Abstract
Among other interesting aspects, this paper presents an alternative approach to larviculture and commercial-scale fingerlings production of commercially important diadromous fish in rural ponds. This approach involves manipulating the natural succession of zooplankton components. This approach allows fish larvae to be offered, in a first phase, on a large scale and at low cost, copepod nauplii (of the appropriate size and with the best bromatological profile), as well as rotifers and other microorganisms (fish larvae benefit by strategically integrating into the food chain that develops in culture ponds). This approach is effective for species with very high fertility, such as marine fish, facilitating the management of enormous quantities of larvae and, importantly, the mass production of fingerlings.
Keywords:Coupled spawning-incubation-hatching system; Extensive larval culture; Manipulation of zooplankton succession in ponds
Introduction
Regarding spawning, fertilization, and hatching, there are technological options that can be used with marine fish. These include coupled spawning and incubation systems for highly fecund pelagic egg-laying species (such as the commercially valuable diadromous marine fish species of the Mexican coast), which allow for automatic egg harvesting and large-scale production of feeding-stage larvae [1].
Likewise, with regard to the larval stage, based on the results achieved in other countries, such as with the Indo-Pacific snook (Lates calcarifer), it would be advisable to test alternative protocols for larval culture and rearing in mesocosms , in rustic ponds, in which the sowing of larvae in the feeding stage (approximately 48 hours after hatching) is coupled with the succession of zooplankton, which occurs naturally in ponds specially prepared for this purpose. In these protocols applied in Australia with L. calcarifer, survival rates ranging from 20 to 40% are reported, under stocking densities of 900,000 larvae/ha, with an additional advantage: a faster growth in larvae is presented with respect to that achieved in intensive systems of high control in small containers [2,3].
Based on experiences in larval culture and fingerlings in rustic ponds with other species [4], the natural succession of zooplankton is manipulated by applying a chemical control agent that acts selectively on aquatic arthropods, thereby achieving a greater abundance of rotifers at the time of larval stocking, ensuring higher survival rates. This option has not been applied to marine fish in Mexico to date, which represents an opportunity.
In the future, it will be useful to conduct a comparative assessment of the effectiveness of production routes (intensive/extensive) for larval culture, in terms of productivity and production costs, which will allow us to determine the best production option.
Coupled Spawning and Incubation Systems
In the controlled production of fry or juveniles on a commercial scale for fattening, this condition represents a major advantage. By applying appropriate management protocols in larval reproduction and culture, it is possible to produce enormous quantities of larvae. Taking advantage of this attribute of highly fecundable fish species, advanced spawning-incubation-hatching systems (Coupled Spawning and Incubation Systems with automatic egg harvesting) were developed in China (the world’s leading aquaculture power) since the late 1970s. Designed specifically for the spawning and mass production of pelagic egg-laying carp larvae (grass, silver, bighead, and black carp), these systems allow for the large-scale production of feeding-stage larvae, with potential for application to marine fish [1].
The model consists of a circular spawning pool with a 1.5 m operating water depth, connected to circulating channel incubation devices (known as Chinese incubators) (Figure 1) by means of a 3” diameter tube that starts from the center of the spawning area and is inserted into the basal part of the incubation chambers (Figure 2). The water supply to the spawning ground is through a 2” pipe that is arranged in a tangential direction in the perimeter wall, which determines the circular movement of the water (Figure 3).

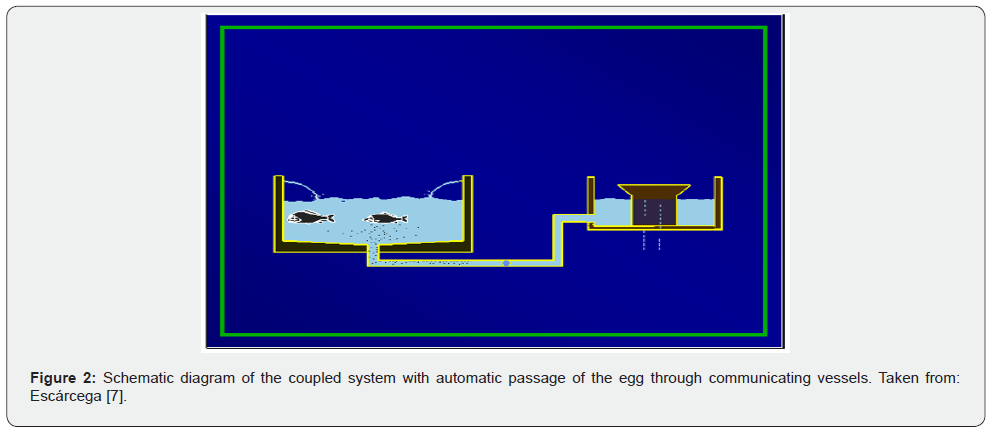
The system’s principle is that both spawning and incubation reproduce the movement conditions in the water column that occur in the natural habitat of the species considered during reproduction. In the case of spawning, the (circular) water current, combined with a high concentration of dissolved oxygen, favors the triggering of ovulation and causes the egg to quickly cluster in the center of the spawning container (Figure 4) [7].
Both the spawning pit and the incubator(s) are maintained at the same operating level in the water column (using the communicating vessel principle), because the water supply to the spawning pit is discharged at the incubator’s discharge level. In this way, as the eggs are released and fertilized, they gradually and automatically move to the incubation chamber, with gentle traction that prevents any damage (Figure 2). It is worth noting that for marine fish such as those in question, with floating viable eggs, a PVC tube (interchangeable) can be inserted into the central outlet duct of the spawning pit, so that the upper part of the tube remains about 10 cm below the operating water level, to conduct the eggs accumulated at the surface level to the incubator of choice.
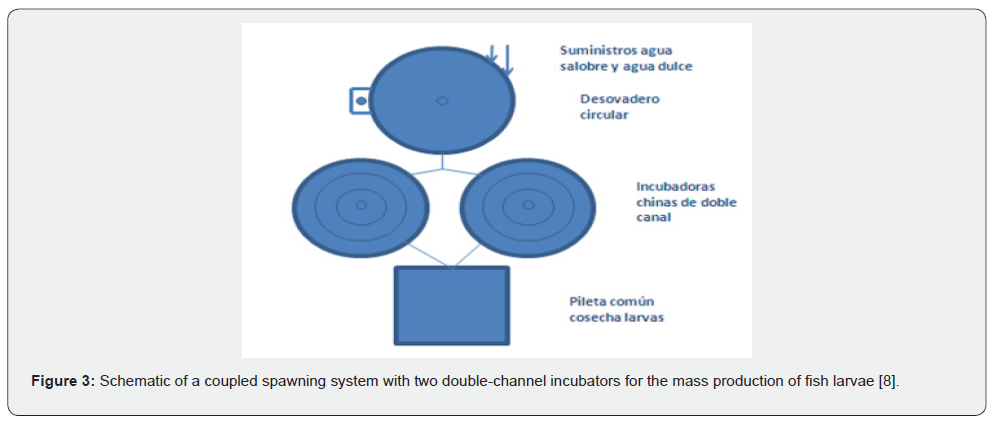

Under the operating principle described above, for largescale egg production, such as that expected with these species, multi-channel incubators can be coupled to a spawning pit, in which water flow and egg delivery are controlled by registers with control valves. This could be highly practical for spawning events with marine fish, which typically occur over two to four consecutive nights (in natural spawning and when hormonal implants are used to induce spawning). In this way, the egg can be channeled in batches to a specific incubation channel to achieve homogeneous batches of larval hatches, greatly optimizing the process (Figure 3) [8].
Background of its Application in Mexico
The application of the Coupled System in Mexico was promoted by the undersigned from the Secretariat of Fisheries and SEMARNAP, since 1994. Given the success achieved in its use at the El Peaje Aquaculture Center, SLP (1993) in the reproduction of grass carp, its application was promoted in the Aquaculture Centers of Tezontepec de Aldama, Hgo. (1999), Zacapu, Mich. (1999) and La Rosa, Coah. (2000), four of the most important federal Aquaculture Centers in the production of carp fry. In Mexico, it has proven to be a very effective system in terms of productivity (viable fry at the feeding stage/kg of weight in females) in the controlled reproduction of pelagic egg fish with high biological potential.
The average efficiency of this system achieved in the CA El Peaje was 69% hatching, in the spawning of the grass carp (Ctenopharingodon idellus), with levels reaching up to 78%, based on the number of eggs released. The average yield, in terms of viable fry/kg of weight In females, it was 62,000 units, with maximum values reaching up to 72,000 fry/kg [1]. Based on these indicators (particularly hatching), with the fertility and biological potential of the females of many species of diadromous marine fish such as those we are concerned with, it is easy to infer the enormous quantities of fry that could be achieved with this system. Details about its operation and results achieved in its application can be found in: Escárcega-Rodríguez [1] and FAO [5].
Extensive Larval Culture. Coupling with Zooplankton Succession in Ponds
The possibility of producing feeding-stage larvae on a mass scale also requires the development and application of larviculture and rearing technologies that can handle the enormous numbers of larvae that can be produced with marine fish, ensuring the supply of fry and juveniles required for commercial grow-out systems.
To date, this phase of larval culture with marine fish in Mexico is in the commercial pilot stage, using highly technical and controlled culture schemes in recirculating systems (RAS), with the parallel controlled production of live feed. The results achieved thus far, while encouraging and of enormous value given the knowledge generated, are still far from being able to absorb the large quantities of larvae that can be achieved in marine fish reproduction.
As mentioned in the previous section, for decades in China,production systems such as the Coupled Spawning and Incubation System have been developed, which have allowed to respond more than adequately to the vast biological potential presented by various species of fish of food and commercial importance, to optimize spawning, incubation and larval production on a large scale, but also with schemes for larval culture in open systems (rustic ponds) that allow the management of large quantities of larvae, with efficiency margins that reach up to 60% survival, for the larval stage in the feeding stage to fry of 3 cm TL.
In the case of marine fish, it has been mentioned that in countries such as Thailand and Australia this alternative is successfully managed for the mass production of Indo-Pacific sea bass (L. calcarifer) fry. The key to the success of this alternative lies in precisely matching the preparation of the ponds for larval culture with the timing of the larvae’s feeding stage (the intersection of two productive pathways), 48 hours after hatching, when the larvae have exhausted their yolk reserves and are ready to feed. In short, the goal is to ensure that at this time the pond has the greatest abundance of rotifers (and copepod nauplii) and the lowest proportion of adult copepods and other zooplankton components that may initially be predators or food competitors of the stocked fish larvae.
Fundamentals of the model
When earthen ponds are used for large-scale production of fish fry, they are integrated from the outset (as larvae) into the food chain established in them (Figure 5).
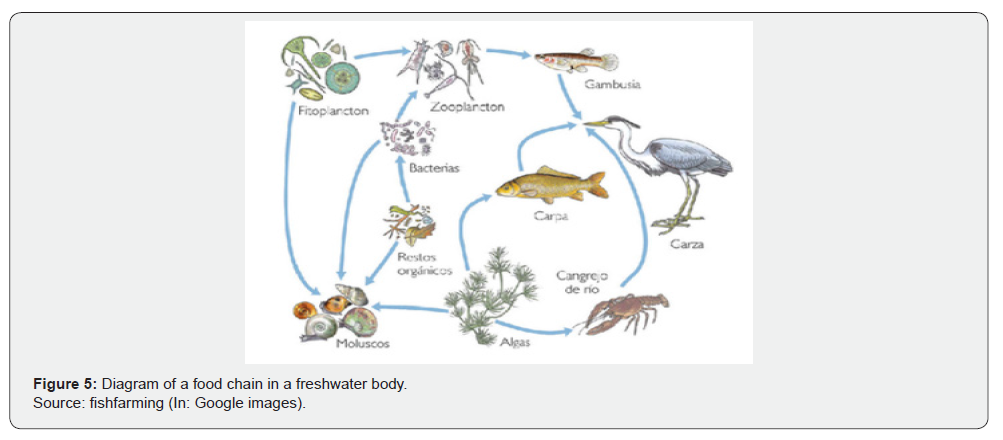
Photosynthesis contributes to the production of plant organic matter in ponds. Phytoplankton serves as the basis for zooplankton development. Thus, fry feed voraciously on zooplankton, selecting individuals whose size fits within their mouths, which are initially rotifers and copepod nauplii (Figure 6). Similarly, some predators feed on fish larvae, and losses can be high if proper management is not applied.
Determining environmental factors
The main environmental factors to consider in the production of marine fish fry during the first stage of fry rearing in ponds are:
a) Water temperature- The appropriate range for the development of tropical marine fish is 25 a (29°Cwith an optimum of 26 a28 °C, in the case of sea bass).
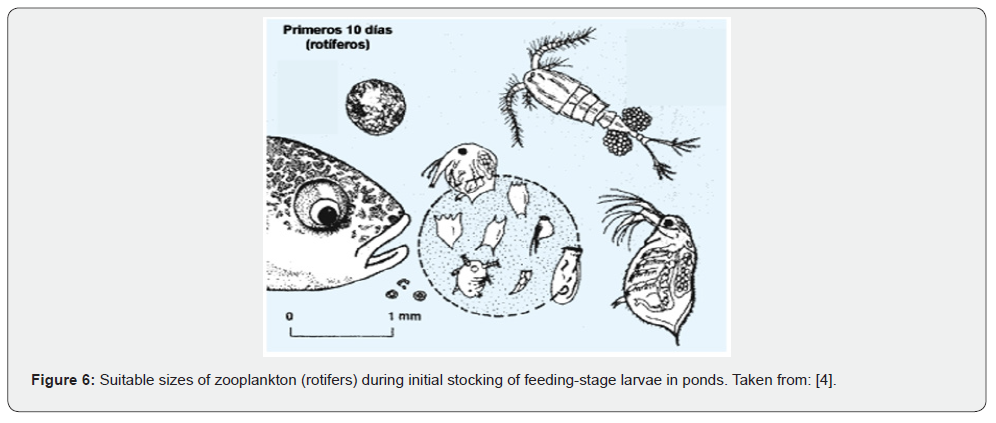
b) The quality and availability of live food- The first fry (larvae in the feeding stage) feed on zooplankton of sizes preferably ≤ 0.08 mm (some rotifers), which fit the size of their mouth.
c) Dissolved oxygen content - which must be at least 5 mg/L.
d) The presence of predators of larvae and fry - such as insect larvae and large copepods, which can cause large losses in this initial fry phase (in virtually all fish species).
e) Weather conditions: Changes in atmospheric pressure, sudden temperature changes and strong winds can significantly reduce survival.
Preparing the pond for receiving the larvae
The most important element for achieving the best results is the proper preparation of the ponds before filling them, including their fertilization:
a) fry ponds is prepared (preferably 0.01 a1.0 ha in area).
b) They are filled to half their capacity, preventing the entry of fish and other wildlife.
c) Selective chemical treatment is applied to the water to eliminate cladocerans, copepods, and other aquatic arthropods, and to facilitate good proliferation of rotifers (the chemical agent is Crustabay ®, which does not affect the latter).
d) pre-fry or larvae in the feeding stage (two days after hatching) are stocked in the ponds.
e) Seven days after stocking, supplementary feed is applied to the fry (this can even be done earlier), larger zooplankton is introduced into the ponds, and the ponds are fully filled.
f) After 21 a30 days from the introduction of the pre-fry, the fry (3 cm) can be obtained and then transported (if desired) to the second fry ponds to reach the desired size, depending on their destination (floating cages, grow-out ponds or for restocking purposes). If the destination is grow-out, the fry 3 cm can be transferred directly to the grow-out ponds to avoid further handling (initial stocking reference of 1 a1.5 fry/m2).
Ponds for this first stage of fry rearing are relatively small and are recommended to be from 100 to 10,000 m21.0 0.01 aha, with an average depth of 1.0 m. A good water supply is required, along with a regularly sloping bottom to ensure complete drainage and harvesting, and the availability of a structure for drainage and water level control (monks with two rows of planks filled with clay are suitable).
First-time fry ponds must undergo a drying period and expose their stock to sunlight (Figure 7). To begin a new fry cycle, they must be prepared with great care:
a) All vegetation is removed from the bottom and burned.
b) Quicklime (dry) is applied homogeneously to the bottom of the pond at a rate of 150 kg/ha (or 1 ton/ha of hydrated lime) to sterilize it and improve the soil structure (Figure 8).
c) organic fertilizer (cattle or pig manure, for example) is spread evenly over the pond template at a rate of 3-5 ton/ha to ensure good development of natural organisms for feeding the fry (Figure 9).
d) Fill the pond to half its capacity, preventing the entry of predatory fish and other unwanted organisms by using fine mesh materials or placing a filter box in the water supply duct or channel.
e) In small ponds (up to 400 m2), inorganic fertilization is done from the shore in two phases:
i. While the pond is being filled, urea (43% nitrogen in the form of nitrates) is added near the water inlet at a rate of 150 kg/ ha. The fertilizer will gradually dissolve and spread throughout the pond.


ii. Once the pond is half full, superphosphate (18% active ingredient) is added at a rate of 100 kg/ha to the edges of the pond.
In the case of alkaline protease, the total amino acid content in the TCA-soluble fractionf) In larger ponds, both fertilizers are mixed and applied from a boat over the entire surface of the water.
Importance of managing the natural succession of zooplankton
Thanks to fertilization, the various components of phytoplankton and zooplankton develop actively, and their relative abundance changes over time. First, the rotifer population develops until it reaches its maximum abundance. Then, small cladocerans begin to proliferate, followed by larger cladocerans and copepods, which then become predominant. This is the natural process of succession of the main components of zooplankton in a pond, provided that they are not chemically treated to selectively eliminate certain organisms.
If we compare the size of the mouthparts of fish larvae with small eggs (such as those of diadromous fish) with the size of various types of zooplankton, it is clear that the larvae’s primary food source is small rotifers and copepod nauplii. During this first phase, rotifers are therefore the most suitable natural food for fish larvae, which consume them voraciously. Therefore, it is necessary to selectively eliminate the larger types of zooplankton from the outset. This will limit the feeding competition of cladocerans to the benefit of rotifers and consequently increase the latter’s survival (to the benefit of fish larvae), while also eliminating the copepods that prey on them. At the same time, the larger copepods, which are also predators of larvae, will be eliminated.
This way, the rotifer population in the pond will be larger and last longer. Furthermore, as the larvae grow, cladocerans and copepods will begin to reappear, now without harming the fry and serving as food. This is the great advantage and the key to the effectiveness of this technology.
Selective treatment
Once the pond is half- filled, it is treated with chemicals (two days after initial filling) to selectively kill cladocerans and copepods. Rotifers are not affected by this treatment. The chemical agent used is Crustabay (90% AI metrifonate), used in Mexico for shrimp farming (to prevent disease and eliminate predators and competitors when stocking post -shrimp in growth ponds). The application rate is 1.0 mg/L (1 g/m3).
To verify whether the treatment has been effective, the presence of zooplankton must be regularly checked. Cladocerans and copepods should not be found for five days after treatment, while the abundance of rotifers should increase. At each specific site, it will be necessary to identify the exact day on which rotifer abundance is greatest. The reference given here is five days after treatment application.
At a mass production level of fry, once the period in which the maximum abundance of rotifers is achieved in the pond has been identified (2 a3 ml of sedimented volume/100 L of water), it will be essential to program and couple accordingly the spawning and the production of the feeding stage larvae to achieve the best results, by stocking the larvae at the precise moment. In the first tests, fish larvae can be stocked at a rate of 140 units/m2 of pond water surface.
Process sequence
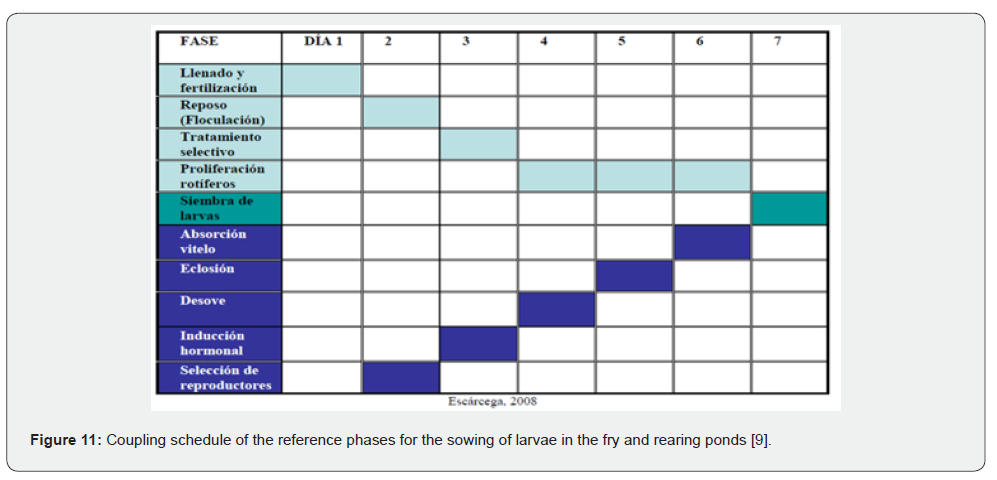
Figure 10 summarizes the sequence of stages in the larviculture and fish rearing process in ponds [9].
Phase coupling: larvae/pond preparation
Figure 11 presents an example of a coupling diagram for both phases (availability of larvae 48 hours after hatching with the time of maximum rotifer abundance) that can serve as a reference. As mentioned, at each site, for each species, the precise times to reach the indicated stages must be determined. In the case of this diagram (taking sea bass as an example), the meeting point of both routes for larval stocking would be on day 7. As can be seen, the key to the success of this production route lies above all in the technological mastery of the reproduction of the target species, which allows for the availability of batches of feeding-stage larvae safely and at the expected times.
Details on the implementation of this alternative for the mass production of offspring in rustic ponds can be found in: Escárcega [7].

References
- Escárcega RS (1996) Evaluation of a coupled spawning and breeding system incubation for the reproduction controlled of the large tent herbivore (Ctenopharyngodon idellus). Fisheries Science, Institute National Fisheries, Secretariat of Environment, Natural Resources and Fishing, Mexico 13: 87-93.
- Rutledge WP, Rimmer MA (1991) Culture of larval sea bass, Lates calcarifer (Bloch), in saltwater rearing ponds in Queensland, Australia. Asian Fisheries Science 4(3): 345-355.
- Barlow C, William K, Rimmer M (1996) Sea bass culture in Australia. INFOFISH International 2/96, p. 26-33.
- Horváth L, Tamás G, Car AG (1986) The large tent common. Part 2. Production massive of fry and fingerlings. Cabbage. FAO: Training. Organization of the Nations United for the Agriculture and the Feeding (FAO). Rome, Italy.
- FAO (1978) Freshwater fisheries and aquaculture in China. Report of the Mission FAO of Fishing (Aquaculture) to China, 21 April - 12 May 1976.
- Caballero CV (2011) Reproduction and fertility of the bass white (Centropomus undecimalis) in southwest Campeche. Fisheries Science. Institute National of Fishing. Mexico 19(1): 35-46.
- Escárcega RS (2005) The sea bass. Biotechnological advances for its breeding. AGT Editor, Mexico pp. 126.
- Escárcega RS (2018) Crop and production commercial of young of bass. PPT presentation. Course prepared for FORSUA, Higher Education in Food.
- Escárcega RS (2008) Practical guide for the cultivation of Pacific black sea bass Oriental Centropomus nigrescens (Günther, 1864). Master's degree in Limnology and Aquaculture. INIRENA-UMSNH. Document of job.
- Tucker JW (1987) Snook and tarpon snook culture and preliminary evaluation for commercial farming. The progressive Fish-Culturist 49: 49-57 (In: SEMARNAP, 1997. Minutes of the technical meetings of the national network of researchers in mariculture. Secretariat of Half Atmosphere, Resources Natural and Fishing, Mexico pp. 192-207).






























