Abstract
The sublethal effects of retained fishing hooks on sharks remain largely unknown, with limited research assessing their long-term impact on individual health and survival. In a previous study, we reported a high prevalence of retained hooks in free-ranging blue sharks observed in the NW Mediterranean Sea. These findings ranked among the highest values reported within the literature. To further investigate this issue, we conducted a study during 2023 and 2024, carrying out 111 pelagic shark surveys (898.7 hours), during which we observed 135 non-YOY blue sharks. We could assess the prevalence of retained fishing hooks (RH) and trailing lines in 121 of these free-ranging blue sharks. A 20% of sharks showed RH or scars derived from hooks. Eight of these specimens exhibited severe lesions (SL) (6.6% of the whole sample): lower jaw dislocation, nictitating membrane protrusion, presence of fibronecrotic tissue and neurological signs, consistent with ataxia, uncoordinated swimming movements and loss of buoyancy. The 33.3% of sharks affected by RH presented SL. SL were found significantly more frequently in specimens affected by RH than those that had not been previously captured. None of the blue sharks without RH or signs of having had them showed evidence of a SL. Such injuries are more frequent than previously suspected and may severely impact the sharks’ ability to feed and survive. Our results reinforce concerns that Western Mediterranean blue shark populations may be seriously exposed to by-catch events and their consequences, classified as a “Critically Endangered” species.
Keywords:Blue shark; Prionace glauca; Hooks; Lesion; Conservation; By-catch; Longline fishery
Keywords:CCC: Cap de Creus Submarine Canyon; dF: Degrees of freedom; IRHP: prevalence of internally retained hooks within the aerodigestive tract; IRHRHP: prevalence of IRH within sharks affected by retained hooks; IUCN: International Union for the Conservation of Nature; NAP: prevalence of active necrotic areas; NARHP: prevalence of NA within sharks affected by retained hooks; NSDMP: SL related to neurologic signs and dysfunctional movements; NSDMRHP: prevalence of NSDM within sharks affected by retained hooks; PVC: Polyvinyl chloride; RH: Retained hook; RHP: Retained hook prevalence; SL: severe lesion; SLEP: prevalence of SL affecting eyes; SLERHP: prevalence of SL affecting eyes within sharks affected by retained hooks.; SLJP: prevalence of SL affecting jaws and their functionality; SLJRHP: prevalence of SL affecting jaws within sharks affected by retained hooks; SLP: prevalence of SL in all sharks observed; SLRHP: prevalence of SL in all sharks affected by retained hooks; TL: Total length; YOY: Young of the Year
Introduction
The pervasive threat of bycatch in pelagic longline fisheries has long been recognized as a primary driver of shark populations decline globally [1,2]. In the Mediterranean Sea, the blue shark (Prionace glauca) has been particularly affected by unintentional fisheries interactions, leading to its designation as Critically Endangered in the region by the IUCN [3]. Historically, bycatch analyses on sharks have predominantly concentrated on immediate and delayed mortality [4,5]; in contrast, sublethal injuries -particularly those resulting from retained fishing hooks- remain a largely unexplored yet critical aspect of these interactions.
Retained hooks, both externally and internally lodged, can induce a variety of lesions ranging from localized fibrosis and tissue necrosis to more complex systemic pathologies [6,7]. These injuries may not be immediately fatal but can compromise vital functions such as feeding, locomotion, and sensory input, potentially leading to starvation, impaired growth, secondary infections, or chronic inflammation [8,9]. Notably, cases of mandibular dislocation, nictitating membrane paralysis, and internal hook retention with associated behavioral dysfunctions suggest the occurrence of severe anatomical and neurological impairments in blue sharks exposed to long line gear [6,10-12]. Despite the critical relevance of these conditions, very little attention has been paid to systematically evaluate the prevalence and pathophysiological consequences of retained hooks in elasmobranchs under natural conditions. In previous works [12,13], a surprisingly high Retained Hook Prevalence (RHP) of over 20% was documented in free-ranging P. glauca from the NW Mediterranean. Moreover, several of these individuals exhibited permanent lesions related to the by-catch capture by means of hooks.
The present study aims to expand on those findings by focusing specifically on severe and functionally significant injuries. Specifically, our objectives were:
a) to identify and classify the range of severe injuries associated with retained hooks and lines
b) to establish whether these cases of severe injuries are found in the entire population of blue sharks or mainly linked to specimens that have presented RH
c) to quantify their prevalence both in the general population and among affected blue sharks over a two-year period (2023-2024) in the Cap de Creus Submarine Canyon (CCC), a main breeding and nursery aggregation area for this species [14-16]
By combining underwater visual assessments with injury classification grounded in comparative pathology, results from this study will provide a more comprehensive understanding of the potential implications of bycatch on sharks’ individual fitness and population sustainability.
Methods
Study area and data collection
This study was located on a section of 25 × 19 km from the upper part of the CCC, in the NE of the Iberian Peninsula. This area is characterized by a permanent marine circulation system, which contributes to its status as a significant biodiversity hotspot in the NW Mediterranean Sea -including for sharks- as demonstrated in previous works [12,14].
We collected data over two consecutive years (2023 and 2024) using a standardized, non-invasive technique. Sharks were studied following the method described by Riera-Renter and col. [12], which involved the attraction of pelagic sharks by means of chumming and perforated Polyvinyl chloride (PVC) tubes containing sardines.
Underwater footage and photographs were initially taken from the boat to document sharks before they potentially left the area. Once sharks were habituated to human presence, researchers entered the water cautiously using snorkelling and freediving to obtain additional close-range images. All images and videos were obtained using a Canon G7X still camera with a Nauticam NA-G7XMKII waterproof case, an Olympus Tg6 camera and two GoPro Hero 10 cameras. Whereas in a part of 2023 sharks were measured by direct comparison between boat equipment and structures, in 2024, the Olympus Tg6 camera was equipped with a device with two parallel lasers (Glowdive, model Acho II) separated by 20 cm, that allowed us to measure the shark’s length with precision [16,17]. These methods enabled the collection of the following information:
a) total length estimation (TL)
b) sex determination
c) presence and characteristics of RH and/or their scars and trailing lines
d) presence of injuries and their classification.
Additionally, underwater footage and photographs were analyzed to identify injuries and abnormal behaviours potentially - but not directly- linked to the impact of hooks, whether currently present or from past exposure. All these parameters aided with the individual recognition of sharks [12].
Age class assessment and classification of hook-related injuries in observed sharks
Sharks were classified into age classes according to their TL estimation [12,16], being defined as follows: young-of-the-year (YOY), juveniles (individuals of ages 1+, 2+ and 3+ years), subadults (4+ years) and adults (5+ o more years).
Scars, stains, spots, abrasions, lacerations, fibronecrotic injuries and other specific but less common symptoms, potentially associated to hook-injuries, were identified as lesions. Among these, severe lesions (SL) were also identified and classified according to their anatomical position (jaw or eyes, for example) when possible. Severe lesions were defined as physical injuries or infections caused by an external cause that produced a permanent physical damage or abnormal behaviour, negatively impacting on their normal life, their fitness or thriving. Each SL was then classified into the following categories: SL affecting jaws and their functionality (SLJ); SL affecting eyes and/or their functional structures, such as the nictitating membrane (SLE); active necrotic areas (NA); SL related to neurologic signs and dysfunctional movements (NSDM); and internally retained hooks within the aerodigestive tract, revealed by lines merging from the mouth (IRH).
Statistical analysis
All observed YOY were excluded from the analysis due to lower catchability with standard hook-fishing methods. First, we calculated the “retained hook prevalence” (RHP), defined as the probability (ranging from 0 to 1) that a shark of a specific age, sex or period exhibited a retained hook and/or derived lesion caused by hooks at the time of observation. We also calculated the probability (prevalence, ranging from 0 to 1) of finding sharks with specific SL, defined as follows:
a) SLJP: prevalence of SL affecting jaws and their functionality
b) SLEP: prevalence of SL affecting eyes and/or their functional structures, such as the nictitating membrane
c) NAP: prevalence of active necrotic areas
d) NSDMP: SL related to neurologic signs and dysfunctional movements
e) IRHP: prevalence of internally retained hooks within the aerodigestive tract
f) SLP: prevalence of SL as a whole (SLP = SLJP + SLEP + NAP + NSDMP). IRHP was not included in this formula because the only affected shark exhibited both an IRH and NSDM.
We also calculated the probability (prevalence, ranging from 0 to 1) of finding sharks with specific SL within only sharks affected by hooks, as follows:
g) SLJRHP: prevalence of SL affecting jaws within sharks affected by retained hooks.
h) SLERHP: prevalence of SL affecting eyes within sharks affected by retained hooks.
i) NARHP: prevalence of NA within sharks affected by retained hooks.
j) NSDMRHP: prevalence of NSDM within sharks affected by retained hooks.
k) IRHRHP: prevalence of IRH within sharks affected by retained hooks.
l) SLRHP: prevalence of SL as a whole within sharks affected by retained hooks (SLRHP = SLJRHP + SLERHP + NARHP + NSDMRHP). Also not including IRHRHP for the same reason.
The Chi-Square test was used to compare the absolute frequencies observed across the different analyses.
Results
Residual hooks, trailing lines and derived lesions
From April 2023 to October 2024, we carried out 111 pelagic shark surveys (898.7 hours), during which we observed 135 non- YOY blue sharks, and only one was seen twice. Among all observed sharks, 121 (87 in 2023, and 34 in 2024) could be studied in detail. Among the two years, 24 sharks showed either retained hooks or scars derived from rejected hooks (RHP=0.20). No significant differences were found between the 2023 prevalence (RHP=0.20) and the 2024 prevalence (RHP=0.20) (χ2 = 0.0113; 1 dF; p=0.916). Among the 24 affected sharks, 54.2% of the specimens also presented trailing lines (8 in 2023 and 5 in 2024).
SL derived from residual hooks
Among the 121 sharks studied in detail, 8 specimens presented SL (SLP = 0.066), differences being not significant between 2023 (SLP = 0.046) and 2024 (SLP = 0.118) (χ2 = 2.034; 1 dF; p=0.154) (Table 1).

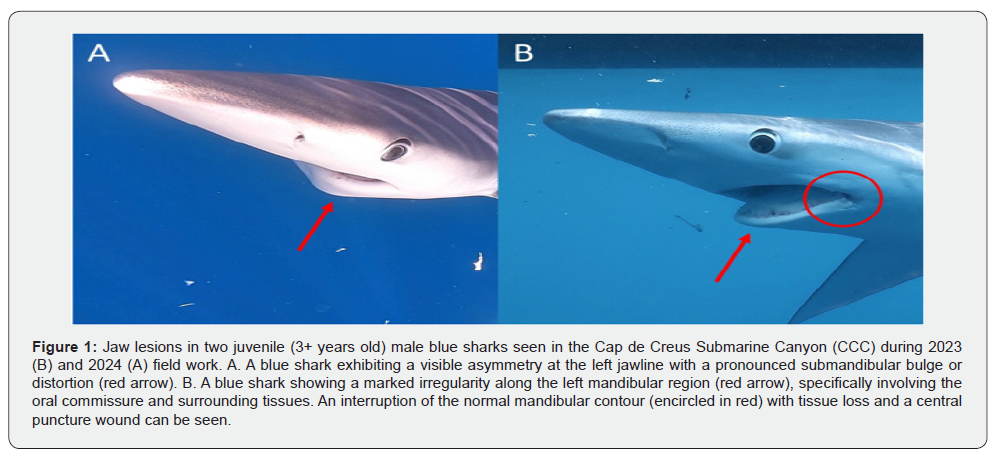
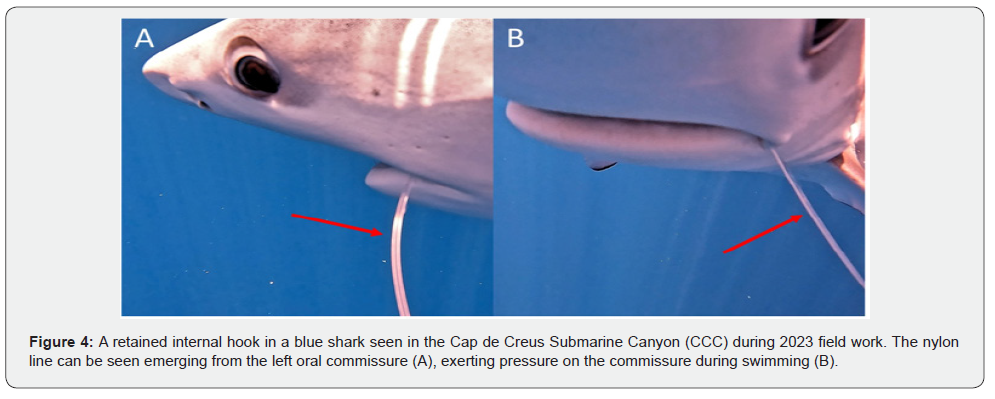
In general, the prevalence of the different types of SL ranged from 0.008 to 0.017. Especially important was the fact that some of these lesions previously considered as rare or infrequent, were observed in different specimens and/or in both years. In both 2023 and 2024 we found sharks with lesions in the jaw compatible with mandible dislocation (Figure 1), and in the eyes (Figure 2). Particularly, one of the last affected sharks exhibited a persistent protrusion of the nictitating membrane, consistent with trauma-induced dysfunction (Figure 2); and the other one showed scleral discoloration, corneal hyperpigmentation, euriblepharon and exophtalmos. In 2023 we found two sharks with fibronecrotic injuries (Figure 3), and in 2024 we detected sharks with no visible external hook but presenting nylon lines merging from the buccal cavity (Figure 4), suggesting an internal hook retention. One of the last specimens also exhibited neurological signs, consistent with ataxia, uncoordinated swimming movements, and loss of buoyancy.
When considering only the sharks affected by retained hooks (n=24), prevalence raised considerably. Nearly one out of three affected-by-hooks sharks presented a severe lesion (SLAHP = 0.333). The prevalence of sharks exhibiting severe lesions was higher in 2024 (SLAHP = 0.571) compared to 2023 (SLAHP =0.235), but differences between both years were not statistically significant (χ2 = 1.2353, with Yates correction factor; 1 dF; p > 0.05) (Table 2). No significant differences in the presence of SL were observed between males and females (χ2 = 2.524; 1 dF; p = 0.112), nor between juveniles and non-juveniles (including preadults 4+ and adults 5+) (χ2 = 0.010; 1 dF; p = 0.920).



Furthermore, SL were found significantly more frequently in specimens presenting retained hooks or evidence of having had them, than those that had not been previously captured using hooks (χ2 = 34.662, with Yates correction factor; 1 dF; p < 0.0001). In fact, all specimens presenting a SL had or had evidence of having a hook previously, while none of the blue sharks without retained hooks or signs of having had them showed evidence of a SL.
Discussion
Our study confirmed that the high prevalence of RH previously reported [12] persists over time and revealed that approximately a quarter of the total blue sharks (Prionacea glauca) surveyed in the NW Mediterranean population exhibit signs of RH or associated lesions. Among the affected individuals by RH, around 33.3% presented visible severe lesions. Notably, all individuals exhibiting severe lesions also showed clear evidence of prior interaction with fishing gear (bycatch events). These findings indicate that fishing gear-related trauma may be more common and detrimental to sharks than previously recognized.
We reported two individuals with mandible dislocation, most likely related to a retained hook due to the presence of a circular perforation in the same oral commissure. In sharks, proper feeding mechanics are dependent on complex jaw kinematics. For instance, in carcharhiniforms such as P. glauca, feeding involves a kinetic suspension system in which the hyomandibulae are posteriorly oriented and the palatoquadrate articulates with the neurocranium via flexible ethmopalatine ligaments [18,19]. During prey capture, the hyomandibulae swing laterally, rotating the upper jaw ventrally and outward to enable a gouging bite, often accompanied by vigorous lateral head movements [20,21]. This mechanism allows these predators to excise tissue from prey too large to be fully engulfed, a strategy that is ecologically essential given their pelagic lifestyle and prey types. Therefore, any type of injury that compromises the functional integrity of the jaw apparatus impairs the shark’s ability to capture and process prey. Given the dependence of pelagic sharks on active predation for survival, such lesions may lead to chronic undernourishment and potentially fatal declines in body condition, particularly in juveniles or subadults, which have high energetic demands [4,6].
Periocular injuries are frequently observed in wild sharks and may result from intraspecific aggression, predator-prey interactions, or anthropogenic sources such as propellers [22]. In our study, we observed two blue sharks with eye lesions. Notably, one individual exhibited a persistent protrusion of the third eyelid (nictitating membrane), along with a nylon line emerging from the same side of the buccal cavity, suggesting a potential link between the ocular trauma and an internal retained hook. In P. glauca, the third eyelid is highly developed and composed of dense connective tissue covered by placoid scales [23]. This nictitating membrane plays a critical role in protecting the eye, particularly during feeding events [22]. Its movement is closely coordinated with jaw opening and relies on proper function of the levator palpebrae nictitans muscle, which is innervated by the oculomotor nerve (cranial nerve III). Traumatic injuries, such as those caused by internal hooks, can damage this nerve, leading to dysfunction of the third eyelid mechanism and for example, lead to prolonged corneal exposure [24]. This may directly affect the shark’s ability to detect and capture prey. In fact, experimental studies have shown that vision loss in sharks can lead to significant behavioral changes and reduced foraging efficiency [25]. As vision plays a critical role in prey detection, any damage to the visual system may have substantial ecological and fitness consequences [26,27]. Although we also observed other ocular lesions, such as permanent exophthalmos and corneal discoloration, their impact on visual functionality remains even less understood. More research is needed to understand the structure and function of the third eyelid and vision in sharks, and the role of these in their ecological and behavioral patterns.
We described two cases of skin lesions in the sharks’ cheeck associated with retained fishing hooks, characterized by granulation tissue, fibrosis and necrosis. In both individuals, either the hooks themselves or residual perforations suggestive of previous hook presence were surrounded by extensive fibronecrotic tissue. Open wounds located near the mouth or other anatomically mobile regions may impair normal function and hinder tissue regeneration due to persistent mechanical stress. This, in turn, can facilitate secondary infections and perpetuate chronic inflammation [9]. What initially presents as a localized infection may subsequently progress to a systemic condition, ultimately compromising the animal’s health and survival [27].
Fishing hooks can also cause significant harm, even when there is no direct visual evidence of their presence. For instance, the emergence of nylon fishing lines from the oral cavity is indicative of hook ingestion, with the hook likely lodged in internal tissues or anatomical structures, potentially resulting in severe internal injuries [7,9]. In our study, we identified two sharks exhibiting external nylon lines protruding from the mouth. One of these individuals also showed signs compatible with neurological impairment, including loss of buoyancy control and erratic swimming behavior. Given the high prevalence of visible hook-associated injuries observed in our sharks, it is likely that the actual incidence is underestimated. This may be due to the presence of hooks without external indicators (e.g., detached from visible lines) that remain undetected, as well as the fact that severe internal injuries may compromise survival, leading to mortality before the affected individuals can be observed or recorded [28]. However, these factors have barely been explored in shark species.
Our findings suggest that the impact of RH on blue sharks and potentially other shark species in the Mediterranean may be greater than previously assumed [13], underscoring the urgency of implementing affective bycatch mitigation strategies. These impacts remain poorly documented and are therefore likely underestimated. A variety of bycatch mitigation strategies have been proposed, including modifications to bait type and gear configuration (e.g., use of nylon leaders, large circle hooks, deeper longline settings), as well as improved handling and release practices such as in-water release and reduced manipulation times [29,30]. While some of these methods have shown promising results in reducing shark bycatch and improving post-release survival, their effectiveness varies by species and fishery [31]. Further investigation, including capture–mark–recapture and telemetry studies, is needed to assess the long-term impacts and true efficacy of these strategies. An additional complementary approach involves the establishment of spatial management measures, such as marine protected areas or temporal fishing closures in known shark aggregation or reproductive zones, such as the CCC for blue sharks in the NW Mediterranean Sea [13]. Evidence suggests that despite the wide-ranging movements of many shark species, spatial protection can be effective for certain species such as the grey nurse shark (Carcharias taurus) [28]. Nonetheless, a deeper understanding of the practical and socio-economic barriers that limit the adoption of these technically validated mitigation strategies is required. Therefore, research (both scientific and economic) is crucial to evaluate the cost–benefit trade-offs of adopting alternative fishing gears and practices. This must include assessing potential changes in target and non-target species captures, initial and ongoing costs of this alternative fishing gear, fisherman extra work requirements, and operational safety concerns associated with gear modifications and shark handling protocols.
Conclusion
Although sharks may initially survive capture and release events during fisheries, the long-term consequences of these interactions remain a significant concern for the conservation and management of these species. Consequences of bycatch may lead to shark starvation, impaired growth, and decreased fitness, ultimately affecting individual survival and reproductive success. In species already under severe demographic pressure, such as P. glauca, classified as Critically Endangered in the Mediterranean by the IUCN [3], these sublethal but debilitating injuries represent a serious conservation concern. However, gaps remain in understanding post-release survival and the long-term impact of retained fishing gear on sharks. Addressing these issues will require targeted capture-mark-recapture and tagging studies, alongside the development and testing of bycatch mitigation strategies.
Acknowledgement
We appreciate the help and collaboration of Elisenda Giró Para, Mireia Riera Giró, Francisco Fernández Rivera, Francesc Carbonell, Elisabeth Salvador, Marc Ruiz-Sagalés, Ana Maria Abril Duro, and all participants of the Cap de Creus Experience programme.
References
- Aslaksen MA, Romarheim OH, Storebakken T, Skrede A (2006) Evaluation of content and digestibility of disulfide bonds and free thiols in unextruded and extruded diets containing fish meal and soybean protein sources. Animal Feed Science and Technology 128(3-4): 320-330.
- Venou B, Alexis MN, Fountoulaki E, Haralabous J (2009) Performance factors, body composition and digestion characteristics of gilthead sea bream, Sparus aurata fed pelleted or extruded diets. Aquaculture Nutrition 15: 390-401.
- Ma F, Li XQ, Li BA, Leng XJ (2015) Effects of extruded and pelleted diets with differing protein levels on growth and nutrient retention of tilapia (Oreochromis niloticus × O. aureus). Aquaculture International 23: 1341-1356.
- Shi Z, Li XQ, Chowdhury MAK, Chen JN, Leng XJ (2016) Effects of protease supplementation in low fish meal pelleted and extruded diets on growth, nutrient retention and digestibility of gibel carp, Carassius auratus gibelio. Aquaculture 460: 37-44.
- Satoh S, Takanezawa M, Akimoto A, Kiron V, Watanabe T (2002) Changes of phosphorus absorption from several feed ingredients in rainbow trout during growing stages and effect of extrusion of soybean meal. Fisheries Science 68(2): 325-331.
- Drew MD, Borgeson TL, Thiessen DL (2007) A review of processing of feed ingredients to enhance diet digestibility in finfish. Animal Feed Science and Technology 138(2): 118-136.
- Adamidou S, Nengas I, Henry M, Grigorakis K, Rigos G, et al. (2009) Growth, feed utilization, health and organoleptic characteristics of European seabass (Dicentrarchus labrax) fed extruded diets including low and high levels of three different legumes. Aquaculture 293(3-4): 263-271.
- Kondo F, Iwai T, Miura C, Sakata J, Ohta T, et al. (2016) Analysis of feeding effects of EP on growth and digestion in cultured bluefin tuna. Nippon Suisan Gakkaishi 82: 923-933.
- Barrows FT, Stone DAD, Hardy RW (2007) The effects of extrusion conditions on the nutritional value of soybean meal for rainbow trout (Oncorhynchus mykiss). Aquaculture 265: 244-252.
- Jover M, Gómez AG, Tomás A, De la Gándara F, Pérez L (1999) Growth of Mediterranean yellowtail (Seriola dumerilii) fed extruded diets containing different levels of protein and lipid. Aquaculture 179: 25-33.
- Takakuwa F, Fukada H, Hosokawa H, Masumoto T (2006) Optimum digestible protein and energy levels and ratio for greater amberjack Seriola dumerili (Risso) fingerling. Aquaculture. Research 37: 1532-1539.
- Thompson BA, Beasley M, Wilson CA (1999) Age distribution and growth of greater amberjack, Seriola dumerili from the north-Central Gulf of Mexico. Fisheries Bulletin 97: 362-371.
- Sato H, Yokoyama S, Yu Y, Koshio S, Ohno T, et al. (2016) Growth performance of juvenile amberjack Seriola dumerili fed a low-fish meal diet under cage culture conditions. Aquaculture Science 64: 1-12.
- Marino G, Mandich A, Massari A, Andaloro F, Porrello S, et al. (1995) Aspects of reproductive biology of the Mediterranean amberjack (Seriola dumerili Risso) during the spawning period. Journal of Applied Ichthyology 11: 9-24.
- Mazzola M, Favaloro E, Sarà G (2000) Cultivation of the Mediterranean amberjack, Seriola dumerili (Risso, 1810), in submerged cages in the Western Mediterranean Sea. Aquaculture 181(3-4): 257-268.
- Nakada M (2002) Yellowtail culture development and solutions for the future. Reviews in Fisheries Science 10: 559-575.
- Sicuro B, Luzzana U (2016) The state of Seriola spp. other than yellowtail (S. quinqueradiata) farming in the world. Reviews in Fisheries Science & Aquaculture 24(4): 314-325.
- Ohta M, Watanabe T (1996) Energy requirements for maintenance of bodyweight and activity, and for maximum growth in rainbow trout. Fisheries Science 62: 737-744.
- Satoh K, Hitaka E, Kimoto K (2000) Effect of water temperature on the protein digestibility of formula feed and mainly-raw-fish diet of young yellow tail. Nippon Suisan Gakkaishi 66: 243-248.
- Watanabe K, Aoki H, Hara Y, Ikeda Y, Yamagata Y, et al. (1998) Energy and protein requirements of yellowtail: a winter-based assessment at the optimum feeding frequency. Fisheries Science 64: 744-752.
- Rodehutscord M, Pfeffer E (1995) Effects of supplemental microbial phytase on phosphorus digestibility and utilization in rainbow trout (Oncorhynchus mykiss). Water Science & Technology 31(10): 143-147.
- Jackson LS, Li MH, Robinson EH (1996) Use of microbial phytase in channel catfish Ictalurus punctatus diets to improve utilization of phytate phosphorus. Journal of World Aquaculture Society 27: 309-313.
- Refstie S, Svihus B, Shearer KD, Storebakken T (1999) Nutrient digestibility in Atlantic salmon and broiler chickens related to viscosity and non-starch polysaccharide content in different soyabean products. Animal Feed Science & Technology 79(4): 331-345.
- Ali Zamini A, Kanani HG, Azam Esmaeili A, Ramezani S, Zoriezahra SJ (2014) Effects of two dietary exogenous multi-enzyme supplementation, Natuzyme and betamannanase (Hemicell), on growth and blood parameters of Caspian salmon (Salmo trutta caspius). Comparative Clinical Pathology 23: 187-192.
- Farhangi M, Carter CG (2007) Effect of enzyme supplementation to dehulled lupinbased diets on growth, feed efficiency, nutrient digestibility and carcass composition of rainbow trout, Oncorhynchus mykiss (Walbaum). Aquaculture. Research 38: 1274-1282.
- Satoh K, Sanada Y, Hitaka E, Kimoto K (2002) Dietary Effects of the Protease Treatments of Fish Meal on the Growth, Feed Efficiency, and Protein Digestibility of Yellowtail During Low Temperature Season. Aquaculture Science 50: 219-226.
- Angel CR, Saylor W, Viera SL, Ward N (2011) Effects of a monocomponent protease on performance and protein utilization in 7- to 22-day-old broiler chickens. Poultry Science 90(10): 2281-2286.
- Ding XM, Li DD, Li ZR, Wang JP, Zeng OF, et al. (2016) Effects of dietary crude protein levels and exogenous protease on performance, nutrient digestibility, trypsin activity and intestinal morphology in broilers. Livestock Science 192: 26-31.
- Liu SY, Selle PH, Court SG, Cowieson AD (2013) Protease supplementation of sorghum-based broiler diets enhances amino acid digestibility coefficients in four small intestinal sites and accelerates their rates of digestion. Animal Feed Science & Technology 183(3-4): 175-183.
- Upadhaya SD, Yun HM, Kim IH (2016) Influence of low or high density corn and soybean meal-based diets and protease supplementation on growth performance, apparent digestibility, blood characteristics and noxious gas emission of finishing pigs. Animal Feed Science & Technology 261: 281-287.
- Yu G, Chen D, Yu B, He J, Zheng P, et al. (2016) Coated protease increases ileal digestibility of protein and amino acids in weaned piglets. Animal Feed Science & Technology 214: 142-147.
- Lowly OH, Rosebrough NJ, Fan AC, Rondall RT (1951) Protein measurement with the folin phenol solution. Journal of Biological Chemistry 193(1): 265-275.
- Chandrasekaran S, Kumaresan SSP, Manavalan M (2015) Production and Optimization of Protease by Filamentous Fungus Isolated from Paddy Soil in Thiruvarur District Tamilnadu. Journal of Applied Biology and Biotechnology 3(6): 66-69.
- Morimura S, Kida K, Sonada Y (1994) Production of protease using waste water from the manufacture of Shochu. Journal of Fermentation and Bioengineering 77(2): 183-187.
- Kumar AG, Nagesh N, Prabhakar TG, Sekharan G (2008) Purification of extracellular acid protease and analysis of fermentation metabolites by Synergistes sp. utilizing proteinaceous solid waste from tanneries. Bioresoure Technology 99(7): 2364-2372.
- Li P, Mai K, Trushenski J, Wu G (2009) New developments in fish amino acid nutrition: towards functional and environmentally oriented aquafeeds. Amino acids 37(1): 43-53.
- Wright PA, Steele SL, Huitema A, Bernier NJ (2007) Induction of four glutamine synthetase genes in brain of rainbow trout in response to elevated environmental ammonia. Journal of Experimental Biology 210(16): 2905-2911.
- Park GS, Takeuchi T, Yokoyama M, Seikai T (2002) Optimal dietary taurine level for growth of juvenile Japanese flounder Paralichthys olivaceus. Fisheries Science 68(4): 824-829.
- Kim SK, Takeuchi T, Yokoyama M, Murata Y (2003) Effect of dietary supplementation with taurine, β-alanine and GABA on the growth of juvenile and fingerling Japanese flounder Paralichthys olivaceus. Fisheries. Science 69(2): 242-248.
- Salze GP, Davis DA (2015) Taurine: a critical nutrient for future fish feeds. Aquaculture 437: 215-229.
- Huxtable RJ (1992) Physiological action of taurine. Physiological Reviews 72: 101-163.
- Goto T, Takagi S, Ichiki T, Sakai T, Endo M, et al. (2001) Studies on the green liver in cultured red sea bream fed low level and non-fish meal diets: Relationship between hepatic taurine and biliverdin levels. Fisheries Science 67(1): 58-63.
- Kuzmina VV, Gavrovskaya LK, Ryzhova OV (2010) Taurine. Effect on exotrophia and metabolism in mammals and fish. Journal of Evolutionary Biochemistry and Physiology 46(1): 19-27.
- Sariha S, Djellata A, Roo J, Cruza CMH, Fontanillas R, et al. (2019) Effects of increased protein, histidine and taurine dietary levels on egg quality of greater amberjack (Seriola dumerili, Risso, 1810) Aquaculture 499: 7.2-79.
- Matsunari H, Hashimoto H, Iwasaki T, Oda K, Masuda Y, et al. (2013) Effect of feeding rotifers enriched with taurine on the growth and survival of larval amberjack Seriola dumerili. Fisheries Science 79: 815-821.
- Matsunari H, Takeuchi T, Takahashi M, Mushiake K (2005) Effect of dietary taurine supplementation on growth performance of yellowtail juveniles Seriola quinqueradiata. Fish Sci 71: 1131-1135.
- Wu GY, Morris SM (1998) Arginine metabolism: nitric oxide and beyond. Biochemistry Journal 336: 1-17.
- Almeida B, Buttner S, Ohlmeier S, Silva A, Mesquita A, et al. (2007) NO-mediated apoptosis in yeast. Journal of Cell Science 120(18): 3279-3288.
- Amber IJ, Hibbs Jr JB, Parker CJ, Johnson BB, Taintor RR, et al. (1991) Activated macrophage conditioned medium: Identification of the soluble factors inducing cytotoxicity and the l-arginine dependent effector mechanism. Journal of Leukocyte Biology 49(6): 610-620.
- Tsai HJ, Shang HF, Yeh CL, Yeh SL (2002) Effects of arginine supplementation on antioxidant enzyme activity and macrophage response in burned mice. Burns 28(3): 258-263.
- Appleton (2002) Arginine: clinical potential of a semi-essential amino acid. Alternative Medicine Review 7: 512-522.
- Pohlenz C, Buentello A, Criscitiello M, Mwangi W, Smith R, et al. (2012) Synergies between vaccination and dietary arginine and glutamine supplementation improve the immune response of channel catfish against Edwardsiella ictaluri. Fish & Shellfish Immunology 33(3): 543-551.
- Pohlenz C, Buentello A, Helland S, Gatlin D (2014) Effects of dietary arginine supplementation on growth, protein optimization and innate immune response of channel catfish Ictalurus punctatus (Rafinesque 1818). Aquaculture Research 45: 491-500.
- Ruchimat T, Masumoto T, Itoh Y, Shimeno S (1998) Quantitative Arginine Requirement of Juvenile Yellowtail Serbia quinqueradiata. Fisheries Science 64(2): 348-349.






























