Synthesis and Uses of Thioacenes and Oligothiophenes for Organic Electronic Devices
Philip Byers*
Department of Chemistry, Farmingdale State College, USA
Submission: September 02, 2021; Published: October 04, 2021
*Corresponding author: Philip Byers, Department of Chemistry, Farmingdale State College, New York, USA
How to cite this article: Philip B. Synthesis and Uses of Thioacenes and Oligothiophenes for Organic Electronic Devices. Trends Tech Sci Res. 2021; 5(2): 555659. DOI: 10.19080/TTSR.2021.05.555659
Abstract
This article outlines the historical background of organic electronic devices, especially ones that utilize thiophenes and oligothiophenes. Original work in the field is highlighted as well as contemporary advances in the field. The synthetic procedures for making these compounds and the materials application in modern organic electronic devices are also discussed. Research shows these compounds can be used in a number of electronic devices and demonstrate improved stability versus carbon-based electronics such as acenes and its derivatives.
Keywords:Thioacene; Oligothiophene; Semiconductors; OLED; OFET; Organic
Introduction
Great attention has been paid to sulfur-containing polycyclic hydrocarbons that possess π-conjugation systems such as thioacenes and oligothiophenes as seen in Figure 1, because these compounds are promising as organic field-effect transistors (OFET), electroluminescence (EL) materials, semiconductors, organic light emitting diodes (OLED), and organic photovoltaics (OPV) [1,2]. Both the importance and the performance of organic electronic devices have increased significantly over the last 25 years, evolving from a field with great promise for new materials and applications to a real industry with several commercial products on the market [3,4]. Broader acceptance of organic semiconductors will hinge on the development of materials with competitive properties and superior processability, yielding high-performance devices from a significantly less expensive fabrication process [5].
Acenes have long been the subject of intense study because of the unique electronic properties associated with their π-bond topology. Due to their unique topological and electrical properties, thioacenes have garnered much interest since the 1990’s [6]. As compared to their hydrocarbon acene analogues, thioacenes possess a lower highest occupied molecular orbital (HOMO) energy level giving it improved stability. The sulfur atoms contained in these acenes also lead to favorable π-π stacking due to the strong S-S and S-π interactions between molecules. When these compounds are packed in the solid state, the solid film is more compact and charge transfer is better as compared with carbon acenes. Thioacenes such as pentathienoacene and hexathienoacene have demonstrated this improved charge transfer and hole mobility [7]. In order to develop acenes with high stability and electrical conductivity as well as good solubility, a range of substituents are introduced. Usually, substituents are introduced at the ends to increase the π-conjugated dimension, improve the planarity, and increase the solubility. In comparison, polycyclic aromatic hydrocarbons (PAHs) containing sulfur-bridged rings are shown to be excellent Weitz-type donors and extended systems exhibit diradical properties [8-12].
Thioacenes and Oligothiophene for OFET, OLED’s and Semiconductors
Over the past several years, a large number of organic semiconductors have been developed for OFETs. Acenes were widely used in the early years of studies into OFETs, and pentacene was considered an outstanding example that could be compared with the field-effect transistor behavior of amorphous silicon [13,14]. However, the low stability of pentacene limits its use in wider applications. To address this, thienoacene-based small molecules were developed by incorporation of thiophene rings into the π-conjugation backbone. This was pursued anticipating that the higher electronegativity of the sulfur atom would favor the stability of these compounds for use as semiconductors [15].
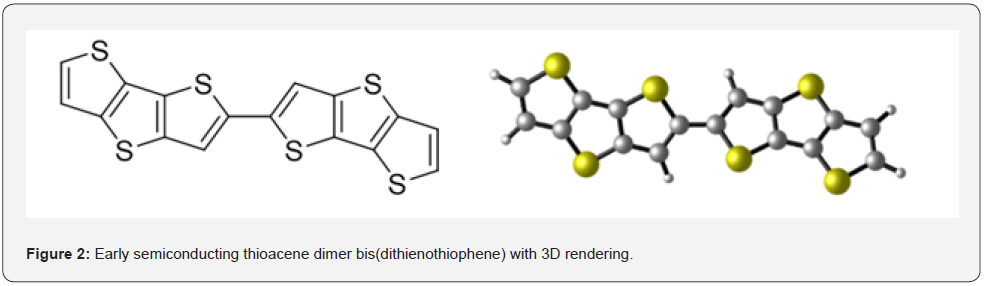
Polythiophenes are some of the most investigated semiconductor materials for applications in organic electronics because of their efficient charge transport properties and compatibility with flexible substrates. Oligothiophenes with the same repeat unit structure as their polymer counterparts offer the opportunity to study the true charge transport nature of these materials, free from structure defects [16- 18]. Bis(dithienothiophene) is an excellent early example of heteroacene-based organic semiconductors with excellent hole mobility as seen in Figure 2 [19]. Thiophene derivatives can be either oxidized in the presence of certain oxidizing agents, such as ferric chloride, or deprotonated and then dimerized by CuCl2 to form a mixture of α-conjugated oligomers as seen in Figure 3a [20]. This process takes advantage of the varying electron density at different positions of the thiophene monomer; the coupling mainly occurs at the positions of highest electron density. One issue with this is with increased conjugation in the precursor molecule, a rapid loss of reactivity of the corresponding cation radical can occur, resulting in short oligomers [21].

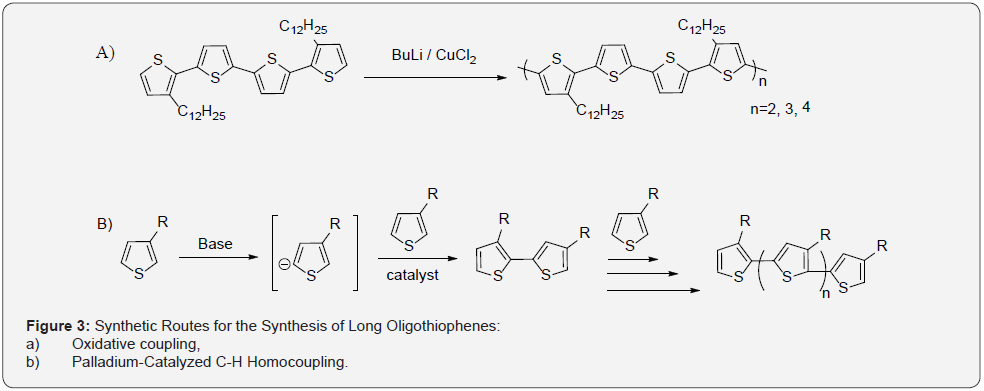
Transition metal-catalyzed cross-coupling has also been explored for the synthesis of polythiophenes. It has been shown that these types of reactions proceed by a chain growth mechanism [22]. A precise synthetic strategy involving palladium catalyzed C-H homocoupling of bromothiophene in the presence of a silver reagent system as an activator was developed by Mori and coworkers [23]. This process results in oligothiophenes with C-Br bonds at the terminal rings, which can then be further functionalized through additional coupling reactions. Treatment of 3-alkylthiophene with 2,2,6,6-tetramethylpiperidinylmagnesium chloride lithium chloride complex (TMPMgCl.LiCl) induces metalation at the 5-position selectively. Subsequent addition of the 2-bromo-3-alkylthiophene and a nickel catalyst leads to the corresponding bithiophene seen in Figure 3b [24].
Work by Bruns, Stahl and coworkers also demonstrated some useful synthetic routes for the synthesis of coupled thiophenes through Pd/Cu oxidative coupling reactions with O2 used as the oxidant [25]. This recent contribution demonstrates the efficient synthesis of homo-coupled 2-bromo-3-hexylthiophenes 1 using nitrogen heterocycles as ligands to facilitate the reaction. Their optimized system utilizes 1,10-phenanthroline-5,6-dione (phd) as a ligand that had not been used previously in Pd-catalyzed aerobic oxidations. The optimized catalyst system, consisting of 3 mol % Pd (OAc)2/phd, Cu (OAc)2, and 1,4-benzoquinone were reacted in the oxidative homocoupling of a series of thiophenes and related heterocycles as seen in Figure 4 [26,27]. High conversions of the thiophene starting material were observed with Pd(OAc)2/ Cu(OAc)2, but little product was obtained. This shows that the use the phd ligand promotes selective formation of product 2. The ternary catalyst system, composed of Pd (OAc)2/Cu (OAc)2/phd, led to substantially improved yields. When loadings of Pd (OAc)2, phd, and Cu (OAc)2 were lowered to 3 mol % the yield of 2 could be improved to 85% by including 3 mol % 1,4-benzoquinone (BQ).

The crucial building block for microelectronics is the fieldeffect transistor (FET), based on inorganic electrodes, insulators, and semiconductors. Instead of competing with conventional silicon technologies, organic FETs based on molecular and polymeric materials (OFETs) may find niche applications in lowperformance memory elements and sensors, [28,29] as well as in integrated optoelectronic devices, such as pixel drive and switching elements in active-matrix organic light-emitting diode (OLED) displays [30]. One of the most common OFET device configurations is that of a thin-film transistor (TFT), in which a thin film of the organic semiconductor is deposited on top of a dielectric with an underlying gate (G) electrode seen in Figure 5. The current between S and D electrodes is minimal when no voltage is applied between G and D electrodes, and the device is in the so called “off” state. When voltage is applied to the gate, charges can be induced into the semi-conductor at the surface with the dielectric layer. Subsequently, the D-S current increases due to the increased number of charge carriers. This is called the “on” state. The major parameters characterizing a FET include the field-effect mobility (μ), which quantifies the average charge carrier drift velocity per unit electric field and the on/off ratio (Ion/Ioff) defined as the D-S current ratio between the “on” and “off” states. The vast majority of organic FETs operate in the p-type accumulation mode, meaning the semiconductor acts as a holetransporting material [31]. To fully see the maximum potential of organic electronics, high-performance electron-transporting (n-type) materials are also needed [32].
Perfluoro n-type molecular and polymeric electronics were developed for specific use in FETs [33]. The fluorocarbonsubstituted oligothiophenes developed and investigated all showed impressive chemical and thermal stability, and volatility, and exhibit similar packing characteristics, strong π-π intermolecular interactions, and comparable lowest unoccupied molecular orbital (LUMO) energies across the conjugated lengths. It was shown that oligothiophenes with repeating units 4-6 are n-type semiconductors with mobilities as high as 0.04 cm2/ (V s). The regioselectivity of the fluorocarbon chain has an important role in semiconducting abilities of these oligothiophenes.
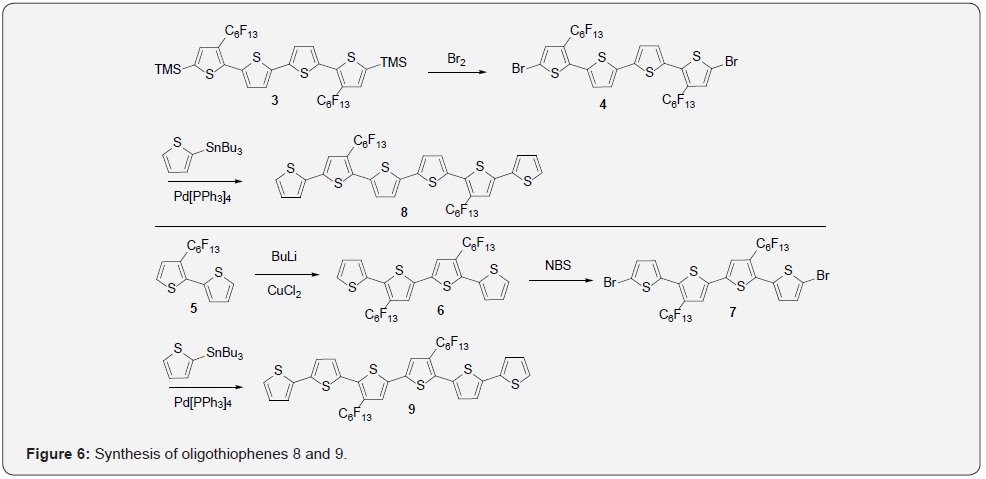
A synthesis of oligothiophenes 8 and 9 has been reported. These compounds demonstrated the most desirable electronic properties as seen in Figure 6. Dibromo-derivative 4 was prepared in 80% yield by a reaction of TMS-protected quaterthiophene 3 with 10% stoichiometric excess of Br2. Stille coupling of 4 with 2-tributylstannylthiophene affords 8 in 87% yield. The isomeric compound was prepared starting from 3-perfluoro-rohexyldithiophene 5, which was lithiated and dimerized with CuCl2 to afford diperfluorohexyl-4T 6 in 44% yield. This was followed by the quantitative bromination of 6 using N-bromosuccinimide (NBS) to afford 7, which was reacted with 2-tributylstannylthiophene to give 9 in a 72% yield. These compounds have shown the regioselective importance of fluorocarbon substituents on oligothiophenes for the production of working n-type materials.

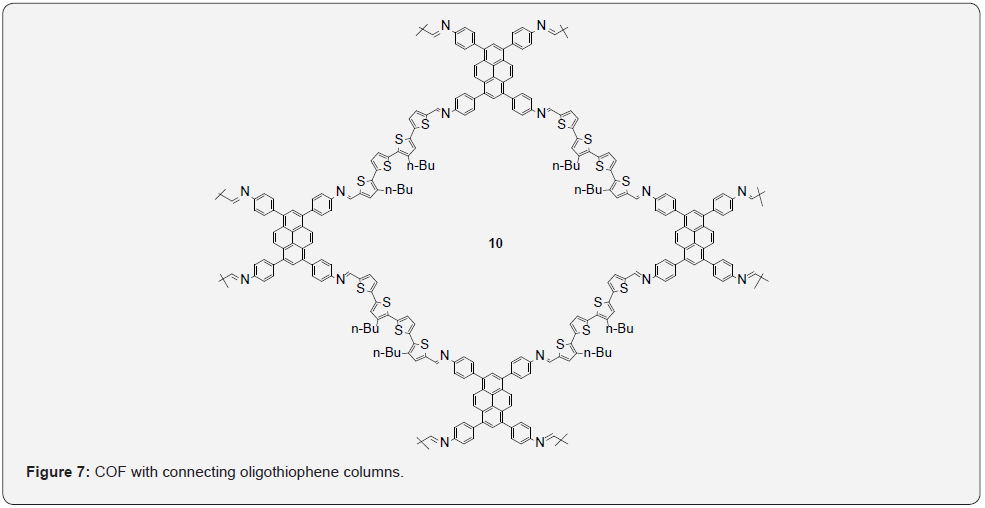
Another interesting application of oligothiophenes has been their incorporation into covalent organic frameworks (COFs) that have uses in organic electronics and optoelectronics [34]. Auras, Bein and coworkers developed a method for the synthesis of highly crystalline quaterthiophene-derived COFs with tunable electronic properties [35]. With respect to the electronic properties of these 2D-COFs, the covalently linked layers are tightly π-stacked, thus forming columns with significant electronic overlap that enable efficient migration of excitations and charge carriers. Solubility issues can arise when synthesizing large 2D surface from strong π-π interactions. These COFs were synthesized through co-condensation of 1,3,6,8-tetrakis(4-aminophenyl) pyrene with 2 equivalents of quaterthiophene dialdehyde bearing asymmetric butyl chains which lead to the formation of the desired 4T COF 10 as seen in Figure 7.
Conclusion
Research and design of thioacenes and oligothiophenes will continue to advance as the compounds have demonstrated an increased stability compared to carbon acenes. These materials have applications in a number of electronic devices including organic field-effect transistors (OFET), electroluminescence (EL) materials, semiconductors, and organic light emitting diodes (OLED). While these materials have proven to be more stable, they have also demonstrated better charge transfer as compared to full carbon acenes due to strong S-S and S-π interactions, a property that is most evident when these materials are applied as thin films. Recent research has been devoted to the investigation and synthesis of oligothiophenes as they have been proven to provide more control with regard to the size and shape of electronic devices. The future is promising as new types of reactions involving these materials are developed every year.
References
- Müller TJJ, Bunz UHF (2007) Functional Organic Materials. (1st), Wiley-VCH, Weinheim, Germany.
- Mullen K, Scherf U (2006) Organic Light Emitting Devices: Synthesis, Properties and Applications. (1st ), Wiley-VCH, Weinheim, Germany.
- Kelley TW, Baude PF, Gerlach C, Ender DE, Muyres D, et al. (2004) Recent Progress in Organic Electronics: Materials, Devices, and Processes. Chem Mater 16: 4413-4422.
- Sun Y, Liu Y, Zhu D (2005) Advances in Organic Field-Effect Transistors. J Mater Chem 15: 53-65.
- Sheats JR (2004) Manufacturing and Commercialization issues in Organic Electronics. J Mater Res 19(7): 1974-1989.
- Mas TM, Rovira C (2011) Role of Molecular Order and Solid-State Structure in Organic Field-Effect Transistors. Chem Rev 111(8): 4833-4856.
- Xiao K, Liu Y, Qi T, Zhang W, Wang F, et al. (2005) A Highly π-stacked Organic Semiconductor for Field-Effect Transistors Based on Linearly Condensed Pentathienoacene. J Am Chem Soc 127(38): 13281-13286.
- Nakasuji K, Kubota H, Kotani T, Murata I, Saito G (1986) Novel Peri-Condensed Weitz-Type Donors: Synthesis, Physical Properties, and Crystal Structures of 3,10-Dithiaperylene (DTPR), 1,6-Dithiapyrene (DTPY), and Some of Their CT Complexes. J Am Chem Soc 108: 3460-3466.
- Nakasuji K, Sasaki M, Kotani T, Murata I, Enoki T (1987) Methylthio- and Ethanediyldithio-Substituted 1,6-Dithiapyrenes and Their Charge- Transfer Complexes: Mew Organic Molecular Metals. J Am Chem Soc 109: 6970-6975.
- Guldi DM, Spänig F, Kreher D, Perepichka IF, van der Pol C, et al. (2008) Contrasting Photodynamics between C60-DithiaPyrene and C60-Pyrene Dyads. Chem -Eur J 14(1): 250-258.
- Wu D, Pisula W, Haberecht MC, Feng X, Müllen K (2009) Oxygen- and Sulfur- Containing Positively Charged Polycyclic Aromatic Hydrocarbons. Org Lett 11(24): 5686-5689.
- Du C, Te S, Liu Y, Guo Y, Wu T, et al. (2010) Fused-Seven-Ring Anthracene Derivative with Two Sulfur Bridges for High Performance Red Organic Light-Emitting Diodes. Chem Commun 46: 8573-8575.
- Zeng Z, Shi X, Chi C, López Navarrete JT, Casado J, et al. (2015) Pro-Aromatic and Anti-Aromatic π-Conjugated Molecules: an Irresistible Wish to be Diradicals. Chem Soc Rev 44: 6578-6596.
- Dong S, Herng TS, Gopalakrishna TY, Phan H, Lim ZL, et al. (2016) Extended Bis(benzothia)quinodimethanes and Their Dications: From Singlet Diradicaloids to Isoelectronic Structures of Long Acenes. Angew Chem Int Ed 55(32): 9316-9320.
- Jurchescu OD, Popinciuc M, van Wees BJ, Palstra TTM (2007) Interface-controlled, high-mobility organic transistors. Adv Mater 19: 688-692.
- Wu W, Liu Y, Zhu D (2010) π-conjugated molecules with fused rings for organic field-effect transistors: design, synthesis and applications. Chem Soc Rev 39(5): 1489-1502.
- Kim EG, Coropceanu V, Gruhn NE, Sánchez CRS, Snoeberger R, et al. (2007) Charge transport parameters of the pentathienoacene crystal. J Am Chem Soc 129(43): 13072-13081.
- Mei J, Diao Y, Appleton AL, Fang L, Bao Z (2013) Integrated materials design of organic semiconductors for field-effect transistors. J Am Chem Soc 135(18): 6724-6746.
- Mishra A, Ma CQ, Bäuerle P (2009) Functional Oligothiophenes: Molecular Design for Multidimensional Nanoarchitectures and Their Applications. Chem Rev 109(3): 1141-1276.
- Li ZC, Sirringhaus H, Garnier F, Holmes AB, Moratti SC, et al. (1998) A Highly π-Stacked Organic Semiconductor for Thin Film Transistors Based on Fused Thiophenes. J Am Chem Soc 120(9): 2206-2207.
- Barbarella G, Zambianchi M, Di TR, Colonna M, Iarossi D, et al. (1996) A Regioselective Oligomerization of 3-(Alkylsulfanyl)thiophenes with Ferric Chloride. J Org Chem 61(23): 8285-8292.
- Osaka I, McCullough R (2008) Advances in Molecular Design and Synthesis of Regioregular Polythiophenes. Acc Chem Res 41(9): 1202-1214.
- Takahashi M, Masui K, Sekiguchi H, Kobayashi N, Mori A, et al. (2006) Palladium-Catalyzed C-H Homo-coupling of Bromothiophene Derivatives and Synthetic Applications to Well-Defined Oligothiophenes. J Am Chem Soc 128(33): 10930-10933.
- Tanaka S, Tamba S, Tanaka D, Sugie A, Mori A (2011) Synthesis of Well-Defined Head-to-Tail-Type Oligothiophenes by Regioselective Deprotonation of 3-Substituted Thiophenes and Nickel-Catalyzed Cross-Coupling Reaction. J Am Chem Soc 133(42): 16734-16737.
- Tereniak SJ, Bruns DL, Stahl SS (2020) Pd-Catalyzed Aerobic Oxidative Coupling of Thiophenes: Synergistic Benefits of Phenanthroline Dione and a Cu Cocatalyst. J Am Chem Soc 142(48): 20318-20323.
- Wendlandt AE, Stahl SS (2014) Bioinspired Aerobic Oxidation of Secondary Amines and Nitrogen Heterocycles with a Bifunctional Quinone Catalyst. J Am Chem Soc 136(1): 506-512.
- Wendlandt A E, Stahl SS (2014) Modular o-Quinone Catalyst System for Dehydrogenation of Tetrahydroquinolines under Ambient Conditions. J Am Chem Soc 136(34): 11910-11913.
- Mushrush M, Facchetti A, Lefenfeld M, Katz HE, Marks TJ (2003) Easily Processable Phenylene-Thiophene-Based Organic Field-Effect Transistors and Solution-Fabricated Nonvolatile Transistor Memory Elements. J Am Chem Soc 125(31): 9414-9423.
- Klauk H, Halik M, Zscieschang U, Eder F, Schmid G (2003) Pentacene organic transistors and ring oscillators on glass and on flexible polymeric substrates. Appl Phys Lett 82: 4175-4178.
- Huitema HEA, Gelinck GH, van der Putten JBPH, Kuijk KE, Hart KM (2002) Active-Matrix Displays Driven by Solution-Processed Polymeric Transistors. Adv Mater 14: 1201-1204.
- Kunugi Y, Takimiya K, Yamane K, Yamashita K, Aso Y (2003) Synthesis and Characterization of Porous and Nonporous Monodisperse Colloidal TiO2 Chem Mater 15: 6-11.
- Facchetti A, Yoon MH, Stern CL, Katz HE, Marks TJ (2003) Building Blocks for n-Type Organic Electronics: Regiochemically Modulated Inversion of Majority Carrier Sign in Perfluoroarene Modified Polythiophene Semiconductors. Angew Chem Int Ed 42(33): 3900-3903.
- Facchetti A, Mushrush M, Yoon MH, Hutchinson GR, Ratner M, et al. (2004) Building Blocks for n-type Molecular and Polymeric electronics. Perfluoroalkyl- versus Alkyl-functionalized Oligothiophenes (nT; n = 2-6). Systematics of Thin Film Microstructure, Semiconductor Performance, and Modeling of Majority Charge Injection in Field-Effect Transistors. J Am Chem Soc 126(42): 13859-13874.
- Dogru M, Handloser M, Auras F, Kunz T, Medina D, et al. (2013) Photoconductive Thienothiophene-based Covalent Organic Framework showing Charge Transfer towards included Fullerene. Angew Chem Int Ed 52(10): 2920-2924.
- Keller N, Bessinger D, Reuter S, Calik M, Ascherl L, et al. (2017) Oligothiophene-Bridged Conjugated Covalent Organic Frameworks. J Am Chem Soc 139(24): 8194-8199.






























