Feeding of Clausocalanus arcuicornis (Dana, 1849) Order Calanoida (Copepoda) in the Coastal Waters of Baniyas City (Eastern Mediterranean)
Wassim Mayya*
Department of Zoology, Damascus university, Damascus, Syria
Submission:January 10, 2024;Published:February 27, 2024
*Correspondence author: Wassim Mayya, Department of Zoology, Damascus university, Damascus, Syria
How to cite this article:Wassim M. Feeding of Clausocalanus arcuicornis (Dana, 1849) Order Calanoida (Copepoda) in the Coastal Waters of Baniyas City (Eastern Mediterranean). Oceanogr Fish Open Access J. 2024; 17(2): 555957. DOI: 10.19080/OFOAJ.2024.17.555957
Abstract
This study, included the feeding of Clausocalanus arcuicornis (Dana,1849) of crustacean zooplankton (Calanoida), by studying the structure of the Mandible and the gut content of this previous species to determine Their favorite food. 76 samples have been collected vertically in period between March and October 2021. The samples were also accompanied with different hydrophysical and hydrochemical measurements in three regions that differ from each other with their environmental properties. The number of members of (C.arcuicornis) that were studied reached (61) individuals, of which (42) are female and (19) are male. On the other hand, determining the morphology, studying its structure, and knowing the content of the gut of the aforementioned species helped in expanding knowledge about the conditions and strategies of feeding it under the influence of environmental factors. The number of algae species (phytoplankton) that C.arcuicornis fed reached (7) species, of which (4) belong to the Dinophyceae, (2) species to Bacillariophyceae earth, and (1) only one species to the group Cryptophyceae. The highest average number of Dinophyceae was (1500), followed by Bacillariophyceae (420) individuals, then the group of Cryptophyceae (140) individuals.
Keywords: The Feeding; Mandible; Gut Content; Hydrophysical and Hydrochemical Measurements; Feeding Strategies
Introduction
Crustacean zooplankton is a Heterotrophic and is an important component of marine ecosystems [1] through the primary role it plays in the food web [2]. As such, it is a biological structure in which the feeding methods vary [3], which increases the complexity and complexity of the food chain [4]. In addition to that herbivorous group dependent on phytoplankton [5], which creates a balanced biological composition [6]. copepods are major components of marine food chains and operate either directly or indirectly as food sources for most commercially important fish [7], and their oral appendices have evolved to suit the nature and quality of their food [8]. On the other hand, the jaw’s leg movement towards the mouth creates a stream of water that raises the pressure inside the mouth, which leads to water entering with food [9], and others are equipped with a special filtration system through which it filters the food particles entering with Water stream [10].
Copepods generally tend to feed on a mixed diet in their natural environment, especially in the first few layers (0-50) m [11], and the survival and success of copepods over the years may be due to their ability to determine prey [12], and the selection of the preferred and most abundant food in the surrounding environment [13]. It is worth noting, however, is the ability of many species of copepodes to shift from a plant-feeding pattern in the absence of it in the medium to feeding on small animals and vice versa as is the case with Glausocalanus arcuicornis [14].
Copepodes are dominant creatures in marine zooplankton [1]. Their diets often include large proportions of Bacillariophyceae that have Silesian structures to protect. Despite this protection, there are many species of copepodes that are capable of breaking and shattering these structures with high efficiency even the most supported and protected species [15]. The composition and shape of mandible teeth at copepodes also differ by species, and studies using electron microscopy have revealed These teeth are of complex microscopic structures that contain in their composition silica and this explains their ability to destroy the structures of Bacillariophyceae [16].
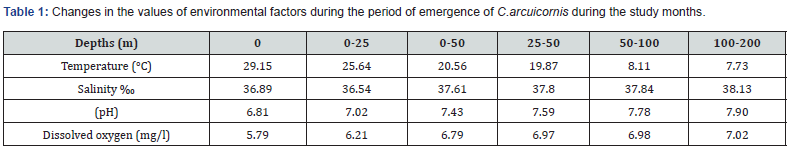
Various environmental factors such as temperature, salinity, dissolved oxygen, pH, and transparency affect marine copepodes and their nutritional activity [9], and the concentration and distribution of food particles in the surrounding medium [17].
The Importance of Research and its Objectives
The research aims to study feeding for C.arcuicornis, determine the intestinal content of food, and study the shape and composition of its species, under the influence of various environmental factors. The importance of economic research lies through clarifying the environmental and nutritional requirements of the studied species, which constitutes a basic rule that facilitates the prediction of the status of these species in terms of productivity, as they are of economic importance and constitute a major food for fish, crustaceans and many other marine creatures.
Materials and Methods
The species collection processes were carried out from the three study areas that were chosen in the coastal waters of the city of Baniyas, which differ from each other in environmental terms, as shown in figure 1, which are
Sanitation area: (A):
35°12′09″N 35°57′08″E
It is located opposite the Baniyas National Hospital, where the sewage of the hospital and the neighborhoods of Al-Morouj flows into a unified liquefaction line (a major sewage line), where its estuary ends in the coastal waters of a city, and this beach is away from the second area (the thermal station area) at a distance of 7 km.
The Thermal Station Area: (Estuary of hot water): (B):
35°10′13″N 35°55′21″E
This region is located opposite the electrical power station in Baniyas, which is one of the five power stations responsible for supplying the country with electrical energy. The thermal plant is 5 km from the third clean area. The thermal water resulting from the cooling of the station and the steam of the boilers that unite with it is poured into marine waters.
Prince Beach Chalets: (C).
35°09′02″N 35°55′20″E
The Prince Chalets Beach, on which the Prince's Resort and Chalets is located, and this beautiful beach is 1 km from the Archaeological Tower of Al-Sabi site. This beach is a very clean area and not exposed to pollution, and therefore it is a destination for tourism and summer vacation.
Each region is divided into three sites (stations):
Zone A: Stations: A3-A2-A1.
Zone B: Stations: B3-B2-B1.
Zone C: Stations: C3-C2-C1.
The process of collecting samples of the two species in each site was as follows:
a) The first location: (50-0) m, (50-25) m, (25-0) m.
b) The second location: (100-0) m, (100-50) m, (50-25) m, (25-0) m.
c) The third location: (200-0) m, (200-100) m, (100-50) m, (50-25) m, (25-0) m.
i. Measurements of the main environmental factors such as: (temperature (t), salinity (s), dissolved oxygen concentration, pH, and transparency) were made by using modern advanced devices with calibration paths, including the Hanna Instruments HI9812-5 device.
ii. To measure salinity and pH, and the Dissolved Oxygen - DO Meter AZ Instrument AZ-8403 to measure the concentration of dissolved oxygen and temperature, while the Seki disk was used to measure transparency, and the global zooplankton collection network with a closing device, with 200µ holes and of the WP2 Closing type, was used. Net in order to obtain the required samples.
iii. I used a submersible lens with a magnification of Specimens of the species Clausocalanus arcuicornis preserved in formalin at a concentration of 4% were studied in the laboratories of the Damascus University according to modern international methods, relying on an advanced and modern microscope, a magnifying glass, and using the microscopic needle necessary to extract the mandible.
iv. The following references were relied upon to identify the phytoplankton found in the intestine, Table 1: [18-21] (Alexandra et al., 2010).
Results
Taxonomic status and general description of the species:
Clausocalanus arcuicornis
Taxonomic status
Phylum: Arthropoda
Subphylum: Crustacea
Class: Copepoda
Order: Calanoida
Family: Clausocalanidae
Genus: Clausocalanus (Giesbrecht, 1888)
Species: Clausocalanus arcuicornis (Dana,1849).
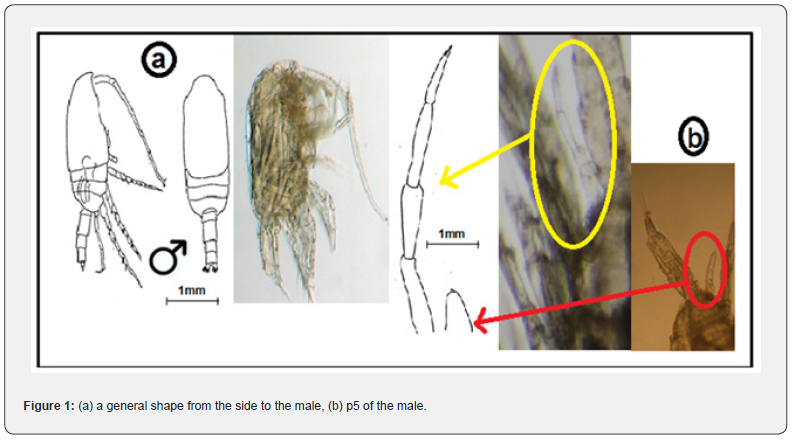
Male
The length of the male (0.7-0.9mm) and in terms of its overall appearance is very similar to the female. The front end of the head was round, while the abdomen extended, and the fifth leg of the legs had a four-legged left leg. As for the right hand, it is atrophied [22] figure 1.
Female
The length of the female (1.1-1.2mm), the front end of the head was round, fifth of the legs has a single branch and two parts [22] figure 2.
Feeding of Clausocalanus arcuicornis
C.arcuicornis appeared in all study areas and stations, and the total number of individuals studied was (64) individuals, of which (44) are female and (20) male are distributed at different depths, and this explains that this species has wide environmental adaptation. Eurybiont with the values of different environmental factors [23] as shown in Table 1. The largest presence was in the layer with depth (0-50) m [24], due to the large number of nutrients in this layer, as it is the primary productivity layer [25], where the phytoplankton that exists Photosynthesis, The presence of marine currents and wave movement, and the exposure of the layer (0-50) m to significant changes in the values of environmental factors, as well as the Estuary drainage, whose water is loaded with organic materials and nutrients and which flows into station (A1), has made this layer a suitable place for the existence of the species Previous (Hidaka et al.;2016), and this species did not exist in station (B1) at all, and the reason is that this station is the mouth of the hot water resulting from cooling the turbine of the station and the boilers. Previous to bear it or even live in its field.

Discussion
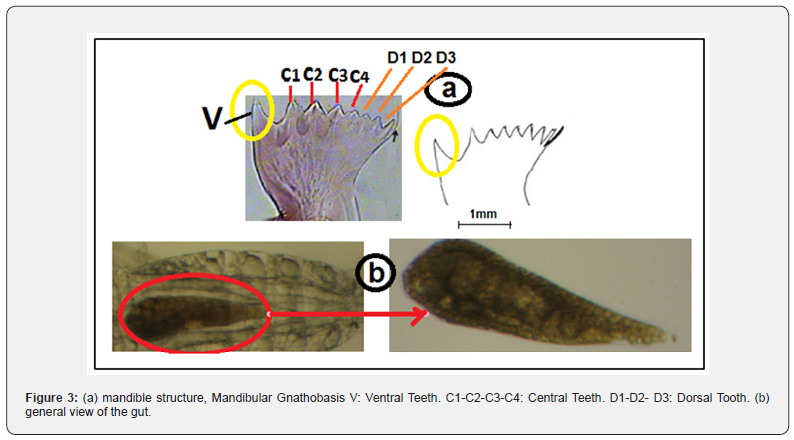
It was found through the study, which was compatible with many international studies, table 2, that C.arcuicornis is herbivorous [26] as shown in table 3, and perhaps the most important food for him is (Dinophyceae) [27,28], knowing that by studying the intestinal content of figure 3 for individuals of the previous species within the layer (50-0) m, it was observed that there are several species of food and this explains that the previous species resort to mixed food [29] and feeding on more than one species due to the high availability of food within them [30], This is illustrated in table 3, whereas at large depths (200-100 m), it resorted to the use of only one species of food, according to what is available in the medium and most likely from Bacillariophyceae [31]. The reason is that these depths Light does not reach it, and phytoplankton does not exist to carry out photosynthesis, and consequently, it is poor layers of food, in which species resort if there is dependence on food available in the medium [7].

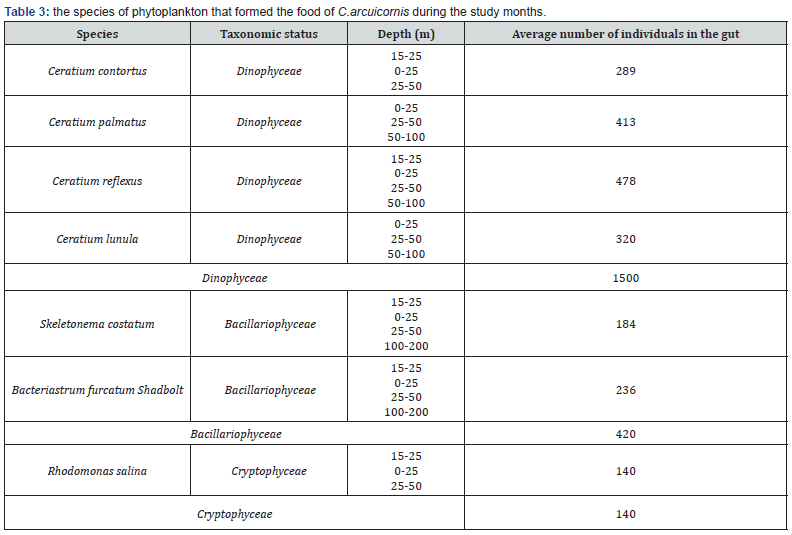
It was found through the current study that the layers with depths (50-0) m, (50-25) m and (15-0) m are the richest layers for the phytoplankton species on which C.arcuicornis feeds, especially Dinophyceae, and the reason for this is due to the presence of light. And for the high and appropriate temperature compared to the deep layers where there is no lighting and the temperature decreases, and thus phytoplankton, which is the main food of the studied species, is unable to exist and live at those depths [27]. Dinophyceae recorded the highest value, as the average number of (1500) individuals within the gut were at the species mentioned above, as in table 3, compared with the rest of the groups. He made its species the main food of the studied species, followed by the Bacillariophyceae (420) individuals in the gut, then the Cryptophyceae (140) individuals, table 3 and this is consistent with [26].
It was observed through studying the gut structure of C.arcuicornis that its preferred species as a suitable food is Ceratium reflexus, and it was found that in the shallow layers it resorts to feeding on a number of species at the same time, including the two species Ceratium palmatus and Ceratium reflexus, where the temperature is high and the salinity is low [25] and occasionally feeding on Skeletonema costatum, Rhodomonas salina, Ceratium lunula, while it resorts to feeding on one species only, such as Bacteriastrum furcatum Shadbolt, in the deep layers due to low temperature and high salinity [30]. Thus, environmental factors, especially temperature and salinity, play an important role in changing the feeding conditions and strategies of the species. The study was studied and adapted to the quantity and quality of food available in the surrounding medium [25]. C.arcuicornis diets include large proportions of Bacillariophyceae that possess protective silicic structures, and despite this protection, the former species is able to break and destroy these structures with high efficiency even the most supported and protected species of Bacillariophyceae [14], and returns the reason for this is the mandible shape of the teeth (4,3), which has short sharp edges that are used for grinding Bacillariophyceae and the rest of the phytoplankton species.
According to many international studies, table 2, which was done using an electron microscope, mandible teeth show a nano particular structure containing a little amorphous silica and a large percentage of crystalline silica that is spread in the form of a network of micro-ketinic fibers [9]. It is also likely that these fibers will serve as the mainstay during the feeding process [26], and the ventral tooth (V) Figure 3 is the most important tooth in mandible at C.arcuicornis and at the rest of the copepods, especially Calanoida, which is the most important teeth varies according to species, on the other hand (MxI) plays an important role in this species in preserving and holding food particles and preventing their exit as a prelude to their entry and push into the body of the organism [30].
The (MxP) also play an important functional role for the previous species in particular and for copepods in general, through their movement with the presence of water currents in collecting food particles from the surrounding medium and surrounding them well and pushing them towards the inside of the body [25].
Conclusion
From the above we find that C.arcuicornis plays with the rest of the copepods a major role in marine food webs, as they form the link between phytoplankton on the one hand and secondary consumers [32-37].
References
- Al Hanoun KS, Zaeni A (2017) Theory of Zooplankton. In: Directorate of University Books and Publications, Tishreen University Press, Syria, pp. 17-295.
- Siokou FI, Bianchi M, Christaki U, Christou ED, Giannakourou A, et al. (2002) Carbon Flow in the Planktonic Food Web along a Gradient of Oligotrophy in the Aegean Sea (Mediterranean Sea). J Mar Syst 33-34: 335-353.
- Aleya L, Michard M, Khattabi H, Devaux J (2006) Coupling of the Biochemical Composition and Calorific Content of Zooplankters with the Microcystis aeruginosa Proliferation in a Highly Eutrophic Reservoir. Published in: Environmental Technology, 27 (11): 1181-1190.
- Avery DE, Altland KK, Dam HG (2008) Sex-related differential mortality of a marine copepod exposed to a toxic dinoflagellate. Limnol Oceanogr 53(6): 2627-2635.
- Bottger SR (2011) Taxonomic re-examination and distribution of copepods reported as Oncaea notopus Giesbrecht, 1891 (Copepoda, Oncaeidae) in the Mediterranean Sea. Marine Biodiversity 41: 325-341.
- Guisande C, Maneiro I, Riveiro I, Barreiro O, Pazos Y (2002) Estimation of copepod trophic nichen in the field using amino acids and marker pigments. Mar Ecol Prog Ser 239: 147-156.
- Sahar K, Nancy F, Mercado S, Pedro MA (2020) Genus level molecular phylogeny of Aegisthidae Gisbrecht, 1893 (Copepoda: Harpacticoida) reveals morphological adaptations to deep-sea and plagic habitats. BMC Evolutionary Biology.
- Battuello M, Mussat SR, Brizio P, Nurra N, Pessani DMC, et al. (2017) The influence of feeding strategies on trace element bioaccumulation in copepods (Calanoida). Ecological Indicators 74: 311-320.
- Łukasz S (2020) Variability of Mandible Shape in the Marine Glacial Relict Eurytemora Lacustris (Poppe, 1887) (Copepoda, Calanoida, Temoridae) 93(3-5): 337-353.
- Mageed AA (2006) Spatial-Temporal variation of zooplankton community in the hypersaline lagoon of Bardawil, north Sina-Egypt. Egyptian Journal of aquatic research 32(1): 186-193.
- Maps F, Record NR, Pershing AJ (2014) A metabolic approach to dormancy in pelagic copepods helps explaining inter- and intra- specific variability in life -history strategies. Journal of Plankton Research 36: 18-30.
- Kang YS, Kim JY, Kim HG, Park JH (2002) Long-term changes in zooplankton and its relationship with squid, Todarodes pacificus, catch in Japan. East Sea, Fish Oceanogr 11(6): 337- 346.
- Kim YO, Shin K, Jang PG, Choi HW, Noh JH, et al. (2012) Tintinnid species as biological indicators for monitoring intrusion of the warm oceanic waters into Korean coastal waters. Ocean Sci J 47: 161-172.
- Valeria CDA, Hoffmeyer MS, Mariana D (2020) Morphology of the mandibular gnathobases of the copepods Calanus australis and Calanoides carinatus: Evidence of omnivory. Zoologischer Anzeiger 286: 64-71.
- Jan M, Stanislav NG (2016) Mandibular Gnathobases of Marine Planktonic Copepods - Structural and Mechanical Challenges for Diatom Frustules, Evolution of Lightweight Structures. Part of the Biologically-Inspired Systems pp. 59-73.
- Abigail ST, Houshuo J, Nicholas SF (2020) Copepod feeding strategy determines response to seawater viscosity: videography study of two calanoid copepod species. Journal of Experimental Biology 223(13): 20-33.
- Brosset P, Lloret J, Munoz M, Fauvel C, Van Beveren, et al. (2019) Body reserves mediate trade-offs between life-history traits: New insights from small pelagic fish reproduction. Royal Society Open Science 21: 214-236.
- William T, Kersey S, Munger P (2010) Guide of Marine Phytoplankton. Col & b/w figs, tabs. Nova Science Publishers, ISBN: 9781607410874, Hardback, pp. 382.
- Mona H, Malte E, Gerhard D (2009) Marine Phytoplankton Selected microphytoplankton species from the North Sea around Helgoland and Sylt. In: ISBN 978-3-510-61392-2, paperback 264: 87.
- Castellani, Claudia, Edwards, Martin (2017) Guide to Marine Plankton: A Practical Guide to Ecology, Methodology, and Taxonomy. Oxford University Press, Oxford pp. 704.
- Anora A, Romano G, Carotenuto Y (2011) Impact of the diatom Oxylipin 15S-HEPE on the Reproductive Success of the copepod. Temora Discaudata. Hydrobiologia 666: 265-275.
- Al Hanoun KS, Zaeni A 2020) Practical Book - Zooplankton -Second Edition, Directorate of University Books and Publications, Tishreen University publications pp. 276.
- Mayya WM (2018) Taxonomical and Ecological study of crustacean zooplankton (Arthropoda) in the coast water of Tartous City Thesis prepared for a master's degree in environment and animal classification. Tishreen University, College of Science pp. 184.
- Al Arraj L (2017) Diversity and Copepods’composition of Moroccan Atlantic Coast (Northwest Africa). European Scientific Journal 13(18): 272-293.
- Tomonari K, Chihong S, Kazuyoshi M, Shigenori N, Kota O, et al. (2019) Evolutionary transformation of mouthparts from particle-feeding to piercing carnivory in Viper copepods: Review and 3D analyses of a key innovation using advanced imaging techniques. Frontiers in Zoology.
- Cornils A, Held C (2014) Evidence of cryptic and pseudocryptic speciation in the Paracalanus parvus species complex (Crustacea, Copepoda, Calanoida). Front Zool 11(1): 19.
- Todd A, Egerton S, Handy M, Whitney LS, Juliette LS, et al. (2020) Characterization of Dinophysis spp. (Dinophyceae, Dinophysiales) from the mid‐Atlantic region of the United States. J Phycol 56(2): 404-424.
- Ibrahim AMM (2014) Marine Plankton and Genus Ceratum in West Coast of the Red Sea. Blue Biotechnal J 3(3): 295-341.
- Jiang H, Paffenhofer GA (2004) Relation of behavior of copepod juveniles to potential predation by omnivorous copepods: An empirical-modeling study. Mar Ecol Prog Ser 278: 225-239.
- Sota K, Susumu O (2020) New genus and species of calanoid copepods (Crustacea) belonging to the group of Bradfordian families collected from the hyperbenthic layers off Japan. ZooKeys 951: 21-35.
- Jan M, Stanislav NG (2015) Mandibular gnathobases of marine planktonic copepods –feeding tools with complex micro- and nanoscalecomposite architectures. Beilstein Journal of Nanotechnology 6: 674-685.
- Al Hanoun KS, Zaeni A (2007) Practical zooplankton. In: (First Edn.), Directorate of University Books and Publications. Tishreen University Press, Syria, pp. 25-138.
- Lena T, Anna S, Wilhelm H, Holger A (2018) Trophic interactions and life strategies of epi- to bathypelagic calanoid copepods in the tropical Atlantic Ocean. Journal of Plankton Research 36(4): 1109-1123.
- Masayoshi S, Koh M, Yuichiro N, Toshi N, Shuhei N (2013) Feeding habits of mesopelagic copepods in Sagami Bay. Progress in Oceanography 110: 11-26.
- Mianrun C, Dongyoung K, Hongbin L, Chang KK (2018) Variability in copepod trophic levels and feeding selectivity based on stable isotope analysis in Gwangyang Bay of the southern coast of the Korean Peninsula. Biogeosciences 15(7): 2055-2073.
- Peter T, Enric S, Thomas K (2013) Sensory capabilities and food capture of two small copepods, Paracalanus parvus and Pseudocalanus sp. Sciences of Limnology and Oceanography 58(5): 1657-1666.
- Ricardo G, González HE (2004) Mandible characteristics and allometric relations in copepods: A reliable method to estimate prey size and composition from mandible occurrence in predator guts. Revista Chilena De Historia Natural 77: 607-616.






























