Abstract
Posidonia oceanica meadows along the Tunisian coast represent a significant ecosystem, providing both ecological and economic benefits. P. oceanica landscapes are heterogeneous, impacted by natural and anthropogenic sources. Our study focuses on sediment analysis and morphological, phenological, and ecological parameters of P. oceanica at nine sites in the Maloula Bay. These sites are categorized into three depths: lower (-3 m), intermediate (-8 m), and upper (-13 m) boundaries. The Posidonia oceanica meadows along the Tunisian coast form a vital ecosystem that provides both ecological and economic benefits. These meadows constitute a heterogeneous landscape influenced by both natural and human activities. Our study examines the morphological and ecological parameters of P. oceanica in nine sites in Maloula Bay. These sites are divided into three depth zones: lower (-3 m), middle (-8 m) and upper (-13 m) limits. Foliar density and morphological parameters (foliar biomass, length, number, leaf category, etc.) show significant values at the lower boundary sites, gradually decreasing with depth. However, epiphyte biomass increases progressively with depth. Furthermore, the examined meadows at the upper and intermediate boundaries (20 years) are older than those at the lower boundary (15 years). In-situ observations and cartographic analysis indicate regressions in the meadows primarily due to natural sources, as evidenced by the Irregularity Source Index (PaSI). These natural regressions manifest as the formation of ‘intermatte’ sand zones. Other sources such as anchoring and fishing activity may also influence meadow regression. Utilizing surface characteristics and intermediate nature, ecological indices (Conservation Index CI, Overall Quality EQR, and PREI) of the meadows were determined. Despite observed regressions, these indices confirm that the meadows of Maloula Bay are preserved and in “good” condition.
Keywords:Underwater ecosystem; Posidonia oceanica; Intermatte; Cartography
Introduction
Magnoliophytes, flowering and rhizomatous plants living exclusively in marine habitats, constitute a group of flowering plants abundantly distributed at the universal level, forming large seagrass beds in surface waters up to 40 m deep [1]. These marine species play a vital role for coastlines by providing beneficial services to marine ecosystems [2].
In the Mediterranean Sea, several Magnoliophytes constitute a significant hotspot characterized by unique, valuable, and
productive habitats. They support high biodiversity and provide essential ecological goods and services, including
a)oxygen production
b)carbon dioxide emissions absorption
c)sediment stabilization
d)coastal protection against erosion [3-5]
Additionally, these marine plants may play a potentially important role in climate change mitigation [6]. Five seagrass species are found in the Mediterranean Sea, specifically Posidonia oceanica, Cymodocea nodosa, Zostera marina, Zostera noltii Hornemann, and Halophilas tipulacea. The most abundant among them is Posidonia oceanica, forming climax species that spread slowly and constitute considerable carbon stocks [7].
In the Mediterranean, Posidonia oceanica is an endemic species highly dominant on the Tunisian coast [8]. It forms bundles of five to eight ribboned leaves, 40 to 80 cm high and one centimeter wide, arranged at the end of an erect rhizome. It builds remarkable structures called “mattes,” composed of intertwined rhizomes and roots, which are not easily destructible and can reach several meters in thickness, persisting for millennia [9].
P. oceanica, a slow-growing plant with low genetic diversity, forms grasslands and ecosystems with greater primary productivity, contributing to neighboring systems [10]. This flowering plant serves multiple ecological functions, such as limiting erosion, supporting biodiversity, and storing carbon [11]. P. oceanica conducts photosynthesis to obtain essential nutrients and energy for its development [12,13]. Its ability to grow in diverse environmental conditions is attributed to physiological and morphological adaptations, sometimes unique to endemic species [14]. Apart from its ecological roles,P. oceanica contributes 11 to 42% of carbon dioxide emissions produced by Mediterranean countries since the industrial revolution, averaging 29 g of carbon per m2 per year at an average depth of 15m [15].
Many primary habitats of P. oceanica in the Mediterranean are currently at risk due to the degradation of seagrass beds caused by reduced water circulation, increased sedimentation, and decreased resuspension of particulate organic matter [16]. These degradations lead to elevated burial rates and hinder the remineralization process due to poor aeration.
The decline of P. oceanica meadows, classified as “priority habitats,” reflects the challenge of distinguishing between climatic and anthropogenic effects [17]. This degradation can be attributed to both pollution and a natural decline of the species. In the Mediterranean, the state of P. oceanica has declined by approximately 34% over the last 50 years [1]. However, this figure is based on a small portion of the total herbarium coverage and different calculation methods, mainly relying on maps. The evolution of mapping techniques over recent decades, affecting precision, is a significant factor contributing to observed differences [18].
Following protection, it is the responsibility of managers to implement conservation measures, assess the state of seagrass beds, and establish protection rules. P. oceanica is utilized as a bioindicator of good water quality by studying its physiology and the structure of seagrass beds, linking them to anthropogenic pressures in the study area [19]. Several synthetic indices have been developed for monitoring coastal waters’ quality and providing quantified and coded results accessible to coastal environment managers [20,21].
However, reasoned management is essential to reconcile the preservation of fragile environments, erosion mitigation, and tourism issues. Data on the distribution and status of Posidonia meadows in the Tabarka region are still fragmentary, with Abidi (2012) being the only researcher to map the Posidonia herbarium in the Tabarka area. Therefore, the present work aims to evaluate P. oceanica meadows as dynamic structuring elements of underwater landscapes. However, before incorporating them into management programs or developing specific applied tools, it is imperative to precisely study their dynamics and ecological state through various analyses such as sedimentology, morphology, and herbarium structure.
Materials and Methods
Sampling Site Presentation
The northern part of Tunisia is divided into two regions, forming a northern Tell in the north (composed of the Kroumirie mountain ranges peaking at around 1,000 meters, the Nefzas at 600 meters, and the Mogods at 500 meters located in Aïn Draham, Nefza, and Bizerte, respectively); and a steppe zone to the south. The coastal area of Tabarka is located in the northwest of Tunisia and covers an area of approximately 330 hectares to a depth of 40 meters. Extending from Tabarka to Cape Blanc, the region features a windy, humid to sub- humid climate, rugged and inaccessible terrain, rocky shores providing varied marine landscapes, extensive dunes, and vast sandy dunes. The natural bathymetric topography exhibits a steep slope, facilitating seawater movement. Consequently, the coastline consists of a series of cliffs, rocky shores, beaches, and dunes, sometimes over short distances.
Diving Exploration Strategy and sites
During the summer in Tabarka, inclement weather had a negative impact on the number of days suitable for exploration. Indeed, exploration is only feasible when the water is very clear to carefully assess the boundaries, the state of the seagrass, associated fauna and flora, sediment composition (rock, sand, etc.). Before starting the survey and sampling, it was necessary to delineate the marine portion of the study area, where the isobaths varied between -3, -8, and - 13 meters. A handheld GPS was used during the exploration phase to specify the bathymetry and relative coordinates of each site. Once in the laboratory, these data were analyzed, sorted, and transferred to an Excel spreadsheet for cartographic analysis.
Assessment of Seagrass Density
Seagrass density was measured at each site through underwater diving using a quadrat of 25 cm x 40 cm. Between six and ten replicates, randomly distributed within the seagrass bed, were conducted at each station [22]. The quadrats were randomly dropped from a height of one meter, and counting was done at the point of impact. The evaluation of foliar biomass benefits from a standardized protocol that allows for differentiating seagrass beds based on their density [23]. In our study, seagrass classification is based on Giraud’s method [23], which relies on the density of shoots/m2 [22] (Giraud, 1977). Simultaneously, bundles of each seagrass meadow were sampled (n = 10) for laboratory analysis.
Biometric Analysis
In the laboratory, the leaves of each bundle are separated to measure various morphological parameters. These measurements allow for defining different parameters related to the biometrics of Posidonia oceanica, including the average number of leaves per bundle, the average length, and the average width of the leaves (adult and intermediate). The measurements obtained enable us to categorize the leaves into three classes (adult, intermediate, and juvenile) following Giraud [23].
Foliar Biomass
Initially, the bundles are rinsed with freshwater to remove some sediment particles from the leaves. The leaves are carefully scraped using a blade along both sides to separate all epiphytes, including encrusting algae [23,24]. Subsequently, the leaves are dried in an oven for 48 hours at 70°C until a constant weight is achieved. Each batch of leaves is weighed using a precision balance to ± 0.001 g.
Epiphyte Biomass on Leaves
The biomass of epiphytes on the leaves is determined using the method of Dauby & Poulicek [24]. The material collected during the scraping of the leaves is rinsed with freshwater, filtered, dried in an oven at 70°C, then weighed before and after drying to 0.001 g. Our results are expressed in mg of dry weight per bundle per site and then multiplied by the density of bundles per square meter [24].
Ecological Indices of Seagrass Beds
To assess the ecological status of the seagrass beds, quality
indices are calculated. Several synthetic indices have been
developed to conduct the necessary monitoring for maintaining
or improving the quality of coastal waters [21]. In our study, the
following indices were determined:
a) Conservation Index (CI) according to Moreno et al.
(2001), which compares the coverage of live P. oceanica to dead
matter using the following equation:
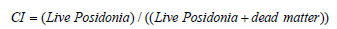
b) Ecological Quality Ratio (EQR) [25] measures the level of ecosystem change due to regression (natural and/or anthropogenic):
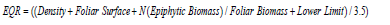
c) Overall Seagrass Quality Index (PREI) [25] calculates the ecological quality ratio including EQR:
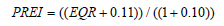
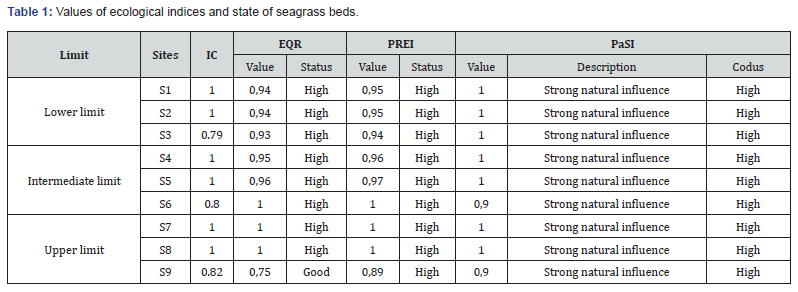
These indices, estimating the health status of P. oceanica seagrass beds, are reported in Table 1 with reference values, providing information on the health status of the ecosystem according to five quality classes [17]. To assess the primary origin of irregularities observed in the P. oceanica seagrass beds, we used the PaSI index developed by Moreno et al. [26], by calculating the areas of the different identified structures. PaSI ranges from 0 to 1 and has no unit.
PaSI = ((Snp)) / ((Snp + Sap)) where Snp: percentage of the area covered by different types of natural patches; Sap: percentage of the area covered by different types of anthropogenic patches.
GIS Process
Once clearly identified, the boundaries of sandy and bare patches in the P. oceanica meadows were traced in GIS software. Their origin was estimated by searching for the presence of human activities near the patches. Additionally, the P. oceanica seagrass beds are represented in maps according to their density and area. The GIS files were then converted into rasters (GeoTIFF grid) and analyzed using the QGIS software program to obtain the desired maps.
Statistical Analysis
The mean values of the parameters studied are expressed with their standard deviations and represented on figures by a vertical line (error bar) and on tables by ±. The comparison of means is estimated using the t-student test between the studied sites. The difference is considered significant for p < 0.05, p < 0.01, and highly significant when p < 0.001.
Results
Description of sampling sites
Our study is conducted on the prospection of the P. oceanica meadow of Maloula Bay (Table 2). To do this, we chose to work by limit (upper, intermediate and lower) and according to depth (-3 m, -8 m and -13 m). For each depth, we prospected 3 sites colonized by the P. oceanica meadow. Generally, the meadows prospected at the lower and intermediate limit are hills, while the upper limit is characterized by striped meadows located on the rocks.
Leaf density of P. oceanica by site
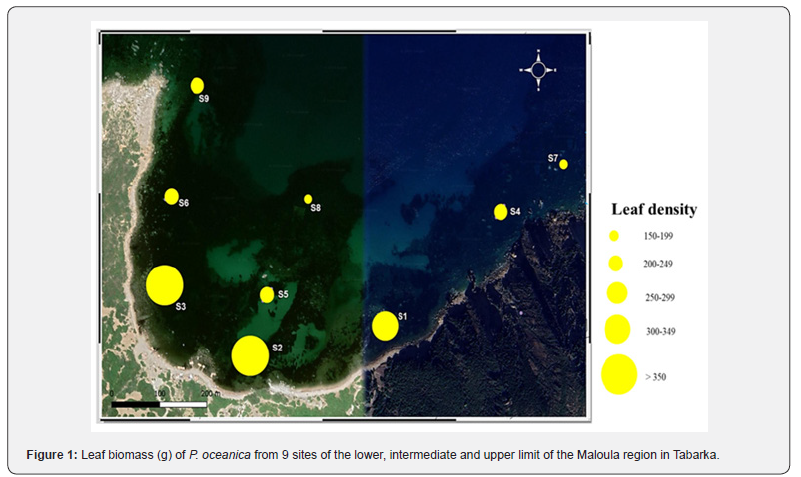
The in-situ examination of the P. oceanica meadows allowed us to determine the leaf density of each site (Table 3 & Figure 1). The results obtained show that the density of the meadows located at -3 m varies between 367m2 and 341 m2. These densities show that P. oceanica of the lower limit is type III (S1, S2 and S3) describing sparse meadows. The classification of the density of these meadows according to depth shows a Subnormal state (≃879m2) The meadows of the intermediate part located at -8 m are very sparse and type IV. A subnormal density according to the classification characterizes the latter by depth. The upper limit is characterized by type IV meadows qualified as very sparse since the number of bundles between 150 and 300 m2. These seagrass beds are classified by a subnormal state according to depth.
The overall comparison of the leaf density of the P. oceanica meadows surveyed during the period of our study shows a remarkable variation (Figure 1). It is observed that the leaf density of the lower limit is very high compared to that of the intermediate part and the upper limit of the P. oceanica meadows of Maloula Bay. This density decreases progressively with depth since it presents average values in the intermediate part of the meadows and low densities at the sites of the upper limit (Figure 1).
Morphological parameters and composition of P. oceanica by site
ND: not determined
Results are presented as mean ± standard deviation
Significant differences between meadows of the same boundary are detected at *p<0.05; **p<0.01 and ***p<0.001. Significant differences between
boundaries are presented by P. oceanic.
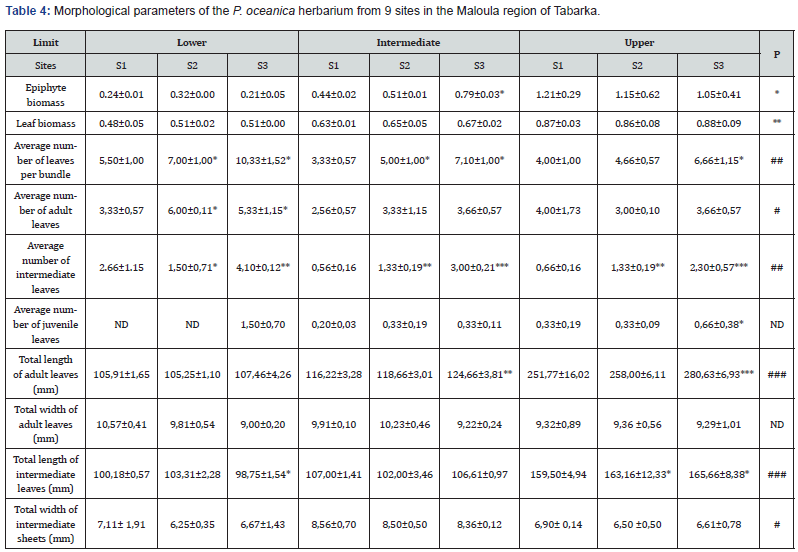
This study presents the morphological differences between the meadows of P. oceanica of the region of Maloula in Tabarka (Table 4). The Posidonia meadows located in the Lower limit have significant average values of leaves per bundle compared to those of the upper limit and the intermediate part. The examination of the main morphological parameters reveals that most of the bundles are adults characterized by lengths greater than 50 mm and benefiting from a petiole (≥ 2 mm) (Table 4). These last ‘adult leaves’ are recorded at the level of the meadows of the Lower limit with large quantity in comparison with those located at -8m and -13m.
The analysis of the composition of the bundle samples in the laboratory allowed us to determine intermediate bundles with average lengths varying between 98.75±1.54 and 165.66±8.38. These bundles are present in all the samples examined. The examination of juvenile leaves shows a significantly lower density compared to the other categories (adult and intermediate) (Table 4). In addition, these leaves are saved in the three sites of the upper limit and the intermediate part. However, note the absence of this category at the level of the seagrass bed of Maloula Tabarka Bay at shallow depth (-3 m) particularly at S1 and S2.
According to Table 4, it is noted that the morphological characteristics of the seagrass bed, particularly the length and width of adult and intermediate leaves, increase significantly with depth. This increase is indicated at the lower limit by lengths that vary between 105.91±1.65mm and 107.46±4.26mm, and between 98.75±1.54mm and 103.31±2.28mm respectively in adult and intermediate leaves. A progressive evolution of this parameter is marked at the level of the herbariums of the intermediate part with adults of lengths between 116.22±22mm and 124.66±3.81mm and intermediate leaves of lengths between 102.00±3.46mm and 107.00±1.41mm. Concerning the upper limit, the Posidonia meadows present are characterized by high morphological parameters with lengths that vary from 159.50±4.34mm to 280.63±6.93mm (Table 4).
Leaf biomass of P. oceanica by site
The leaf biomass determined at 9 sites in the Maloula region of Tabarka is presented in Table 4. Generally, the leaf biomass varies similarly at the sites of each limit from where no significant difference was recorded in the sites at the same depth. Furthermore, a progressive and significant increase in leaf biomass is noted at the level of the Posidonia meadow sample of the intermediate part. This biomass also increases and marks high values at the level of the Posidonia sample of the upper limit. These results allowed us to conclude that the leaf biomass increases according to the depth.
Epiphyte Biomass of P. oceanica by Site
Epiphyte biomass varies in a similar way to leaf biomass, i.e. it increases progressively with depth (Table 4). At the lower limit, epiphyte biomass is slightly small at S3. In the intermediate part of the meadow, epiphyte biomass is higher at S6 compared to S4 and S5. Significant biomass is reported at the upper limit sites with significant increases observed at S7, S8 and S9.
Ecological indices of the state of the seagrass beds
Conservation index (CI): The evaluation of the conservation index (CI) of the P. oceanica seagrass beds in the Maloula region of Tabarka allowed us to elucidate values that exceed the reference interval of the ecological indices (≥ 0.9) (Table 1). These results indicate that the P. oceanica seagrass bed is in high conservation (in blue). Other results are observed in the western part of the Maloula Bay at depths of -3 m, -8 m and -13 m. These results confirm that the posidonia of these three sites is in Good condition since the values of the CI conservation index are between 0.7 and 0.9 (in green).
Ecological Quality Ratio (EQR) and Overall Seagrass Quality Index (PREI):The ecological indices EQR and PREI presenting the quality of P. oceanica seagrass beds in the Maloula region of Tabarka are illustrated in Table 1. These indices show that the seagrass beds surveyed at all depths have high ecological indices (0.89 – 1) according to the reference intervals adopted internationally. However, only the seagrass bed located at S9 (-13 m) in the western part of Maloula Bay is characterized by good quality with a value of 0.75 associated with the EQR.
Irregularity Source Index (PaSI): The survey of the studied sites allowed us to determine the irregularity source index (PaSI) (Table 1). This index shows that the P. oceanica meadows of Maloula Bay are subject to a strong natural influence. This natural influence is based on the PaSI values that are recorded per site and range between 0.8 and 1.
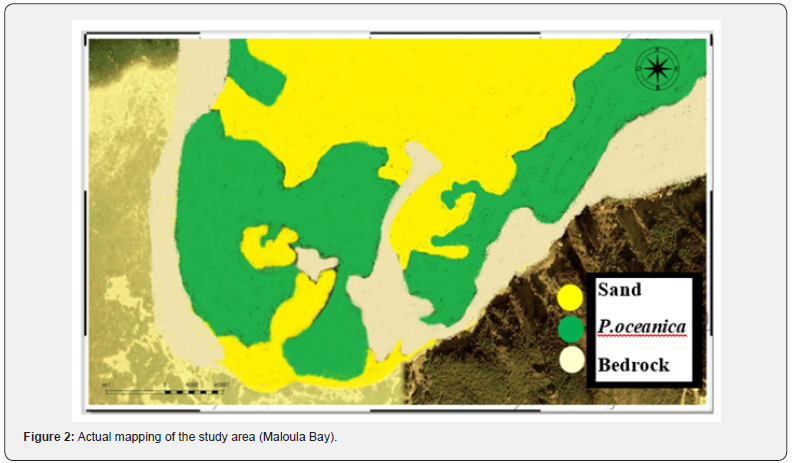
Cartographic distribution of the P. oceanica meadow in Maloula Bay in Tabrka
The bathymetric structure of Maloula Bay shows a sharp increase in depth from the shore to the open sea. This area is characterized by cliffs and coves where significant depths exceed 10 meters are noted. Also, there are shallow parts that are located far from the coast, this is related to the nature of the coast and the accumulation of rock blocks, particularly at Cap de Maloula.
The cartographic analysis is based on “real mapping” which is coupled with the certainty of what exists in the ecosystem. The map of Maloula Bay shows a varied structure composed of P. oceanica meadows on sandy and also rocky substrates. In addition, there is a deep zone where the P. oceanica meadow does not exist or is rarely reported.
Discussion
Currently, Posidonia meadows are adopted as key systems given the ecosystem benefits they provide [1]. Their ecological roles range from raw material production, coastal support for erosion, water purification, carbon sequestration and shelter for biodiversity. These are interesting sites for several tourism and leisure activities. Similarly, Posidonia is a biological material that is widely studied in scientific research [27]. The present study focused on the morphological, phenological and ecosystem characteristics of P.oceanica meadows in Maloula Bay as a function of depth. This study shows that the density of recorded seagrass beds gradually decreases with depth since their number is significantly lower at sites at -13 m depth compared to those located at -5 m. The large number of adult leaves is observed at a shallow depth. Our results also show that the intermediate limit and the upper limit are characterized by low values relative to adult leaves. Nevertheless, we note the presence of juvenile sheets at these two limits compared to that of the lower limit. In this context, authors have reported the dominance of juvenile leaves in significant depths, which is explained by the slowdown in development of intermediate leaves [28,29].
In general, the morphological parameters analyzed during this work are important in the western region of Maloula Bay, regardless of depth. Comparing the depths, the morphological parameters of the leaves examined are more important at –5 m depth compared to –8 m and – 13 m. These observations may be related to strong hydrodynamics and significant turbidity at great depths, inhibiting or dampening the growth of seagrass beds [30]. However, the abundance of nutrient elements at shallow depths is a key element in the growth of seagrass beds [31].
Leaf and epiphyte biomass increase progressively with depth, with very high values at -13 m and very low values at -5 m. Our results are relatively low compared to the leaf density in the Tabarka region in 2013 of around 556 m2 [29]. Nevertheless, the leaf density of the seagrass beds of Maloula Bay is close and similar to those of the Tamentfoust region in Algeria [32] and the Ventimigila region in Italy [33]. During this study, we found the dominance of the biomass of epiphytes, early warning indicators and descriptors of changes in the water column [34], compared to leaf biomass especially at the level deep sites. This observation is possibly due to the enrichment of nutrients and the reduction in water quality at these sites, which disrupts the posidonia and limits the absorption of available light [35]. This could be due to pollutants carried by shore currents and discharges from numerous boats (pleasure and fishing) which stop in the bays during the summer period. According to Ruiz et al. [36], the richness of epiphyte biomass can be a major cause in leaf degradation, this coincides with our in-situ prospecting. From an ecological point of view, the state of theP.oceanica meadows is consulted by diving where significant natural regressions have been detected followed by regressions caused by fishing activity (mooring and nets). This observation is corroborated by significant regressions of their distribution on the scale of Tunisia and on a global scale [37,38] (Pergent et al., 2012). Also, the PaSI regression index showed values close to 1, which explains a strong natural influence exerted on the seagrass beds of Maloula Bay.
These regressions can constitute in the long term a net loss in terms of carbon sinks and an indirect risk of release of all or part of the carbon sequestered at the level of the mattes, but also of the contaminants, trapped within these structures [39]. To confirm these regressions, we used the cartographic analysis, already adopted, in previous works as a tool reliable reflecting reality [40]. The results found during this work show a remarkable regression at the level of P.oceanica seagrass beds compared to the results of Abidi et al [40].
From an ecological point of view, an classification of the property of seagrasses, from poor to good quality, has been established based on several vitality parameters (dead leaf, living, etc.). During our work, “good” values relating to the conservation index (CI) of seagrass beds are recorded at the level of P.oceanica in Maloula Bay, describing a “high” ecological state according to the reported classification in the literature [19]. The EQR landscape descriptor and the PREI index are consistent and indicate a better state of health for the seagrass beds of Maloula Bay, despite the regressions observed [41-71]. At the end of this work, we can deduce that the roles of P.oceanica are influenced by its ecological state, thus confirming the way in which human disturbances (fishing, boating, etc.) on ecosystems can hinder functioning by generating a reduction in ecosystem services [72-89].
Conclusion
The information presented in this study represents a significant contribution to the description and study of P.oceanica meadows in the Maloula Bay. In addition, the results provided can serve as basic information to guide future studies concerning the abundance, growth, regression, and quality of these meadows. Generally, P.oceanica meadows in Maloula Bay are sparse to very sparse with a subnormal state. Most morphological parameters, except for epiphyte biomass, decrease with depth, marking minimal values at -13 m. The ecological status of P.oceanica in Maloula Bay is high, confirming the good quality of these meadows, despite the regressions observed during dives and through the Irregularities Index (PaSI). All these data strongly suggest that the current P.oceanica beds constitute a relic population that should be preserved.
References
- Telesca L, Belluscio A, Criscoli A, Ardizzone A, Apostolaki ET, et al. (2015) Seagrass meadows (Posidonia oceanica) distribution and trajectories of change. Science Reports, 5.
- Costanza R, D Arge R, De Groot S, Farber M, Grasso K, et al. (1997) The value of the world’s ecosystem services and natural capital. Nature 387: 253-260.
- Orth RJ, Carruthers TJB, Dennison WC, Duarte CM, Fourqurean JW, et al. (2006) A Global Crisis for Seagrass. Ecosystems. Bioscience 56: 987-996.
- Coll M, Piroddi C, Steenbeek J, Kaschner K, Ben RLF, et al. (2010) The biodiversity of the Mediterranean Sea: Estimates, patterns, and threats. PLoS ONE 459 5(8).
- Pergent MC, Pergent G, Monnier B, Boudouresque CF, Mori C (2021) Contribution of Posidonia oceanica meadows in the context of climate change mitigation in the Mediterranean Sea. Marine Environmental Research 165: 105236.
- Duarte CM, Losada IJ, Hendriks IE, Mazarrasa I, Marbà N (2013) The role of coastal plant 844 communities for climate change mitigation and adaptation. Nature Climate Change 3(11): 961-968.
- Björk M, Short F, McLeod E, Beer S (2008) Managing seagrasses for resilience to climate change. IUCN, Gland, p. 56.
- Sghaier YR, Zakhama SR, Charfi CF (2011) Posidonia oceanica meadows along the eastern coast of tunisia: feature and health status. Bull Inst Natn Scien Tech Mer de Salammbô, p. 38.
- Boudouresque CF, Pergent G, Pergent MC, Ruitton J, Thibaut T, et al. (2016) The necromass of the Posidonia oceanica seagrass meadow: fate, role, ecosystem services and vulnerability. Hydrobiologia 781: 25-42.
- Mannino AM, Micheli C (2020) Ecological Function of Phenolic Compounds from Mediterranean Fucoid Algae and Seagrasses: An Overview on the Genus Cystoseira sensu lato and Posidonia oceanica (L.) Delile. Journal of Marine Science and Enginering 8(1): 19.
- Alcoverro T, Manzanera M, Romero J (2001) Annual metabolic carbon balance of the seagrass Posidonia oceanica: the importance of carbohydrate reserves. Marine Ecology Progress Series 211: 105-116.
- Elkalay K, Frangoulis N, Skliris C, Goffart A, Gobert S, et al. (2003) A model of the seasonal dynamics of biomass and production of the seagrass Posidonia oceanica in the Bay of Calvi (Northwestern Mediterranean). Ecological modelling 167: 1-18.
- Velimirov B, Lejeune P, Kirschner A, Jousseaume M, Abadie A, et al. (2016) Estimating carbon fluxes in a Posidonia oceanica system: Paradox of the bacterial carbon demand. Estuarine, Coastal and Shelf Science 171: 23-34.
- Gnisci V, Cognetti MS, Belmonte A, Micheli C, Piermattei V, et al. (2020) Assessment of the ecological structure of Posidonia oceanica (L.) Delile on the northern coast of Lazio, Italy (central Tyrrhenian, Mediterranean). Italian botanic 9: 1-19.
- Abadie A (2012) Evolution of Posidonia oceanica (L.) Delile meadows in the Bay of Calvi (Corsica, France) and influence of anchoring in the Bay of Alga. Professional Master's degree "Marine Environment". STARESO, Calvi, France p. 45.
- Litsi MV, Efthymiadis PT, Gerakaris V, Serrano O, Tsapakis M, et al. (2023) Decline of seagrass (Posidonia oceanica) production over two decades in the face of warming of the Eastern Mediterranean Sea. New Phytologist Foundation. 239(6): 2126-2137.
- Rigo I, Montefalcone M, Morri C, Paoli C (2019) Use of ecological indices to assess the health status of Posidonia oceanica meadows in Eastern Liguria. In: Gargiulo C, Zoppi C (Eds.), Planning, nature and ecosystem services. Naples: Fed OA Press pp. 271-280.
- Bonacorsi M, Pergent MC, Breand N, Pergent G (2013) Is Posidonia oceanica regression a general feature in the Mediterranean Sea? Mediterranean Marine Science 14(1): 193-203.
- Montefalcone M (2009) Ecosystem health assessment using the Mediterranean seagrass Posidonia oceanica: A review. Ecological Indicators 9(4): 595-604.
- Romero J, Martinez CB, Alcoverro T, Perez M (2007) A multivariate index based on the seagrass Posidonia oceanica (POMI) to assess ecological status of coastal waters under the water framework directive (WFD). Marine Pollution Bulletin 55(1-6): 196-204.
- Lopez RC, Pergent G, Pergen MC, Casazza G (2010) Seagrass (Posidonia oceanica) monitoring in western Mediterranean: implications for management and conservation. Environmental Monitoring and Assessment 171: 365-380.
- Pergent G, Pergent MC, Boudouresque CF (1995) The use of the meadow of Posidonia oceanica as a biological indicator of the water quality in the Mediterranean Sea: state of the knowledge. Bulletin du Museum d’Histoire Naturelle de Marseille 54: 3-27.
- Giraud G (1979) On a method for measuring and counting the leaf structures of Posidonia oceanica (Linnaeus) Delile. Bulletin of the Museum of Natural History of Marseille 39: 33-39.
- Dauby P, Poulicek M (1995) Methods for removing epiphytes from sea grasses: SEM observations on treated leaves. Aquatic Botany 52: 217-228.
- Gobert S, Sartoretto S, Rico RV, Andral B, Chery A, et al. (2009) Assessment of the ecological status of Mediterranean French coastal waters as required by the Water Framework Directive using the Posidonia oceanica Rapid Easy Index: PREI. Marine Pollution Bulletin 58(11): 1727-1733.
- Moreno D, Aguilera PA, Castro H (2001) Assessment of the conservation status of seagrass (Posidonia oceanica) meadows: implications for monitoring strategy and the decision- making process. Biological Conservation 102(3): 325-332.
- Vassallo P, Paoli C, Rovere A, Montefalcone M, Morri C, et al. (2013) The value of the seagrass Posidonia oceanica: A natural capital assessment. Marine Pollution Bulletin 75(1-2): 157-167.
- Balestri E (2004) Flowering of the seagrass Posidonia oceanica in a north-western Mediterranean coastal area: temporal and spatial variations. Marine Biology 145: 61-68.
- Sghaier YR, Zakhama SR, Charfi CF (2013) Patterns of shallow seagrass (Posidonia oceanica) growth and flowering along the Tunisian coast. Aquatic Botany 104: 185-192.
- Vacchi M, De Falco G, Simeone S, Montefalcone M, Morri C, et al. (2017) Biogeomorphology of the Mediterranean Posidonia oceanica seagrass meadows. Earth surface processes and landforms Earth Surf Process Landforms 42: 42-54.
- Pace M, Borg JA, Galdies C, Malhotra A (2016) Influence of wave climate on architecture and landscape characteristics of Posidonia oceanica meadow Marine Ecology 38(1).
- Semroud R (1993) Contribution to the knowledge of the ecosystem of Posidonia oceanica (L.) Delile in the region of Algiers (Algeria). Study of some compartments. Thesis Oceanography, Houari Boumédienne University of Science and Technology, Alger: pp. 218.
- Peirano A, Damasso V, Montefalcone M, Morri C, Bianchi CN (2005) Effects of climate, invasive species and anthropogenic impacts on the growth of the seagrass Posidonia oceanica (L.) Delile in Liguria (NW Mediterranean Sea). Marine Pollution Bulletin, 50(8): 817- 822.
- Giovannetti R, Alibabaei L, Petetta L (2010) Aggregation behaviour of a tetracarboxylic porphyrin in aqueous solution. Journal of Photochemistry and Photobiology A: Chemistry 211(2-3): 108-114.
- Cancemi G, De Falco G, Pergent G (2003) Effects of organic matter input from a fish farming facility on a Posidonia oceanica meadow. Estuarine. Coastal and Shelf Science 56: 961-968.
- Ruiz JM, Romero J (2003) Effects of disturbances caused by coastal constructions on spatial structure, growth dynamics and photosynthesis of the seagrass Posidonia oceanica. Marine Pollution Bulletin 46: 1523-1533.
- Montefalcone M, Albertelli G, Bianchi CN, Mariani M, Morri C (2006) A new synthetic index and a protocol for monitoring the status of Posidonia oceanica meadows: a case study at Sanremo (Ligurian Sea, NW Mediterranean). Aquatic Conservation: Marine & Freshwater Ecosystems 16: 29-42.
- Waycott M, Duarte CM, Carruthers TJB, Orth RJ, Dennison WC, et al. (2009) Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proceedings of the National Academy of Sciences of the United States of America 106(30): 12377-12381.
- Renzi M, Guerranti C, Anselmi S, Provenza F, Leone M, et al. (2022) Multidisciplinary Approach to Posidonia oceanica Detritus Management (Port of Sperlonga, Italy): A Story of Turning a Problem into a Resource. Water 14: 2856.
- Abidi A (2010) Characterization of the seabed of the Tabarka region: Optimization of mapping methods using geographic information systems. Master, National Agronomic Institute of Tunisia (INAT), p. 96.
- Abadie A, Lejeune P, Pergent G, Gobert S (2016) From mechanical to chemical impact of anchoring in seagrasses: the premises of anthropogenic patch generation in Posidonia oceanica meadows. Marine Pollution Bulletin 109(1): 61‐71.
- Arnaud HS, Migliaccio M, Diaz AE, Teixeira S, Susanne VDV, et al. (2007) Vicariance patterns in the Mediterranean Sea: east–west cleavage and low dispersal in the endemic seagrass Posidonia oceanica. Journal of Biogeography 34(6): 963-976.
- AOAC (2005) Official Methods of Analysis of the Association of Analytical Chemists (AOAC) In: Horwitz W, George W, Latimer JR, 18th Washington DC: AOAC International. Gaithersburg, MD U.S.A. Official methods.
- Ben AH (1972) Distribution and conditions of establishment of Posidonia oceanica Delile and Cymodocea nodosa Ascherson in the Gulf of Tunis. Bulletin of the Oceanographic Institute 2: 331-416.
- Den HC (1970) The seagrasses of the world. Proceedings of the Royal Netherlands Academy of Sciences. Department of Physics, Section, 2, 59(1): 1-275.
- Ben MK, Hattour (1992) Posidonia seagrass beds of the Tunisian coasts. Notes n.s. National Institute of Oceanographic Fisheries Science and Technology, Salammbô, 2: 42.
- Ben MK, Hattour A, Mhetli M, El Abed A, Tritar B (1999) State of the benthic communities from the infra and circalittoral levels of the Gulf of Gabes. Bulletin of the National Institute of Marine Sciences and Technologies of Salammbô, 26: 5-48.
- Ben MK, Komatsu T, Hattour A, Sammari Ch, Zarrouk S, et al. (2002) Tunisian mega benthos from infra (Posidonia meadows) and circalittoral (Coralligenous) sites. Bulletin de l'Institut National des Sciences et Technologies de la Mer de Salammbô 29: 23-35.
- Bonamano S, Piazzolla D, Scanu S, Mancini E, Madonia A, et al. (2021) Modelling approach for the evaluation of burial and erosion processes on Posidonia oceanica Estuarine, coastal and shelf science 254: 107321.
- Bortone SA, Davis WP, Bundrick CM (1989) Morphological and behavioral characters in mosquitofish as potential bioindication of exposure to kraft mill effluent. Bulletin of Environmental Contamination and Toxicology 43(3): 370-377.
- Boudouresque CF, Meinesz A (1982) Discovery of the Posidonia seagrass meadow. Pare Nat Port-Cros notebook 4: 1-79.
- Boudouresque CF, Mayot N, Pergent G (2006) The outstanding traits of the functioning of the posidonia oceanica seagrass ecosystem. Biological Marine Mediterranean 13(4): 109-113.
- Boudouresque CF, Bianchi CN (2013) A new idea: the protection of marine species GIS Posidonie: more than 30 years of service to the protection and management of the marine environment. In: Le Diréach L, Boudouresque CF (eds.), GIS Posidonie publ, Marseille p. 85-91.
- Brock JF (1954) Survey of the world situation on kwashiorkor. Annals of the New York Academy of sciences. 57(6): 696-713.
- Bulteel P, Coulon P, Jangoux M (2020) Population densities of dominant echinoderm species in the Posidonia meadow of Lacco Ameno (Ischia Island, Italy): Preliminary observations. In: Echinoderm Research.
- Celebi B, Gucu AC, OK M, Sakinan S, Akoglu E (2006) Hydrographic indications to understand the absence of Posidonia oceanica in the Levant Sea (Eastern Mediterranean).
- Conference: The Mediterranean Seagrass Workshop At: Malta Volume: Biologia Marina Mediterranea 13(4): 34-38.
- Derbal F, Kara MH (2013) Age, growth and reproduction of the drum seabream Diplodus cervinus cervinus (Sparidae) from the coasts of eastern Algeria. Cybium, 37(4): 247-254.
- Dumestre M, Janson AL, De Bettignies T (2022) Feasibility study for the Red List of marine phanerogam ecosystems in metropolitan France. PatriNat (OFB-CNRS-MNHN) p. 56.
- Fourqurean JW, Duarte CM, Kennedy H, Marbà N, Holmer M, et al. (2012) Seagrass ecosystems as a globally significant carbon stock. Nature Geoscience 5: 505-509.
- Giakoumi S, Gerovasileiou V, Mazor T, Beher J, Possingham HP, et al. (2013) Ecoregion-based conservation planning in the Mediterranean: dealing with large- scale heterogeneity. PLoS One 8: e76449.
- Gobert S, Cambridge MT, Velimirov B, Pergent G, Lepoint G, et al. (2006) Biology of Posidonia. In: Larkum AWD, Orth RJ, Duarte CM, Seagrasses: Biology, Ecology and Conservation, (eds). Springer: Dordrecht pp. 387-408.
- Hemminga MA, Duarte CM (2000) Seagrass Ecology. Cambridge University Press, UK, pp. 298.
- Holmer M, Frederiksen MS (2007) Stimulation of sulfate reduction rates in Mediterranean fish farm sediments inhabited by the seagrass Posidonia oceanica. Biogeochemistry 85: 169-184.
- Hutchinson TH, Ankley GT, Segner H, Tyler R (2006) Screening and Testing for Endocrine Disruption in Fish - Biomarkers As “Signposts,” Not “Traffic Lights,” in Risk Assessment. Environmental Health Perspectives 114: 106-114.
- Kubitzki K (1998) The families and genera of vascular plants. In: Chapter VII Flowering plants Dicotyledons Springer, Germany pp. 167-275.
- Kuo J, Den HD (2006) Seagrass morphology, anatomy, and ultrastructure. In: Larkum AWD, Orth RJ, Duarte CM (eds.). Seagrasses: Biology, Ecology and Conservation. Springer, Netherlands. p. 20.
- Labrosse S, Poirier JP, Le Mouël JL (2001) The age of the inner core. Earth and planetrary Science letters 10(3-4): 111-123.
- Lizaso JS, Guillén JE, Espla AAR (1990) The regression of Posidonia oceanica meadows in el Campello (Spain).
- Marbà N, Diaz AE, Duarte CM (2014) Mediterranean seagrass (Posidonia oceanica) loss between 1842 and 2009. Biological Conservation 176: 183-190.
- Mayot N, Boudouresque CF, Leriche A (2005) Unexpected response of the seagrass Posidonia oceanica to a warm-water episode in the North Western Mediterranean Sea. Comptes Rendus Biologies 328: 291-296.
- Meinesz A, Cirik Ş, Akcali B, Javel F, Migliaccio M, et al. (2009) Posidonia oceanica in the Marmara Sea Aquatic Botany 90(1): 18-22.
- Modigh M, Lorenti M, Mazzella L (1998) Carbon assimilation in Posidonia oceanica: biotic determinants. Botanic Marine 41: 249-256.
- Montefalcone M, Giovannetti E, Morri C, Peirano A, Bianchi CN (2013) Flowering of the seagrass Posidonia oceanica in the NW Mediterranean: is there a link with solar activity? Mediterranean Marine Science 14(2): 416-423.
- Molinier R, Picard J (1954) Elements of marine bionomics on the coasts of Tunisia. Salammbo Oceanography Bulletin 48, p. 47.
- Nemec KT, Raudsepp HC (2013) The use of geographic information systems to map and assess ecosystem services. Biodiversity Conservation 22: 1-15.
- Pasqualini V, Pergent MC, Clabaut P, Pergent G (1998) Mapping of Posidonia oceanica using aerial photographs and side-scan sonar: application of the islands of Corsica (France). Estuarine Coastal Shelf Science 47(3): 359-367.
- Pergent MA, Leoni V, Pasqualini V, Ardizzone GD, Balestri E, et al. (2005) Descriptors of Posidonia oceanica meadows: Use and application. Ecological indicators 5(3): 213-230.
- Pergent G, Leonardini R, Lopez RC, Mimault B, Pergent MC (2008) Implementation of a Posidonies Surveillance Network along the Corsican coast. Synthesis report Office of the Environment of Corsica. Water Agency Rhône Méditerranée – Corsica, Regional Directorate of the Environment of Corsica, GIS Posidonie Center of Corsica, pp. 273.
- Pergent G, Bazairi H, Bianchi CN, Boudouresque CF, Buia MC, et al. (2014) Climate change and Mediterranean seagrass meadows: a synopsis for environmental managers. Mediteranean Marine Science 15(2): 462-473.
- Potter IC, Rose TH, Huisman JM, Hall NG, Denham A, et al. (2021) Large variations in eutrophication among estuaries reflect massive differences in composition and biomass of macroalgal drift. Marine Pollution Bulletin 167: 112330.
- Stipcich P, Apostolaki ET, Chartosia N, Efthymiadis PT, Jimenez CE, et al. (2022) Assessment of Posidonia oceanica traits along a temperature gradient in the Mediterranean Sea shows impacts of marine warming and heat waves. Frontiers in Marine Science, 9.
- Stanbury KB, Starr RM (1999) Applications of Geographic Information Systems (GIS) to habitat assessment and marine resource management. Oceanologica Acta 22(6): 699-703.
- Sraied ZR, Sghaier YR, Charfi F (2011) Raising awareness of the importance of Posidonia oceanica beds in protecting sandy beaches: Participatory approach. Sandy beaches and coastal zone management. Proceedings of the fifth International Symposium on Sandy Beaches, 19th-23rd October 2009, Rabat, Morocco Works of the Scientific Institute, Rabat, general series, 6: 115-119.
- Somaschini A, Gravina ML, Ardizzone GD (1994) Polychaete depth distribution in Posidonia oceanica bed (rhizome and matte strata) and neighbouring soft and hard bottoms. Marine Ecology 15(2): 133-151.
- Vela A (2006) Functioning and primary production of Posidonia oceanica (L.) Delile meadows in the Mediterranean. These pp. 155.
- Wernberg T, Filbee DK (2019) Missing the marine forest for the trees. Environmental Science 612: 209-215.
- Wentworth CK (1922) A scale of grade and class terms for clastic sediments. Journal of Geology 30: 377-392.
- Zenone A, Badalamenti F, Alagna A, Gorb SN, Infantes E (2022) Assessing Tolerance to the Hydrodynamic Exposure of Posidonia oceanica Seedlings Anchored to Rocky Substrates. Frontiers in Marine Science 8.






























