Abstract
Fish and their products are considered an important source of food all over the world. Many countries have huge potential for the development of the fish industry as they possess coastlines. Though global fish farming has reached a substantial level, unfortunately, there is a severe threat from pathogens such as viruses, bacteria, parasites, etc. Specifically, parasites damage the fish population, which is one of the major concerns in fisheries. However, the identification of certain parasites through conventional morphological methods is difficult due to their huge number and vast morphological variations. Therefore, molecular studies will help to understand the accurate systematic position of certain fish parasites. In addition, histopathological studies of parasitized fish reveal the infection levels and severity of the disease. Physiological, biochemical and molecular aspects disclose the alteration in the behavior of the host, growth and development, including fish-parasite interaction at the genetic level. Furthermore, this review discussed the mitigation methods of parasitic diseases. Overall, this review intends to summarise the developments in fish parasite authentication, post events of fish infected with various parasites and mitigation strategies for the benefit of the fishing industry.
Keywords:Fish; Parasite; Classification; Biochemical; Molecular; Mitigation
Introduction
Fish serves as the best source of animal protein, healthy fats and nutrients. Therefore, it stands as one of the major contributors that solves the problem of food insecurity caused due to population explosion [1]. Globally, there is huge potential for the development of fisheries as it is bestowed with extensive coastlines and a huge number of rivers, canals, reservoirs, lakes and ponds [2]. Though there was a considerable increase in fish products, the industry is facing a substantial setback due to sudden disease outbreaks caused by viruses, bacteria, fungi, parasites, etc. Among the aquatic pathogens, parasites appear to be more common and pathogenic [3]. Parasites exist in all ecological systems and have evolved in a diverse way of life to ensure their perpetuation in the nutritionally abundant and immunologically hostile environment of their host [4]. Fish parasites of both fresh and marine water environments were broadly divided into protists including ciliates, flagellates, oomycota, sporozoans and metazoans consisting of myxozoans, monogeneans, trematodes, nematodes, leeches and crustaceans [5]. Metazoan parasites are more numerous, and some of them are not yet identified due to their diversified habitat and varied ecological conditions.
Both ecto and endo parasites are the source of damage in fish and cause various diseases [6]. These parasites cause mechanical injury and introduce toxic metabolic by-products into their hosts. On the other hand, they act as carriers of secondary pathogens and make the fish more susceptible to diseases, thus resulting in severe economic loss worldwide by causing mass mortalities [7]. According to national bank for agriculture and rural development (NABARD) the state of Andhra Pradesh in India produces more than 40,000 tonnes of major carps worth more than 600 million Indian rupees (INR) per year and reports a loss of about 40 million Indian rupees due to sudden disease outbreaks by the pathogens [8]. Most of the ecto and endo parasite research works is carried out in both fresh and marine water fishes with a main focus on systematic, morphological, histopathological, physiological and host-pathogen interactions [4,9-10]. In addition, both biochemical and molecular studies have been conducted to know the molecular insights of the fish-parasite interaction as well as details of damage/disease [10]. Molecular studies such as genomics, transcriptomics, proteomics and metabolomics are the key to understanding the mechanisms of fish-parasite interaction and the immune defense of the fish [11]. Though considerable progress has been achieved in parasite eradication, still unexplored facts are to be identified for enhancement in fish production. Therefore, several reviews will be helpful to acquire knowledge of both conventional and molecular mechanisms of fish parasites.
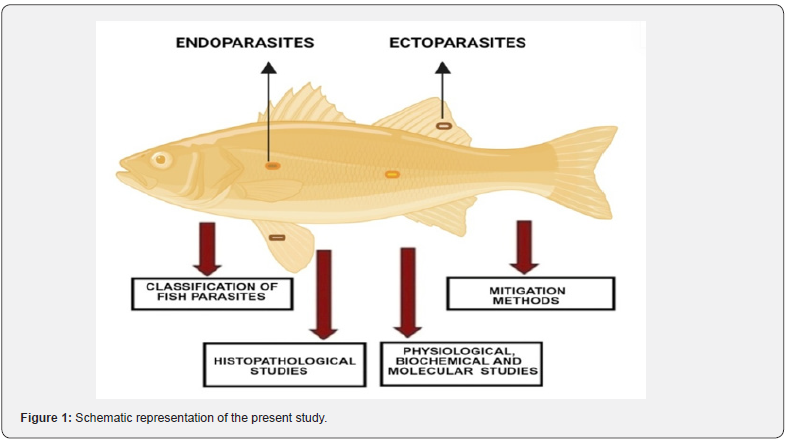
In the present review, the importance of the systematic position of parasites and post-events of fish infected with these pathogens was emphasized through histopathological, physiological, biochemical and molecular approaches. In addition, the mitigation of parasitic diseases using different methods was discussed. Figure 1 illustrates the fish-parasite aspects covered in this review. Overall, this review focuses on summarizing the existing knowledge on parasites of fishes, keeping in view the excellent prospects.
Identification and Classification of Fish Parasites
The study of parasitic diseases of fish can be achieved by identification of the parasite, thorough knowledge of its life history, host specificity and symptoms [12,13]. Parasite groups possess a complex habitat nature and live in a wide range of freshwater and marine water fishes. Identification of certain parasites is complicated due to their huge number, high morphological variation and insufficient and differences in taxonomical knowledge [13,14]. To date, most of the classifications of diverse parasites of fish have been carried out majorly using conventional morphological methods along with the help of histopathological and physiological analyses [15].
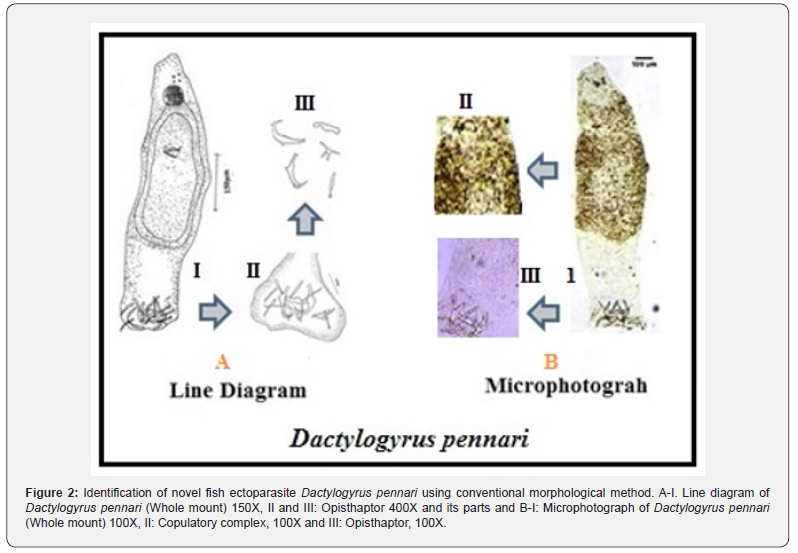
However, these morphological methods, through microscopic and microtome tools, need careful observation and expertise in classical taxonomy. With both morphological and pathological information, our laboratory at Yogi Vemana University identified a novel parasite Dactylogyrus pennari, recently, which is not always possible with all the parasites (Figure 2). Hence, molecular evidence is essential apart from classical taxonomic data for accurate identification of the diverse parasites.
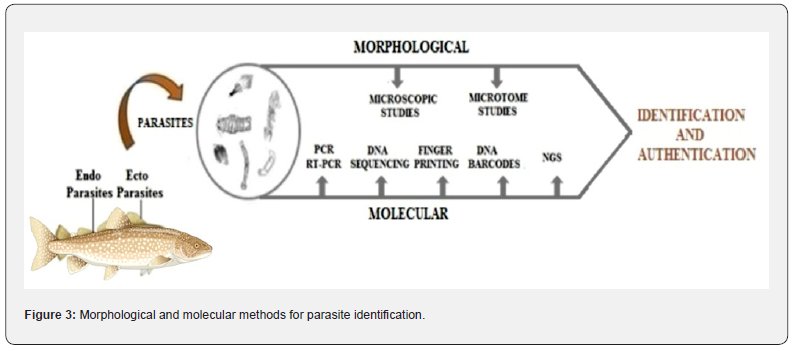
Several studies were conducted for certain genes of parasites using PCR and RT-PCR to determine their systematic position [16,17]. In addition, DNA sequencing and fingerprinting or molecular markers such as RAPD (random amplified polymorphic DNA), RFLP (restriction fragment length polymorphism) etc., techniques were applied to determine the systematic position of the species [18-20]. Specifically, sequencing and construction of a DNA barcode are the widely applied basic tools to validate the parasites of fish [21,22]. The importance and usage of universal cytochrome c oxidase subunit 1 (COI), internal transcribed spacers (ITS1 and ITS2), NADH dehydrogenase 1 (NDI), etc., markers for the identification of various parasites were emphasized by several researchers [22,23]. Figure 3 explains the morphological and molecular methods used for the identification and authentication of fish parasites.
Parasitic copepods, including Lepeophtheirus salmonis (Salmon louse) and Caligus clemensi (Sea lice), were identified in three-spine stickleback fish (Gasterosteus aculeatus) and molecular analysis were also carried out using 18S rDNA [24]. Boxshall [12] explained in detail the controversies and unresolved problems of crustacean classification. Similarly, Song et al. [25] applied molecular tools for the identification and authentication of copepods using 18S and 28S rDNA sequences. Multiple gene analyses of caligid copepods were carried out for Caligus evolution [26]. Graca et al. [22] highlighted the internal transcribed spacers 1 and 2 markers in the authentication of platyhelminth parasites in fishes along with other markers. Our laboratory recently identified the Paraorygmatobothrium floraformis parasite from the spiral intestine of elasmobranch fish using the 28S rDNA to determine the phylogenetic position [20].
At present, forensically informative nucleotide sequencing
(FINS) and environmental DNA (eDNA) are in use for the
identification and authentication of parasites [27]. Lebedeva et
al. [21] used ITS-5.8S-ITS2 nuclear ribosomal gene sequencing
for the identification of salt-tolerant Gyrodactylus species.
Muñoz-Caro et al. [23] identified Anisakis by comparing the
mitochondrial cytochrome oxidase subunit 1 gene. The Goussia
parasite of
Histopathological Studies of Fish Infected with Parasites
Fish disease caused by parasites is one of the concerns for fisheries, and the severity of the disease can also be determined through histopathological techniques. Several research groups worked on histopathological studies using parasitized fish to learn more about disease and damage patterns. Monogenean and myxosporea-infected gills of Piaractus mesopotamicus and Prochilodus lineatus were analyzed through histopathological experiments by Campos et al. [29]. Ojha & Hughes [30] noticed the branchial parasite (Ergasilus bengalensis)-induced gill damage through the histopathological technique and observed the reduction of oxygen uptake in the infected fish. Histopathological changes in the gills of several economically important food fishes were observed by the ergasilid copepod infections [31]. Feist & Longshaw [32] highlighted the importance of histopathological studies for fish-parasite interactions. Figure 4 explains the histopathological studies conducted to know the infection levels of an ectoparasite, Dactylogyrus fotedari, in Labeo calbasu in our laboratory.
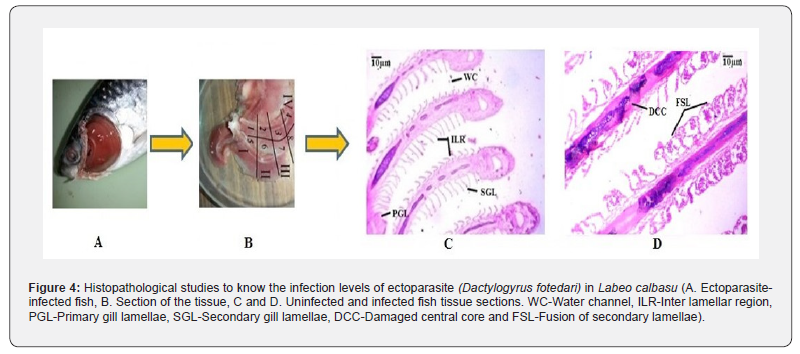
Mohammadi et al. [33] emphasized the impact of parasitic infection, which includes mechanical damage, damage of internal parts, the influence of infestation on non-specific sites and the influence on the growth of the host using histopathological technique. Infection of Tetracotyle metacercaria in the heart of freshwater spiny eel was observed through histopathological technique [34]. Monogenean and copepod-infected gills of Labeo rohita and Hypophthalmichthys molitrix were analyzed using histopathological tools [35]. In addition, Vankara [36] reported the mode of attachment of the cestode Circumonchobothrium shindei in the intestine of Mastacembelus armatus and subsequent pathological events. Gudivada et al. [6] conducted a histopathological analysis to determine the infestation levels of parasite (Raorhynchus polynemi) in marine fish species. Recently, histopathological analysis was carried out using Wallago attu for the identification of certain ectoparasites [15]. In total, the histopathological technique will always be useful and economical, even though we have an advanced facility to capture the images.
Physiological, Biochemical and Molecular Studies of Fishes Infected with Parasites
Fisheries are economically beneficial, but the parasites in the fish often cause damage and make them less suitable as food, which affects the economy. Major fish organs such as gills, intestine, liver, kidneys, muscles, skin, etc., were severely impaired due to both ecto and endo parasite infections [3]. These effects can be determined by examining the physiological, biochemical and molecular performance of differentially parasitized fish under diverse conditions [5]. Specifically, parasitized fishes were compared using various physiological parameters such as swimming efficiency, restlessness, body weight, blood flow, respiratory distress, excretion and overall health [4,30,37]. It is a well-known fact that physiological effects are generally linked to biochemical and molecular aspects. Particularly, the immune system of fish is meant to protect itself against infection by parasites, which in turn depend on the species [38]. In general, nutrients regulate the biochemical reactions of fish and parasites. Metabolites such as certain lipids and proteins in fish were altered by parasitisation. In the following paragraphs, certain physiological, biochemical and molecular changes in the parasitized fish were highlighted.
The effects of Lepeophtheirus salmonis infection on Atlantic salmon (Salmo salar) were studied by measuring the levels of cortisol and glucose in the blood, macrophage respiratory burst activity, phagocytosis and non-specific defence mechanisms [39]. Thomas [40] observed the biochemical and physiological modifications in salmonid fish hosts when helminth parasites were infected. Wegner et al. [41] proved that multiple parasites control the major histocompatibility complex (MHC) genes in three-spined stickleback fish. Masvaer et al. [42] proved that parasite infection alters the immunity levels and sex traits in Salvelinus alpines (Arctic charr). Gollock et al. [37] noticed the alteration in cortisol, glucose and hemoglobin levels in Anguillicola crassus-infected European eels. The appearance of rodlet cells in Cyprinus carpio and the role of their secretions in defence against parasite infection were described by Mazon et al. [43]. Infection of the nematode parasite (Anguillicola crassus) declines the population of European eel (Anguilla anguilla) by altering the growth, osmoregulation and stress tolerance levels and also alters the gene expression [44]. Marty et al. [45] noticed that the Pacific herring, i.e. Clupea pallasii, population was reduced due to parasite infection.
It was proved that the Sparus aurata fed with vegetable oils did not show any effect on the intestine transcriptome but found modulation of the transcriptomic response after infection with Enteromyxum leei [46]. Fundulus parvipinnis (California killifish) infected with trematode parasite (Euhaplorchis californiensis) displayed conspicuous swimming behaviour [47]. Morphological plasticity and phylogeny were noticed in monogeneans when infected in wild and reared fishes. This data indicates mild gene flow between parasites in cultured and wild fish [48]. Mimicry proteins produced by the cestode (Schistocephalus solidus) exhibited the phenotypic modifications of three-spined stickleback, i.e. Gasterosteus aculeatus [49]. Yin et al. [50] analyzed the transcriptome of Cryptocaryon irritans parasite that affects the fish in low temperature. Haase et al. [51] proved the immunity levels in three-spined sticklebacks during a parasite attack and listed its consequences. Jorgensen [52] highlighted the Ichthyophthirius multifiliis (Ich) impact on host fish immune responses. Souza et al. [53] demonstrated the differential immune responses of teleosts to helminth infection. Gene editing alters the fish species positively to reach a phenotype for disease resistance in turn for better yield [54].
Marcus-Lopez & Rodger [55] emphasized the amoebic gill disease in Atlantic salmon caused by Neoparamoeba perurans and addressed the pathological changes, immune response and mechanism underlying the epithelial proliferation. Modification in distribution and expression of E-cadherin protein was noticed in the intestine of both turbot and gilt-head bream when infected with Enteromyxum species [56]. Holzer et al. [10] described in detail the fish immune responses against myxozoan infections. Hunt et al. [11] noticed the transcriptomic alterations over day-night cycles, i.e. around 50 % of annotated genes exhibited significant daily rhythms in the fish louse parasite (Argulus foliaceus). The disease, namely cryptocaryonosis, was noticed in marine fishes by the parasitic ciliate, Cryptocaryon irritans [57]. They also discussed about the host-defence mechanism and remedies including development of vaccines. Hasegawa & Poulin [4] reviewed the fish body condition after infection with a parasite using several examples. All these studies will help to identify physiological, biochemical and molecular changes in a parasitized fish, which in turn is useful to know the treatment and control methods. Also, the molecular knowledge will be helpful to develop effective targeted therapeutics against parasites and the immune reactions in fish.
Mitigation of Parasitic Diseases
Mitigation of parasitic diseases includes various stages such as prevention, control and treatment. All these practices completely depend on the type of parasite. Initially, it is suggested that possible hygiene conditions must be implemented in fish industries. In general, water quality, pH, temperature, nutrients, season, etc., are the crucial abiotic factors for parasite growth [58]. Particularly, the quality of water determines the parasite infestation. For instance, Labeo calbasu was infected with more monogeneans in Somasila backwater (Nellore Dt., A.P., India) when compared to Chennur dam (Kadapa Dt., A.P., India) due to high pollution in the earlier one [59]. Similarly, certain copepods grow well in W. attu in Somasila backwater when compared to another one (Figure 5).
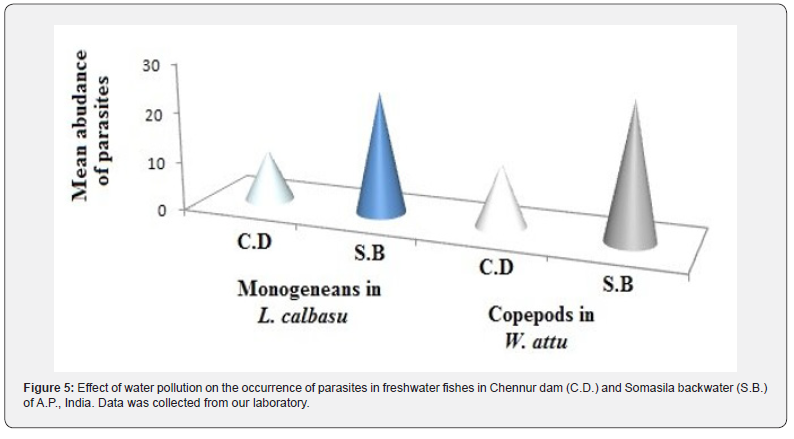
Variations in parasite prevalence in the fish can be attributed to alterations in temperature in different seasons [60]. Moreover, certain parasites grow in acidic conditions and some others in alkaline conditions. Several works emphasized that nutrients, DO, TDS, calcium hardness, chlorides, nitrates, etc., are crucial for the proliferation of parasites [60,61]. Different medications must be used to control parasitic diseases in fish. Certain chemicals such as common salt, copper sulfate, ferric sulfate, hydrogen peroxide, sodium percarbonate, potassium permanganate, etc., were used to suppress the growth of certain parasites [9,14, 62- 64]. Biological control is one of the best practices for controlling parasitic diseases. Natural extracts such as pyrethroids, including deltamethrin, were also useful to eradicate certain fish parasites [9,63]. In addition, organophosphates such as malathion, parathion, azamethiphos, etc., and organohalogens were used to mitigate parasitic diseases [9,14,64]. Overall, the immunity level of fish is a crucial factor in combating with the parasite [65]. It was proved that the skin mucus of fish is involved in the defence mechanism against parasites [66]. Certain antibiotics and vaccines will also be used to eradicate the parasitic diseases in fish [9].
Recently, nanoparticles have been applied to control the parasitic diseases of fish as well as parasite growth. Antiprotozoal effects of silver and zinc oxide, including gold nanoparticles, against Ichthyophthirius multifiliis were established by Saleh et al. [67]. In vitro anthelmintic effect of silver nanoparticles against adults and eggs of monogeneans was noticed [68]. Mathews et al. [69] used oral drug delivery nanoparticles to treat the intestinal parasite infection. Our laboratory is also using nanoparticles for disease management practices in fish [2]. Though it is beginning, future work with nanoparticles may be useful to control parasitic diseases in fish.
Future Prospects
Fish production should be more than double the size to meet the current demand, which can be possible by improving fisheries. In contrast, fish diseases caused by parasites constitute one of the most important concerns and dealing with them is a challenge to fish culturists. Identification and authentication of certain parasites in fish is possible through the characterization of intraspecific genetic divergence to establish the limits between species. Contamination with other organisms is a major concern for the molecular identification of parasites because of the presence of other organisms’ DNA along with parasite DNA. Moreover, fish parasites are the hosts for secondary pathogens such as bacteria and viruses. Therefore, the selection of an appropriate molecular marker or primer plays a key role in the molecular identification of parasites. Extensive usage of eDNA and FINS tools is also helpful for the accurate identification of fish parasites. In histopathological experiments, the preparation of tissue material is crucial for both infected and control samples. Using advanced microscopes such as scanning electron microscope (SEM) and transmission electron microscope (TEM) will be helpful for the accurate morphological identification of parasites.
Time-to-time analysis of water quality, including levels of nutrients, temperature, pH, DO, TDS, total hardness, etc., using advanced tools is mandatory for the fish industry. Biochemical and molecular studies on parasite-fish relations might require more finesse. Apart from genomics, focusing on both transcriptomics and metabolomics will be helpful to know the fish and parasite interaction in turn for better yield. Using the advanced biochemical techniques for the development of vaccines, antibodies, and biological agents may be helpful to control the parasites in the fisheries. Practice of advanced CRISPR/Cas9 (clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9) technique will be useful in editing the genome of fish to get resistance against parasites or editing the genome of the parasite to reduce the pathogenicity. Strong safety measures, environmental risk assessment of fish parasites and an integrated approach of both conventional and advanced biotechnological methods will be helpful to take precautionary measures to protect the fisheries from parasites. Overall, fishing industries need to perform certain environmental precautions before starting fish cultures to avoid certain unwanted growth of parasites.
Conclusion
Fish are important source of food, and therefore fisheries need to take precautions to improve the yield. However, fisheries witness severe loss due to pathogens such as viruses and bacteria including parasites. Among the various diseases, infections due to ecto and endo-parasites appear to be more common and pathogenic. In the present review, the importance of systematic studies of fish parasites was emphasized for accurate identification and authentication. In addition, histopathological studies were highlighted to know the damage levels of fish by parasitisation. Physiological, biochemical and molecular aspects of fish infected with various parasites were highlighted to understand the behaviour of the host, metabolic regulation of the host, growth and yield levels and enhance the knowledge of parasite-fish interaction at the genetic level. Moreover, the methods of mitigation of parasitic diseases in fish were emphasized. Furthermore, the prerequisites and necessity of advanced technology to know the insights of fish parasites were discussed. Overall, the present review may be useful to improve the fish yield, which in turn improves food security and the economy.
Acknowledgment
We are highly thankful to late Dr. Asha Kiran Modi (Ph. D student of VAP), Yogi Vemana University, India for the experimental data.
References
- Bennett A, Patil P, Kleisner K, Rader D, Virdin J, et al. (2018) Contribution of fisheries to food and nutrition security: Current knowledge, policy, and research. NI Report 18-02. Durham, NC: Duke University.
- Rajeswari D, Anu PV, Riazunnisa K, Venkata RTS, Vijayalakshmi D, et al. (2024) Role of nanoparticles in fish disease management: A review. Biocatal Agricul Biotechnol 58: 102218.
- Dezfuli BS, Scholz T (2022) Fish parasites (special issue). Parasitology 149(14): 1811-1814.
- Hasegawa R, Poulin R (2025) Effect of parasite infections on fish body condition: a systematic review and meta-analysis. Int J Parasitol In Press.
- Modi AK, Vankara AP (2021) Prevalence and spatial distribution of the ectoparasites on the gills of Mystus vittatus from River Penna flowing through YSR District, Andhra Pradesh, India. J Parasit Dis 45(1): 43-49.
- Gudivada M, Vankara AP, Chikkam V (2021) Pathological changes in the intestine of the marine fish Polydactylus sexfilis (Val. 1831), due to heavy infestation of the Acanthocephalan parasite, Raorhynchus polynemi from Eastern Coast of Bay of Bengal. J Env Bio-Sci 35: 47-53.
- Sures B, Nachev M, Selbach C, Marcogliese DJ (2017) Parasite responses to pollution: what we know and where we go in environmental parasitology. Parasit Vect 10: 65.
- NABARD (2018) Sectoral paper on fisheries and aquaculture. Farm sector policy department Nabard Head office, Mumbai. p. 1-86.
- Banu H, Rathinam RB (2023) Myxozoan fish diseases: possible treatment and zoonoses. J Parasitic Dis 47(2): 215-223.
- Holzer AS, Piazzon MC, Barrett D, Bartholomew JL, Sitjà BA (2021) To react or not to react: The dilemma of fish immune systems facing myxozoan infections. Front Immunol 12: 734238.
- Hunt R, Cable J, Ellison E (2022) Daily patterns in parasite processes: diel variation in fish louse transcriptomes. Int J Parasitol 52(8): 509-518.
- Boxshall GA (2007) Crustacean classification: On-going controversies and unresolved problems. Zootaxa 1668: 313-325
- Jowers MJ, Xavier R, Lasso AOM, Quintero TE, Nunes JLS, et al. (2023) First molecular identification of a goussia parasite from a new world invasive blenny. Acta Parasitol 68(2): 458-462.
- Paladini G, Longshaw M, Andrea G, Shinn A (2017) Parasitic diseases in aquaculture: Their biology, diagnosis and control. In: Diag Control Dis Fish Shellfish 37: 107.
- Vankara AP, Thummala CS, Khateef R, Peddinti RA (2022) Histopathological evaluation of Wallago attu (Bloch & Schneider, 1801) infested by ectoparasites. The J Basic Applied Zoology 83: 32.
- Iida AN, Harada T, Kawai T, Yokoyama H, Kawatsu K (2018) Development of a real-time PCR assay for detection of Kudoa iwatai (Myxosporea: Multivalvulida) in Japanese Seabass (Lateolabrax japonicus). J Food Protection 81(8): 1346-50.
- Jaruboonyakorn P, Tejangkura T, Chontananarth T (2022) Multiplex PCR development for the simultaneous and rapid detection of two pathogenic flukes, Dactylogyrus and Centrocestus formosanus, in ornamental fishes. Aquaculture 548: 737660.
- Rokicka M, Lumme J, Ziętara MS (2007) Identification of Gyrodactylus ectoparasites in Polish salmonid farms by PCR-RFLP of the nuclear ITS segment of ribosomal DNA (Monogenea, Gyrodactylidae). Acta Parasitologica 52(3): 185-195.
- Sahoo PK, Mohanty J, Garnayak SK, Mohanty BR, Kar B, et al. (2013) Genetic diversity and species identification of Argulus parasites collected from major aquaculture regions of India using RAPD-PCR. Aquaculture Res 44: 2.
- Chadamala SK, Vankara AP (2018) Morphological and molecular identification of Paraorygmatobothrium floraformis in Rhizoprionodon acutus off Nellore Coast, Bay of Bengal, India. Trends Bionform 11: 25-32.
- Lebedeva D, Munoz G, Lumme J (2021) New salinity tolerant species of Gyrodactylus (Platyhelminthes, Monogenea) on intertidal and supratidal fish species from the Chilean coast. Acta Parasitol 66(3): 1021-1030.
- Graça RJD, Fabrin TMC, Gasques LS, Prioll SMA, Prioll AJ, et al. (2016) Molecular markers in studies on fish parasites (Platyhelminthes): Review. Acta Scienti Biol Sci 38(4): 495-500.
- Muñoz CT, Machuca A, Morales P, Verdugo J, Reyes R, et al. (2022) Prevalence and molecular identification of zoonotic Anisakis and Pseudoterranova species in fish destined to human consumption in Chile. Parasitology Res 121(5): 1295-1304.
- Jones S, Prosperi PG, Kim E, Callow P, Hargreaves N (2006) The occurrence of Lepeophtheirus salmonis and Caligus clemensi (Copepoda: Caligidae) on three-spine stickleback Gasterosteus aculeatus in coastal British Columbia. The J Parasitol 92(3): 473-480.
- Song Y, Wang GT, Yao WJ, Gao Q, Nie P (2008) Phylogeny of freshwater parasitic copepods in the Ergasilidae (Copepoda: Poecilostomatoida) based on 18S and 28S rDNA sequences. Parasitol Res 102(2): 299-306.
- Freeman MA, Anshary H, Ogawa K (2013) Multiple gene analyses of caligid copepods indicate that the reduction of a thoracic appendage in Pseudocaligus represents convergent evolution. Parasit Vect 6: 336.
- Rusch JC, Hansen H, Strand DA, Markussen T, Hytterod S, et al. (2018) Catching the fish with the worm: a case study on eDNA detection of the monogenean parasite Gyrodactylus salaris and two of its hosts, Atlantic salmon (Salmo salar) and rainbow trout (Oncorhynchus mykiss). Parasit Vectors 11(1): 333.
- Cheng J, Zou H, Li M, Wang J, Wang G, et al. (2023) Morphological and molecular identification of Dactylogyrus gobiocypris (Monogenea: Dactylogyridae) on gills of a model fish, Gobiocypris rarus (Cypriniformes: Gobionidae). Pathogens 12(2): 206.
- Campos CM, Moraes JRE, Moraes FR (2001) Histopathology of gills of Piaractus mesopotamicus (Holmberg, 1887) and Prochilodus lineatus (Valenciennes, 1836) infested by monogenean and myxosporea, caught in Aquidauana River State of Mato Grosso do Sul, Brazil. Brazilian J Vet Parasitol 20(1): 67-70.
- Ojha J, Hughes GM (2001) Effect of branchial parasites on the efficiency of the gills of a freshwater catfish Wallago attu. J Zool 255(1): 125-129.
- Vinobada P (2007) Histopathological changes induced by ergasilid copepod infections on the gills of food fish from Batticaloa Lagoon Sri Lanka. Sri Lanka J Aquatic Sci 12: 77-87.
- Feist S, Longshaw M (2008) Histopathology of fish parasite infections- Importance for populations. J Fish Biol 73: 2143-2160.
- Mohammadi F, Mousavi SM, Rezaie A (2012) Histopathological study of parasitic infestation of skin and gill on oscar (Astronotus ocellatus) and discus (Symphysodon discus). Aquaculture, Aquarium, Conservation & Legislation Bioflux 5: 88-93.
- Vankara AP, Chikkam V (2013) Histopathology of heart of freshwater spiny eel, Mastacembelus armatus naturally infected with Tetracotyle metacercaria (Trematoda: Strigeidae). Res J Parasitol 8: 45-54.
- Singh S, Kaur P (2014) Histology of gills of Labeo rohita and Hypophthalmichthys molitrix infested by monogenean and copepod parasites. Int J Fisheries Aqua Studies 1(6): 01-06.
- Vankara AP (2018) Mode of attachment and pathogenecity of Circumonchobothrium shindei (Eucestoda: Ptychobothriidae) in Mastacembelus armatus of River Godavari, Andhra Pradesh, India. Int J Zool Res 14: 1-7.
- Gollock MJ, Kennedy CR, Brown JA (2005) Physiological responses to acute temperature increase in European eels Anguilla anguilla infected with Anguillicola crassus. Dis Aquatic Organ 64(3): 223-228.
- Rohlenová K, Morand S, Hyršl P (2011) Are fish immune systems really affected by parasites? an immunoecological study of common carp (Cyprinus carpio). Parasit Vect 4: 120.
- Mustafa A, MacWilliams C, Fernandez N, Matchett K, Conboy GA, et al. (2000) Effects of sea lice (Lepeophtheirus salmonis Kröyer, 1837) infestation on macrophage functions in Atlantic salmon (Salmo salar). Fish Shellfish Immunol 10(1): 47-59.
- Thomas JD (2002) The ecology of fish parasites with particular reference to helminth parasites and their salmonid fish hosts in Welsh rivers: a review of some of the central questions. Adv Parasitol 52: 1-154.
- Wegner KM, Reusch TB, Kalbe M (2003) Multiple parasites are driving major histocompatibility complex polymorphism in the wild. J Evol Biol 16: 224-232.
- Masvaer M, Liljedal S, Folstad I (2004) Are secondary sex traits, parasites and immunity related to variation in primary sex traits in the Arctic charr? Proc Biol Sci 7: 271.
- Mazon AF, Huising MO, Taverne TAJ, Bastiaans J, Verburg VKBM (2007) The first appearance of Rodlet cells in carp (Cyprinus carpio L.) ontogeny and their possible roles during stress and parasite infection. Fish Shellfish Immunol 22(1-2): 27-37.
- Fazio G, Monée H, Da Silva C, Simon LG, Allienne JF, et al. (2009) Changes in gene expression in European eels (Anguilla anguilla) induced by infection with swim bladder nematodes (Anguillicola crassus). J Parasitol 95(4): 808-816.
- Marty GD, Hulson PJ, Miller SE, Quinn TJ, Moffitt SD, et al. (2010) Failure of population recovery in relation to disease in Pacific herring. Dis Aquatic Organ 18: 1-14.
- Calduch GJA, Sitjà BA, Davey GC, Cairns MT, Kaushik S, et al. (2012) Dietary vegetable oils do not alter the intestine transcriptome of gilthead sea bream (Sparus aurata) but modulate the transcriptomic response to infection with Enteromyxum leei. BMC Genom 13: 470.
- Shaw JC, Overli O (2012) Brain-encysting trematodes and altered monoamine activity in naturally infected killifish Fundulus parvipinnis. J Fish Biol 81(7): 2213-2222.
- Mladineo I, Šegvić BT, Stanić R, Desdevises Y (2013) Morphological plasticity and phylogeny in a monogenean parasite transferring between wild and reared fish populations. PLoS One 8(4).
- Hebert FO, Phelps L, Samonte I, Panchal M, Grambauer S, et al. (2015) Identification of candidate mimicry proteins involved in parasite-driven phenotypic changes. Parasit Vect 15: 225.
- Yin F, Sun P, Wang J, Gao Q (2016) Transcriptome analysis of dormant tomonts of the marine fish ectoparasitic ciliate Cryptocaryon irritans under low temperature. Parasit Vect 9(1): 280.
- Haase D, Rieger JK, Witten A, Stoll M, Bornberg BE, et al. (2016) Immunity comes first: The effect of parasite genotypes on adaptive immunity and immunization in three-spined sticklebacks. Dev Comp Immunol 54(1): 137-144.
- Jørgensen LVG (2017) The fish parasite Ichthyophthirius multifiliis-Host immunology, vaccines and novel treatments. Fish Shellfish Immunol 67: 586-595.
- Souza DCM, Santos MCD, Chagas EC (2019) Immune response of teleost fish to helminth parasite infection. Brazilian J Vet Parasitol 28(4): 533-547.
- Wargelius A (2019) Application of genome editing in aquatic farm animals: Atlantic salmon. Transgenic Res 28(2): 101-105.
- Marcas LM, Rodger HD (2020) Amoebic gill disease and host response in Atlantic salmon (Salmo salar L.): A review. Parasite Immunol 42(8).
- Ronza P, Estensoro I, Bermúdez R, Losada AP, Pérez CG, et al. (2020) Effects of Enteromyxum spp. (Myxozoa) infection in the regulation of intestinal E-cadherin: Turbot against gilthead sea bream. J Fish Dis 43(3): 337-346.
- Jiang S, Huang X (2023) Host responses against the fish parasitizing ciliate Cryptocaryon irritans. Parasite Immunol 45(3).
- El-Naggar AM, Mashly MI, Hagras AM, Alshafei HA (2017) Monogenean microfauna of the Nile catfish, Clarias gariepinus as biomonitors of environmental degradation in aquatic ecosystem at the Nile delta, Egypt. IOSR J Env Sci Toxicol Food Technol 11: 45-62.
- Modi AK, Thummala C, Vankara AP (2021) Water quality assessment based on gill monogenean parasites of Oreochromis niloticus (Linneaeus, 1758) and Labeo calbasu (Hamilton, 1822) fishes of river Penna (India). Appl Biol Res 23(3): 235-244.
- Modu BM, Saiful MF, Kartini M, Kassim Z, Hassan M, et al. (2012) Spatial distribution of monogeneans and their prevalence in two freshwater fish species in relation to some physico-chemical parameters of Lake Kenyir. Malaysian J Sust Sci Manag 7: 179-185.
- Peddinti RA, Thummala C, Khateef R, Vankara AP (2021) Ectoparasitic community ecology of freshwater fishes of river Penna, YSR Kadapa district, Andhra Pradesh, India. Int J Biol Inno 3: 241-256
- Jithendran KP, Natarajan M, Azad IS (2008) Crustacean parasites and their management in brackish water finfish culture. Aquac Asia Mag 75: 47-50.
- Noga EJ (2010) Fish disease: diagnosis and treatment. In: 2nd Ed, Blackwell Publishing pp. 519.
- Buchmann K (2022) Control of parasitic diseases in aquaculture. Parasitol 149(14): 1985-1997.
- Sahoo PK, Mishra M, Mohapatra A, Parida S, Mohanty J (2021) Vaccination approach to prevent Argulus siamensis infection-success, challenges and preparedness, Fish and Shellfish Immunology Reports 100023: 2.
- Saleh M, Abdel BAS, Dkhil M, El-Matboul M, Al-Quraishy S (2021) Proteins of the ciliated protozoan parasite Ichthyophthirius multifiliis identified in common carp skin mucus. Pathogens 10(7): 790.
- Saleh M, Abdel BAA, Dkhil MA, El-Matbouli M, Al-Quraishy S (2017) Antiprotozoal effects of metal nanoparticles against Ichthyophthirius multifiliis. Parasitol144(13): 1802-1810.
- Pimentel ACA, Morales SFN, Chávez SMC, Lara HH, Pestryakov A, et al. (2019) Efficacy of silver nanoparticles against the adults and eggs of monogenean parasites of fish. Parasitol Res 118(6): 1741-1749.
- Mathews PD, Patta ACMF, Madrid RRM, Ramirez CAB, Pimenta BV, et al. (2023) Efficient treatment of fish intestinal parasites applying a membrane-penetrating oral drug delivery nanoparticle. ACS Biomaterials Science & Eng 9(6): 2911-2923.






























