E)-2-undecenal (3.0 %), (E)-2-hexenal (2.3%), 6-methyl- 5-hepten-2-one (1.6%) and octanal (1.5%).
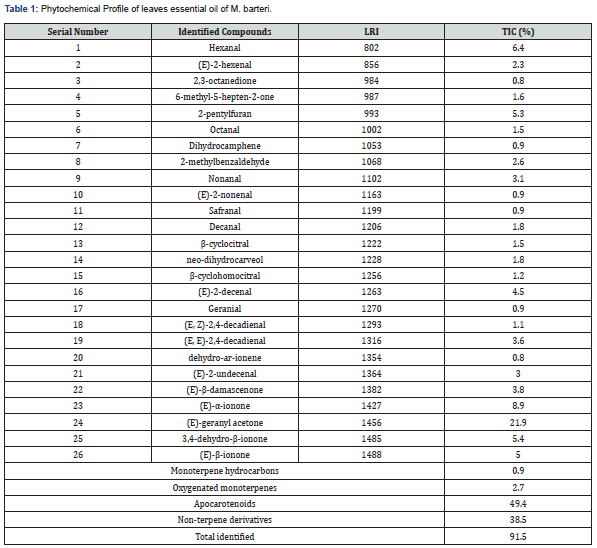
LRI - Linear Retention Index, TIC: Total ion concentration in percentage
Cytotoxicity Assay
The nauplii were exposed to leaf essential oil of M. barteri at different concentrations for 24hrs. The number of mortalities were calculated to determine the effectiveness of the essential oil. The cytotoxicity of M. barteri
essential oil and doxorubicin against nauplii (IC50) was 2822.46 μg/mL and 114.92 μg/mL respectively.Antimicrobial Activity of the leaf essential oil of M. barteri
Experimental data obtained from the in vitro antimicrobial activity of M. barteri leaf essential oil is summarized in Table 2. The essential oil of M. barteri leaves exhibited significant antimicrobial activities with inhibitory zones of 22-34 mm against bacterial strains and 16-25mm against fungal strains when compared to the controls (Gentamicin: 40-45 mm and Tioconazole: 33 mm).
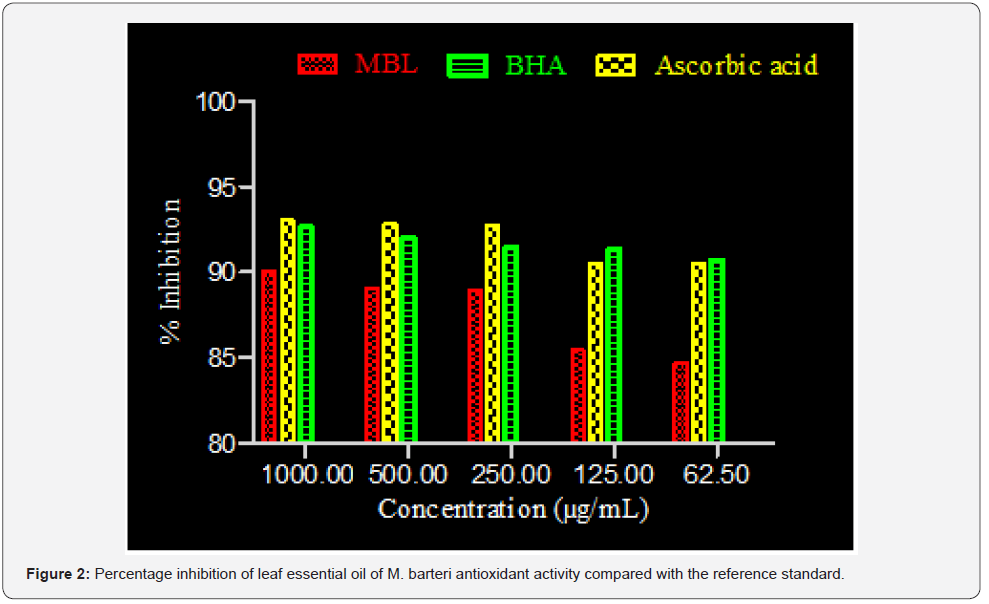
Antioxidant Activity
Results of the scavenging ability of leaf essential oil of M. barteri on DPPH* assay and IC50 values are summarized in Table 3, Figure 2. The percentage inhibition of the essential oil was concentration dependent.
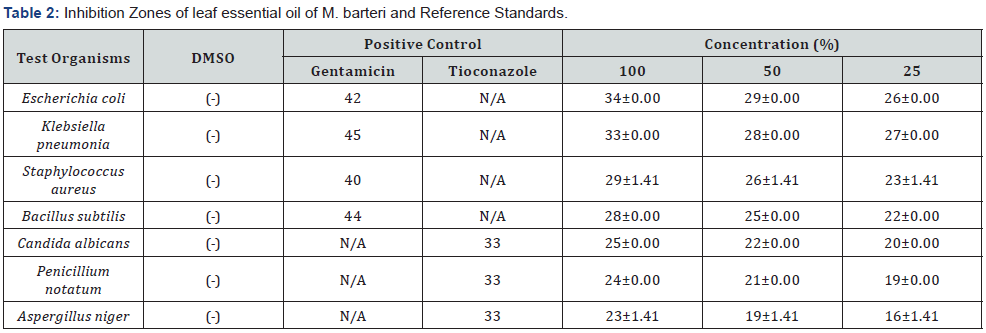
The inhibitory zone was measured in millimeter, mm; N/A: Not applicable, (-): No inhibition, ± = Standard deviation.
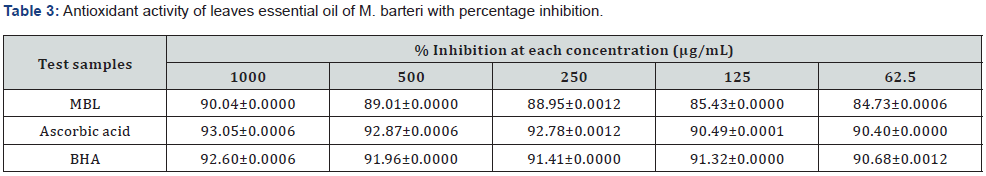
MBL: leaf essential oil of M. barteri, BHA: Butyl hydroxyanisole, ± = Standard deviation.
Discussion
The reputed medicinal properties of M. barteri leaves in folk medicine might be due to the presence of relatively high amounts of apocarotenoids in the essential oil. (E)-geranyl acetone has been reported to possess strong antimicrobial activity [20-22]. β-Ionone has been shown to demonstrate potent anticancer activity both in vitro and in vivo and also suppresses DMBA-initiated mammary cancer in the rat [23]. α-ionone and β-ionone inhibited growth against algae [24] and are used in cosmetics such as perfumes [25]. Apocarotenoids also have wide applications in food, feed, nutritional, pharmaceutical and personal care. Their remarkable nutrition and health importance cannot be overemphasized [26-28].
Brine shrimp lethality assay is a method employed to evaluate the level of toxicity in plant extract and others based on the ability of the plant extract to kill a laboratory cultured larvae (nauplii) [17]. According to standard guidelines for determining the level of toxicity of samples, IC50 > 1000 μgmL−1 is considered nontoxic [29]. Thus, the essential oil in this study is non-toxic (IC50 of 2822.46 μg/mL) when compared to the doxorubicin (IC50 of 114.92 μg/ mL).
The antimicrobial activities of M. barteri leaves essential oil and the test organisms were concentration dependent. The essential oil showed significant antibacterial activity against both the gram-positive (23-28 mm) and gram-negative bacteria (26-34 mm). Generally, the gram-negative reference strains were more sensitive to M. barteri leaves essential oil than the gram-positive at all concentrations. Furthermore, E. coli and B. subtilis were more susceptible to the essential oil among the test strains by inhibitory zones of 26-33 mm and 22-28 mm respectively. With the antifungal assay, M. barteri leaves essential oil was most active against Candida albicans by a zone of inhibition of 20-25mm. Essential oils that was dominated with apocarotenoids such as (E)-geranyl acetone, α-ionone and β-ionone were reported to inhibit the growth of some microorganism [20-22,24]. The polar content such as apocarotenoids in the leaves essential oil may account for the strong inhibition of growth in the test microorganisms as polar compounds have been found to have better interaction with microorganisms - cell walls leading to their death [30].
DPPH* assay is considered a valid, accurate, easy and economic method to evaluate radical scavenging activity of antioxidants since the radical compound is stable [19, 31]. The mechanism of this assay follows the change in color of DPPH from purple to yellow as the radical is reduced by the antioxidant agent either by donating hydrogen atom or electron transformation of DPPH into its reduced form DPPH-H, becoming a stable diamagnetic molecule [30]. It was observed that the leaf essential oil of M. barteri exhibited significant scavenging ability at all concentrations with percentage inhibition between 90.04-84.73 (Figure 2 and Table 3), comparable to that of ascorbic acid (93.05-90.40) and BHA (92.60 - 90.68). Statistical analysis of the data subjected to analysis of variance (ANOVA) showed that there was significant difference between the leaf essential oil and the reference standard at p<0.05. Thus, the presence of apocarotenoids in the essential oil might be responsible for the antioxidant activity [32].
Conclusion
Twenty-six compounds were identified in the leaf essential oil of M. barteri. The essential oil is non-toxic and displayed significant antioxidant and antimicrobial activities. The essential oil is rich in apocarotenoid (49.4%). Apocarotenoid has been found to exhibit numerous biological activities in in-vitro and in-vivo experiments, including free radical scavenging, sunburn preventive, chemo preventive, antimicrobial, insect repellent and attractive effects. The essential oil of M. barteri displayed a significant free radical scavenging activity with percentage inhibition ranging from 90.04-84.73%. The leaf essential oil showed susceptibility against bacterial and fungal strains and the brine shrimp lethality test revealed that the essential oil was non-toxic. These results justify the medicinal applications of M. barteri in ethno-medicine.
Authors’ Contributions
PAO, Organic Chemistry-Ph.D. was involved in all the laboratory work, conceptualization, result interpretation and writing (editing); MGI- M.Sc. Organic Chemistry-Ph.D. candidate carried out the sample collection, extraction of the essential oil, cytotoxicity, antioxidant, antimicrobial assays, results interpretation and write up; OOO-Ph.D. was involved in the GC-MS analysis and interpretation of the essential oil; GKO-Ph.D. was involved in the antioxidant assay; GF, Professor (Associate) of Pharmacy-Ph.D. carried out the GC-MS analysis of the essential oil. All authors read and approved the final manuscript.
Acknowledgments
The authors are grateful to the Departments of Chemistry and Pharmaceutical Microbiology, University of Ibadan, Nigeria for providing the laboratory space and facilities for the extraction and biological analysis of the essential oils and to University di Pisa, Pisa, Italy for the GC-MS analysis of the essential oil.
References
- Lingan K (2018) A Review on Major Constituents of Various Essential Oils and its Application. Transl Med (Sunnyvale) 8(1): 201.
- Bassolé IH, Juliani HR (2012) Essential oils in combination and their antimicrobial properties. Molecules 17(4): 3989-4006.
- Victoria FN, Lenardão EJ, Savegnago L, Perin G, Jacob RG, et al. (2012) Essential oil of the leaves of Eugenia uniflora , antioxidant and antimicrobial properties. Food and Chemical Toxicology 50(8): 2668-2674.
- Iqbal T, Hussain AI, Chatha SAS, Naqvi SAR, Bokhari TH (2013) Antioxidant activity and volatile and phenolic profiles of essential oil and different extracts of wild mint (Mentha longifolia) from the Pakistani Flora. Journal of Analytical Methods in Chemistry 2013: 536490.
- Bhalla Y, Gupta VK, Jaitak V (2013) Anticancer activity of essential oils: a review. Journal of the Science of Food and Agriculture 93(15): 3643-3653.
- Thormar H (2011) Lipids and Essential Oils as Antimicrobial Agents. John Wiley & Sons, Ltd. Chichester, UK, pp. 336.
- Ogbuagu MN, Agu B (2008) Fruit nutritive composition of Maesobotrya barteri, an under-exploited tropical African tree. Fruits 63(6): 357-361.
- Keay RWJ (1989) Trees of Nigeria. Clavendon Press Oxford, UK, 11(3): 322.
- Burkill HM (1994) The useful plants of West Tropical Africa, 2nd Royal Botanic Gardens, Kew, London, United Kingdom, pp. 99-100.
- Essiett UA, Ajibesin KK (2010) Antimicrobial activities of some Euphorbiaceae plants in the traditional medicine of Akwa Ibom State of Nigeria. Ethnobotanical Leaflet 4(6): 654-664.
- Etukudo MM, Osim SE (2017) Assesment of Mineral, Proximate and Phytochemical Composition of leaf, stem and root of Maesobotrya barteri (BAILL) from Secondary Forest in Akwa Ibom State. Int J Adv Res 6(1): 500-505.
- Udo UE, Akan NE, Udoh EM (2017) Qualitative phytochemical screening and antimicrobial activity of methanol extract of Maesobotrya barteri (Baill.) Hutch. Journal of Scientific and Engineering Research 4(9): 351-355.
- Peters DE, Ezendiokwere EO, Njoku UC, Wegwu MO (2017) Antimalarial Activity of Ethanolic Leaf Extract of Maesobotrya barteri and Effects on Some Hematological and Biochemical Parameters of Mice. Journal of Applied Life Sciences International 12(4): 1-15.
- Ogwuche CE, Amupitan JO, Ndukwe IG, Ayo RG (2014) Isolation and biological activity of the triterpene Β-amyrin from the aerial plant parts of Maesobotrya barteri (Baill). Chem 4(11): 729-733.
- Onocha PA, Oloyede GK, Afolabi QO (2011) Chemical Composition, Cytotoxicity and Antioxidant Activity of Essential Oils of Acalypha hispida International Journal of Pharmacology 7(1): 144-148.
- Adams RP (2001) Identification of Essential Oil Composition by Gas Chromatography/ Quadrapole. Allured Publishing, Carol Stream, USA, pp. 804.
- Oloyede GK, Oladosu IA, Shodia AF (2010) Chemical composition and cytotoxicity of the essential oils of Crinum ornatum (Ait.) Bury. Afr J Pure Appl Chem 4(3): 35-37.
- Kalemba D, Kunicka A (2003) Antibacterial and antifungal properties of essential oils. Curr Med Chem 10(10): 813-829.
- Brand-Williams W, Cuvelier M, Berset E (1995) Use of a free-radical method to evaluate antioxidant activity. Food Science and Technology 28(1): 25-30.
- Ghannadi A, Tavakoli N, Mehri-Ardestani M (2003) Volatile Constituents of the Leaves of Ziziphus spinachristi (L.) Willd. From Bushehr, Iran. Journal Essential Oil Research 15(3): 191-192.
- Jirovetz L, Buchbauer G, Schmidt E, Stoyanova AS, Denkova Z, et al. (2007) Purity, antimicrobial activities and olfactoric evaluations of geraniol/nerol and various of their derivatives. J Essent Oil Res 19(3): 288-291.
- Bonikowski R, Świtakowska P, Kula J (2015) Synthesis, odour evaluation and antimicrobial activity of some geranyl acetone and nerolidol analogues. Flavour and fragrance Journal 30(3): 1-7.
- Jianan S, Cao c, Xu J, Zhou C (2020) Research Advances, Biosynthesis, Regulation and Biological Activities of Apocarotenoids Aroma in Horticultural Plants. J of Chem 2020: 11.
- Kumar PP, Kumaravel S, Lalitha C (2010) Screening of antioxidant activity, total phenolics and GC-MS study of Vitex negundo. Afr J Biochem Res 4(7): 191-195.
- Curtis T, Williams DG (1994) Introduction to Perfumery. Ellis Horwood Limited, Hemel Hemstead, UK, pp. 752.
- Kidd P (2011) Astaxanthin, cell membrane nutrient with diverse clinical benefits and antiaging potential. Alternative Medicine Review 16(4): 355-364.
- Power R, Coen RF, Beatty S, Mulcahy R, Moran R, et al. (2018) Supplemental retinal carotenoids enhance memory in healthy individuals with low levels of macular pigment in a randomized, double-blind, placebo-controlled clinical trial. Journal of Alzheimer’s disease 61(3): 947-961.
- Bernstein PS, Li B, Vachali PP, Gorusupudi A, Shyam R, et al. (2016) Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid based nutritional interventions against ocular disease. Progress in Retinal and Eye Research 50: 34-66.
- Gad-Shayne C (2009) Alternatives to in vivo studies in toxicology. In. General, and Applied Toxicology, John Wiley & Sons Inc, USA.
- Olaoluwa OO, James DA, Adigun OA (2018) Volatile oil analysis of aerial parts of Boerhavia coccinea (Mill.). Nat Prod Res 32(8): 959-962.
- Sanchez-Moreno C, Larrauri JA, Saura-Calixto F (1998) A Procedure to measure the antiradical efficiency of polyphenols. J Sci Food Agri 76(2): 270-276.
- Bukhari SI, Manzoor M, Dhar MK (2018) A comprehensive review of the pharmacological potential of Crocus sativus and its bioactive apocarotenoids, Biomedicine & Pharmacotherapy 98: 733-745.






























