Enhancement of Shelf Life of the Climacteric Fruits: A Review on Application of CRISPRi Technology
Surajit Pathak1*, Sushmitha Sriramulu1, Sathya Priya Thandavan1, Ganesan Jothimani1, Antara Banerjee1 and Francesco Marotta2
1FacuJty of Allied Health Sciences, Chettinad Academy of Research and Education, Kelambakkam, India
2ReGenera R&D International for Aging Intervention, Milano, Italy & Montenapoleone Clinic, Healthy Aging Unit by Genomics & Biotechnology, Milano, Italy
Submission: January 31, 2018; Published: February 23, 2018
*Corresponding author: Surajit Pathak, Department of Allied Health Sciences, Chettinad Hospital & Research Institute (CHRI), Chettinad of Research and Education (CARE), Kelambakkam, Chennai-603103, India, Tel: +91-44-47429050/00919002737622; Email: surajit.pathak
How to cite this article: Sushmitha Sriramulu, Sathya Priya Thandavan, Ganesan Jothimani, et al. Enhancement of Shelf Life of the Climacteric Fruits: A Review on Application of CRISPRi Technology. Trends Tech Sci Res. 2018; 1(2): 555557. DOI: 10.19080/TTSR.2018.01.555557
Abstract
Ripening of climacteric fruits is found to be a major hitch to enhance the commercial value of several countries. As it is considered to be one of the most abundantly consumed fruit varieties, it is imperative to increase the shelf life of the climacteric fruits. The significant reason for ripening is the production of ethylene hormone in the fruits. The commercial value of these fruits seems to be high and the export rate has also been drastically increasing due to the demand. Currently, CRISPRi is the emerging technology to show the prospect to silence the Aminocyclopropane- 1-carboxylic acid synthase gene which is mainly responsible for the ethylene biosynthesis, thus this process will retain the freshness of the fruits and thereby the ripening will also be delayed. This review majorly discusses about the existing methods and also focuses on new approach to increase the shelf life of climacteric fruits.
Keywords: Climacteric; Commercial; Ethylene; Ripening; shelf life
Abbreviations: SAM: S-Adenosyl Methionine; GA: Gibberellic Acid; AVG: Aminoethoxy Vinyl Glycine; ACS: ACC Synthase; ACO: ACC oxidase; NO: Nitric oxide; Cnr: Colorless Non-Ripening; 1-MCP: 1-methyl cyclopropane
Introduction
Based upon the pattern of respiration and the productivity of ethylene, fruits are generally categorized into climacteric and non-climacteric varieties [1]. Climacteric fruits or ethylene dependent fruits have the capability to ripen after the harvest with the help of ethylene production. Non-climacteric fruits are not capable of ripening after it has been taken off from the parent plant whereas the climacteric fruits can ripen [2]. The issue left behind is that after the removal of climacteric fruits from the tree it takes generally around 8 days and it needs a condition of about 15 °C to ripen. But for the export of certain companies and various issues, if the climacteric fruits begin to over ripe then it will start to decay and will be of no use [3]. These climacteric fruits which are harvested at full maturity can be ripened off from the parent plant. The respiration rate and ethylene formation though being minimal at maturity stage, they raise dramatically to a climacteric peak at the onset of ripening after which it begins to decline [4]. It clearly shows that the life expectancy of the climacteric fruit is very low. As it is considered to be one of the most abundantly consumed fruit varieties, it is imperative to increase the shelf life of climacteric fruits (Table 1). The only way to implement is by delaying the ripening period of the climacteric fruits. The significant reason for ripening is the production of ethylene hormone in the fruits. Since the climacteric fruits are highly consumed all over the world, the commercial value seems to be high and the export rate has also been drastically increasing due to the demand of the people day by day [5-7]. It is necessary to delay the ripening of climacteric fruits until it reaches the destination. Currently, delaying of the ripening process has become indispensable in the commercial aspects. Though, there are several studies on delaying the ripening process, still there is a need for intense research. In this review, we majorly focus on the existing methodologies that have been utilized and new approach to delay the ripening process by inhibiting the ethylene biosynthetic pathway.
Process of ripening
Ripening is considered to be a biological activity that generally occurs in each climacteric fruit and even in few non-climacteric fruits which helps the fruit to attain its desirable changes such as color, texture, flavor, aroma, quality and palatable nature which also includes conversion of starch to sugar [8]. It is an irreversible occurrence. There are two ethylene biosynthetic systems where one in climacteric and another one in non-climacteric fruits [9,10]. In non-climacteric fruits, biosynthesis begins through a normal vegetative growth and it causes the base level of ethylene synthesis whereas in climacteric fruit varieties the ripening is characterized by the autocatalytic ethylene responses [11-13].
Ethylene, a phytohormone and simple olefin is the one which regulates the ripening process and affects its rate [14,15]. It is usually present in gaseous form in the physiological conditions and it also involves in the senescence process. Cousins HH [16] suggested that the plants usually produce ethylene gas which is the key factor for its growth and development for the first time and they also proved that during the mixed commercial fruit export, ethylene which is originally produced from other fruits can also affect the rate of ripening of the climacteric fruits. Yang et al. [17] has shown the detailed explanation for the production of ethylene and its regulation mainly in the higher plants. Studies show that the ethylene biosynthesis has a number of precursors such as linolenic acid, propanal, I3-alanine, acrylic acid, I3-hydroxypropionic acid, ethionine, ethanol, ethane, acetic acid, fumaric acid and methionine [9,18,19] (Figure 1).
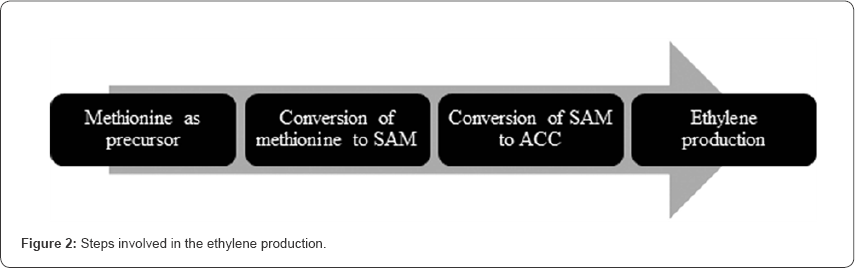
Lieberman et al. [20] discovered chemical system utilizing methionine, which was developed and evidence has been submitted to prove that methionine is the effective precursor for ethylene production. The biosynthesis of ethylene begins due to the activation of multiple 1-aminocyclo-propane 1-carboxlyic (ACC) synthase [21,22] genes due to which there is an increased production of ACC synthase [23]. The first step is the conversion of methionine to S-AdenosylMethionine (SAM) and second step is the conversion of s-adenosylmethionine to ACC in the presence of ACC synthase. The ACC synthase is a pyridoxal-5'-dependent enzyme and it is the main regulating enzyme of the ethylene biosynthesis [19,24]. ACC is then converted by oxidation to ethylene by ACC oxidase enzyme. It has been evident that a metallic compound is involved in the ethylene activation. When the ethylene is produced by ACC oxidase enzyme it is said to be bind to the silver metal ion of the same order so that the ethylene is instigated in the plants [25]. Normally, ethylene diffuses at a t half rate of 2 to 10 minutes from the site of production. The steps involved in ethylene production shown in Figure 2. After the production of ethylene, the ethylene receptor helps in the activation of the ripening process [5] (Figure 2).
General existing methods to arrest the ripening process
Chemical methods: Post-harvest treatment of the Gibberellic Acid (GA) hold up the ripening of the climacteric fruits and its effect has been indicated by the lowered respiratory rate and procrastinated color change. Gibberellic acid probably increases the peroxidase and catalase activities.
Aminoethoxy Vinyl Glycine (AVG), an ethylene inhibitor which also has the ability to reduce fruit abscission, generally slathered on the surface of the climacteric fruits before lucrative harvest to lessen ethylene production that showed comparatively delayed ripening [26]. 1-methylcyclopropene (1- MCP) has proven to inhibit ethylene production by obstructing the escalation of two major predominant enzymes such as ACC Synthase (ACS) and ACC oxidase (ACO) that take part in ethylene biosynthesis [27]. Nitric oxide (NO) treatment found to have similar mechanism of 1-MCP and although 1-MCP delay the ripening by hindering the ethylene perception, whereas NO suppresses ethylene production by targeting the mechanism of senescence delay. Recent studies have recognized that NO absorption is faster than its oxidation in the air so that the rate of NO absorption by each horticultural goods will be higher and effective when compared to other techniques [28].
Ethylene oxide, an endogenous metabolic antagonist of CH4 (methane) has shown a novel delay in the ripening of mangoes after treatment. Vitamin K and vitamin K3 has demonstrated to inhibit the ripening of banana at a temperature as high as 140 °F. When the climacteric fruits were treated with cycloheximide, they begin to ripe as usual but the ripening proceeds at a reduced rate. Similar effects were also detected on the pre-climacteric fruits treated with Actinomycin-D. Ethylene has not reversed the complete inhibition of ripening imposed by Actinomycin-D.
Physical methods and gaseous treatments
Waxing, low O2, High CO2, and ripening inhibitors are mixed to prolong the storage life. Biggs et al. [29] studied the effect of temperature in the climacteric fruits during ripening and found that high temperature stress has an obvious effect on ACC synthase but it varies distinctly. Although the temperature stress altered the amount of production of ethylene by the tomato, the maximum ethylene production was observed constantly in harvested fruits and constant heat stress treatment resulted in decreased production of ripening- associated ethylene. This delayed the ripening process by altering the metabolism behind the ethylene production. Irradiation using radiation such as y-irradiation has the major impact on the retardation of the ripening process [30].
Banana is normally packed in the film bag containing potassium permanganate to absorb ethylene. Commercial absorbents like Purafil are commonly used to control the ripening during the export. Alkaline potassium permanganate on a silicate barrier proved to be effective in the complete absorption of ethylene from bananas detained in sealed polythene bags.
Genetic engineering approach
Ethylene biosynthesis can be retarded by either insertion or suppression of particular gene responsible for the production of ethylene. The four different ways which are generally implemented so far are as follows:
Concealment of ACC synthase gene expression
ACC synthase is the key enzyme involved in the process of conversion of SAM to ACC in the ethylene biosynthetic pathway by suppressing the gene, which encodes the enzyme ACC synthase. This conversion will be controlled and so that the ethylene production can be retarded. Gene expression of ACC synthase enzyme can be mired when an antisense ("mirror- image”) or a condensed copy of the synthase gene is inserted into the climacteric fruit plant's genome.
Enclosure of ACC deaminase gene
Enzyme ACC deaminase is responsible for the conversion of ACC into Ammonia and a-ketobutyrate. The gene coding for the ACC deaminase enzyme is attained from Pseudomonas chlororaphis, a common non-pathogenic soil bacterium that alters the ACC to a different compound, thereby insertion of ACC deaminase coding enzyme leads to dropping the amount of ACC accessible for ethylene production.
Enclosure of SAM hydrolase gene
The gene coding for the enzyme is obtained from Escherichia coli T3 bacteriophage. In this, the approach is similar to ACC deaminase wherein the ethylene production is mired when the amount of its precursor metabolite is condensed. In this event, SAM has been altered to homoserine. Thus, there would not be much SAM available for the ethylene production.
Concealment of ACC oxidase gene expression
ACC oxidase is the enzyme which catalyzes the oxidation of ACC to ethylene, the last step in the ethylene biosynthetic pathway. Through anti-sense technology, down regulation of the ACC oxidase gene results in the suppression of ethylene production, thereby delaying the fruit ripening [31]. The gene coding for the ACC oxidase enzyme is acquired from Pseudomonas chlororaphis, a common non-pathogenic soil bacterium that converts ACC to a different compound thereby dropping the amount of ACC available for ethylene production.
Role of mutation in ripening
Recent studies have found that in tomato, LeACS1A and LeACS4 are the two prominent genes responsible for the initiation of ripening ethylene but they are still under progressive control. These genes found to be induced during the onset of the ripening process, and usually weakened by mutation at the ripening-inhibitor (rin) locus [32]. Rin fruits are reactive towards exogenous ethylene when it is induced by expression of an ethylene-regulated gene, thus it will not ripen [33].
LeMADS-RIN, a MADS box transcription factor encoded by the rin locus, which also has been inferred to conduct control over ripening of climacteric ethylene synthesis with the combination of the above mentioned-phenotypes viz., LeACS1A and LeACS4 [34]. LeACS6, an ACS gene which is mainly responsible for pre-ripening ethylene synthesis can be blocked to prevent ripening by ethylene synthesis [35]. Apart from ACS gene activity, in most plant tissues excess of ACO gene activity been observed, and are induced during ripening process in accordance to ethylene which eventually leads to autocatalytic ethylene synthesis [36]. Studies found that the Colorless Non-Ripening (Cnr) mutation also results in comprehensive ripening inhibition.
Although the above mentioned genetic approaches are widely used for delaying the ripening of climacteric fruits by retarding the ethylene biosynthesis at gene level, both approaches has pros and cons equally. To overcome the shortcoming of already available methods, in this study we focused on highly efficient technology, CRISPRi which is a feasible technique when compared to available techniques in the market.
CRISPRi gene technology: an alternative technique
The CRISPRi (Clustered Regularly Interspaced palindromic Repeats) system comes from the Streptococcus pyrogene CRISPR system pathway. It is an RNA based repression system orthogonal system. The CRISPRi system is a complex of catalytically inactive Cas9 (Caspase9) gene and single guide RNA derived from Streptococcus pyrogene. The Cas9 and sgRNA (single guide RNA) are responsible for the repression of the targeted gene sequence. The Cas9 is catalytically inactive and it does not have endonucleolytic activity whereas the single guide RNA has 102nt (nucleotides) non-coding RNA. This whole RNA consists of 20nt of target specific complementary region, 42 nt of Cas9 binding RNA structure and 40nt transcription terminator. This takes place by the way that the Cas9 and sg RNA binds to the DNA complementary to the sgRNA which blocks the transcription elongation, RNA polymerase binding and transcriptase factor binding. The sgRNA and the dCas9 complex have a complementary region to any gene of interest so that it helps in the binding of the specific gene. Thus, CRISPRi system can be useful in the gene silencing of the ACC synthase gene which is responsible for the ethylene production. Mutation to the single guide RNA by single or more mutations will be helpful in tuning the repression of the gene. The efficiency of the gene repression can also be enhanced by the activity of multiple single guide RNA to target the same DNA gene sequence [37].
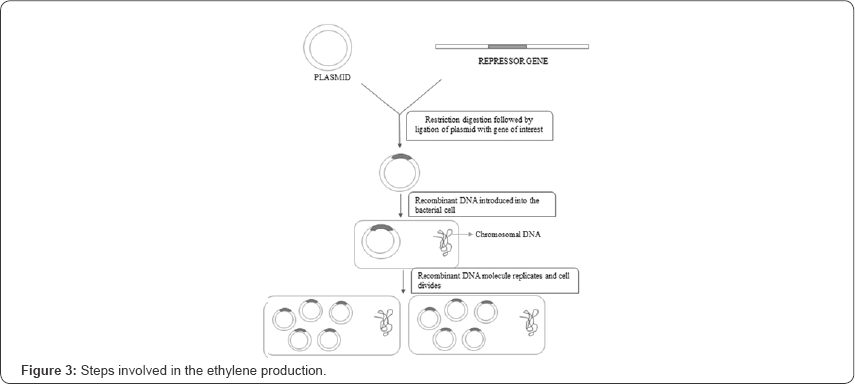
Recombinant DNA technology (r-DNA) is used to repress the activity of ACC synthase gene is where the plasmid vector system has been utilized. The dCas9 and sgRNA gene from the Streptococcus pyrogene can be restricted using the restriction polymerase enzyme and joined into the plasmid vector gene using DNA ligase enzyme. In the plasmid vector gene, the circular gene has been cut so that there will be sticky ends at the spliced region, as the sticky ends will be useful in the binding of the dCas9 and sgRNA gene using the complementary regions. The plasmid DNA with dCas9 and sg RNA can be incorporated into Agro bacterium species so that it can be multiplied into several clones. As it was discussed above, multiple single guide RNA can help in the better repression of the gene activity. From the Agro bacterium species, it can be incorporated into the plant (Figure 3).
Activity of CRISPRi gene using rDNA technology
Using the plasmid vector DNA, the dCAs9 and sg RNA gene called the CRISPRi (Clustered Regularly Interspaced Palindromic Sequences interference) system is multiplied by inserting them into the Agrobacterium species. After the insertion, the repressor gene can be cloned into multiple copies. DNA containing the repressor gene is injected into the banana plant. After injection, dCas9 and sgRNA complex in the DNA will act on the 1-aminocyclopropane 1-carboxylic synthase gene and the 1-aminocyclopropane 1-carboxylic oxidase gene.
As discussed, these two genes are responsible for the production of ethylene and the activation of the ethylene receptor. The dCas9 and sgRNA complex binds to the nontemplate strand of the double stranded DNA of the ACC synthase gene where the sgRNA has a series of complementary strand to that of the ACC synthase gene. It will bind at the promoter sequence of the ACC synthase so that the RNA polymerase could not bind at the promoter sequence. After the binding of the dCas9 and sgRNA gene, the transcription elongation will not take place. If the transcription doesn't take place, there will be no gene expression. After the repression of ACC synthase, S-adenosyl methionine cannot be converted to 1-aminocyclopropane 1- carboxylic acid so that the ethylene cannot be produced. The ethylene biosynthesis will be halted. If the ethylene productivity is halted, then the ethylene receptor will not be activated. Thus, ripening process will be inhibited. The CRISPR interference system will repress the ACC synthase so that the fruit can be unripening for few days by increasing the t half period. It does by the way that it does not knockout the whole gene instead of that it will silence it. It does not disrupt the ACC synthase so that there will not be any mutation due to gene silencing or repression. This is the main advantage of the CRISPR interference system. The activity of multiple Cas9 and single guide RNA complex can lead to better repression of the gene activity, thereby reducing the ripening period of the fruit.
After the repression of the ripening, it must be activated so that it must be given artificially. The ethylene treatment is done by giving 0.1 parts per million ethylene so that it induces the activity of ripening whereas in the commercial aspects it should be given at the rate of 100 to 1000 parts per million. After the induction of artificial ethylene the ripening starts and within 2 to 5 days it will get ripens. This technique can be easily done when compared to the other conventional methods of using RNA interference and 1-methyl cyclopropane (1-MCP) compound as it is not that viable and there are many flaws in it. In the future work, this technique can be used to repress the activity of ethylene induced ripening in any fruits as ethylene is the only compound responsible for natural ripening.
Conclusion
Even though there are various methods to delay the ripening of climacteric fruits, it doesn't show effective results that would last for longer time and mostly all the processes are seem to be reversible. Researchers are working with effective techniques towards inhibiting the ethylene biosynthesis at the gene level, since it is the major step involved in fruit ripening. CRISPRi is currently the emerging technology which has showed the prospect to silence genes responsible for the ripening process, and several studies found that prominently the gene ACC synthase is responsible for the ethylene biosynthesis and ripening process. Thus, focusing on silencing the ACC synthase gene using CRISPRi technique, the freshness of the fruits will be retained for longer period. This technique does not interfere with the mechanism behind the maintenance of health benefits of climacteric fruits. In near future, this technique can be used to enhance the shelf life of climacteric fruits.
Acknowledgement
The authors are thankful to Chettinad Academy of Research and Education (CARE), Chennai, India for the research facilities and support.
Funding
This study was partially financially supported by grants from Chettinad Hospital & Research Institute (CHRI), Chettinad Academy of Research and Education (CARE), Kelambakkam, Chennai-603103, India.
References
- Coombe BG (1976) The development of fleshy fruits. Annual Reviews of Plant Physiology 27: 207-28.
- Prasanna V, Prabha TN, Tharanathan RN (2007) Fruit ripening phenomena-An overview. Crit Rev Food Sci Nutr 47(1): 1-19.
- Brady CJ, PBH O'Connell (1976) On the significance of increased protein synthesis in ripening banana fruits. Australian Journal of Plant Physiology 3(3): 301-310.
- Gamage TV, MS Rehman (1996) Handbook of Food Preservation: Postharvest handling of foods of plant origin. In: Rehman MS (Eds.), p. 1146.
- Ferguson IB (1984) Calcium in plant senescence and fruit ripening. Plant Cell Environment 7(6): 477-489.
- Grierson D, Tucker GA, Robertson NG (1981) The molecular biology of ripening. 51: 147-158.
- Hobson GE (1979) Commentaries in Plant Science: What factors are involved in the onset of ripening in climacteric fruit? Current Advances in Plant Sciences 2(37): 1-11.
- Bapat VA, Trivedi PK, Ghosh A, Sane VA, Ganapathi TR, et al. (2010) Ripening of fleshy fruit: Molecular insight and the role of ethylene. Biotechnol Adv 28(1): 94-107.
- McMurchie EJ, McGlasson WB, Eaks IL (1972) Treatment of fruit with propylene gives information about biogenesis of ethylene. Nature 237(5352): 235-236.
- Mousdale DA, M Knee (1982) Indole-3-acetic acid and ethylene levels in ripening apple fruits. Journal of Experimental Botany 32: 753-58.
- Brecht JK, Kader AA (1984) Regulation of ethylene production by ripening nectarine fruit Prunus persica as influenced by ethylene and low temperature. Journal of American Society of Horticultural Sciences 109(6): 869-872.
- Bufler G (1984) Ethylene-enhanced 1-aminocyclopropane-l-carboxylic acid synthase activity in ripening apples. Plant Physiol 75(1): 192-195.
- Bufler G (1986)Ethylene-promotedconversion ofl-aminocyclopropane- l-carboxylic acid to ethylene in peel of apple at various stages of fruit development. Plant Physiol 80(2): 539-543.
- Burg SP, Burg EA (1967) Molecular requirements for the biological activity of ethylene. Plant Physiol 42(1): 144-152.
- Hoffman NE, SF Yang (1980) Changes in l-amino-cyclopropane-l- carboxylic acid content in ripening fruits in relation to their ethylene production rates. J Amer Soc Hort Sci 105(4): 492-495.
- Cousins HH (1910) Handbook of Plant and Crop Physiology: Agricultural experiments. Citrus. Department of Agriculture, Jamaica annual report 7-523.
- Yang SF, NE Hoffman (1984) Ethylene biosynthesis and its regulation in higher plants. Plant Physiology 135: 155-189.
- Bulens I, Poel BV, Hertog ML, Cristescu SM, Harren FJ, et al. (2014) Dynamic changes of ethylene Biosynthesis in 'Jonagold' apple. Scandinavian Plant Physiol Society 150(2): 161-173.
- Mizrahi Y, Dostal HC, Cherry JH (1975) Ethylene induced ripening in attached rin fruits, a non-ripening mutant of tomato. Horticultural Sciences 10: 414-415.
- Lieberman M, Kunishi AT, MapSon LW, Wardale DA (1965) Ethylene production from methionine. Biochem J 97(2): 449-459.
- Su LY, T McKeon, D Grierson, M Cantwell, SF Yang (1984) Development of I-aminocyclopropane-I- carboxylic acid synthase and polygalacturonase activities during the maturation and ripening of tomato fruit. Horticultural. Sciences 19: 576-78.
- Terai HS, Mizuno S (1985) Changes in l-aminocyclopropane-l- carboxylic acid and ethylene forming enzyme activity in growing and ripening fruits of tomato and cucumber. Journal of the Japanese Society of Horticultural Sciences 53(4): 467-73.
- Kende H (1989) Enzymes of ethylene biosynthesis. Plant Physiol 91(1): 1-4.
- Liu Y, Hoffman NE, Yang SF (1985) Promotion by ethylene of the capability to convert l-aminocyclopropane-I-carboxylic acid to ethylene in pre-climacteric tomato and cantaloupe fruits. Plant Physiol 77(2): 407-411.
- Bregolia AM, Scaramaglib S, Costaa G, Sabatinia E, Ziosib V, et al. (2002) Peach (Prunus persica) fruit ripening: aminoethoxyvinylglycine (AVG) and exogenous polyamines affect ethylene emission and flesh firmness. Physiol Plant 114(3): 472-481.
- Sisler EC, M Serek (1999) Compounds controlling ethylene receptor Bot Bull Acad Sin 40: 1-7.
- Nakatsuka A, Shiomi S, Kubo Y, Inaba A (1997) Expression and internal feedback regulation of ACC synthase and ACC oxidase genes in ripening tomato fruit. Plant Cell Physiol 38(10): 1103-1110.
- Manjunatha G, Lokesh V, Neelwarne B (2010) Nitric oxide in fruit ripening: Trends and opportunities. Biotechnol Adv 28(4): 489-499.
- Biggs MS, Woodson WR, Handa AK (1988) Biochemical basis of high temperature inhibition of ethylene biosynthesis in ripening tomato fruits. Physiol Plant 72(3): 572-578.
- Mahajan PV, Caleb OJ, Singh Z, Watkins CB, Geyer M (2014) Postharvest treatments of fresh produce. Philos Trans A Math Phys Eng Sci 372: 20130309.
- Apelbaum A, Wang S, Burgoon AC, Baker J, Lieberman M (1981) Inhibition of the conversion of ACC to ethylene by structural analogs, inhibitors of electron transfer, uncouplers of oxidative phosphorylation and free radical scavengers. Plant Physiol 67(1): 74-79.
- Lincoln JE, Fischer RL (1988) Regulation of gene expression by ethylene in wild-type and rin tomato (Lycopersicon esculentum) fruit. Plant Physiol 88(2): 370-374.
- Vrebalov J, Ruezinsky D, Padmanabhan V, White R, Medrano D, et al. (2002) A MADS-box gene necessary for fruit ripening at the tomato ripening-inhibitor (rin) locus. Science 296(5566): 343-346.
- Barry CS, Blume B, Bouzayen M, Cooper W, Hamilton AJ, et al. (1996) Differential expression of the 1-aminocyclopropane-1-carboxylate oxidase gene family of tomato. Plant J 9(4): 525-535.
- Barry CS, Llop-Tous MI, Grierson D (2000) The regulation of 1-aminocyclopropane-1-carboxylic acid synthase gene expression during the transition from system-1 to system-2 ethylene synthesis in tomato. Plant Physiol 123(3): 979-986.
- Larson MH, Gilbert LA, Wang X, Lim WA, Weissman JS, et al. (2013) CRISPR interference (CRISPRi) for sequence-specific control of gene expression. Nat Protoc 8(11): 2180-2196.
- James DW (2007) Recombinant DNA: Genes and Genomes: A short Course. San Francisco: Freeman WH ISBN 0-7167-2866-4.






























