The Diet of Cephalopholis Cruentata: A functional Perspective of a Predator in a Natural Protected Area of the Mexican Caribbean
Alfrancis Teresa Arredondo Chávez1, Carlos Alberto Niño Torres2, Francisco Martínez Servín3, Omar Domínguez Domínguez3 and José Adán Caballero Vázquez1*
1Scientific Research Center of Yucatán, Water Sciences Unit Laboratory of Ecology and Biodiversity of Aquatic Organisms, Mexico
2University of Quintana Roo, International Foundation for Nature and Sustainability (FINS), Mexico
3Aquatic Biology Laboratory, Michoacana University of San Nicolás de Hidalgo, Mexico
Submission:January 09, 2024;Published:January 25, 2024
*Correspondence author: DJosé Adán Caballero Vázquez, Scientific Research Center of Yucatán, Water Sciences Unit Laboratory of Ecology and Biodiversity of Aquatic Organisms, Mexico
How to cite this article:Alfrancis Teresa Arredondo C, Carlos Alberto Niño T, Francisco Martínez S, Omar Domínguez D, José Adán Caballero V. The Diet of Cephalopholis Cruentata: A functional Perspective of a Predator in a Natural Protected Area of the Mexican Caribbean. Oceanogr Fish Open Access J. 2024; 17(1): 555955. DOI: 10.19080/OFOAJ.2024.17.555955
Abstract
Groupers (Family: Serranidae) are ecologically and commercially important fishes for the marine ecosystems and coastal communities of the Greater Caribbean. Groupers are considered generalist carnivores, however, detailed trophic information on the diet of most of the species in the group is not available, such is the case of Cephalopholis cruentata. This work aims to describe the diet of C. cruentata in Puerto Morelos Reef National Park in the Mexican Caribbean. From October 2014 to May 2015, 134 individuals were collected from 18 sites in PMRNP. The roving diver method was used to find and capture the organisms. The size (standard length, cm) and weight (g) of each fish were obtained. Fishes were eviscerated, and the stomach was fixed in 96% alcohol. In the laboratory, the items obtained were identified to the lowest possible taxonomic level. To assess the composition of contents of gastrointestinal tracts, contents the numerical (%N) and gravimetric (%W) percentages were calculated, and the percentage of frequency of occurrence (% FO) was obtained and used to determine the index of relative importance (% IRI). Twenty-four alimentary items were identified as part of the C. cruentata diet, representing 16 families, 18 genera, and 15 species. The dietary Index of Relative Importance reaches 64.19% for crustaceans and 35.81% for fish, suggesting that C. cruentata is a generalist carnivore. The most important prey species were the crustaceans Neogonodactylus curacaoensis (30.3%), Periclimenes rathbunae (6.7 %) and Pseudosquilla ciliata (3.8%), and the fish species Clepticus parrae (16.6%), Caranx ruber (5.8%), Chromis cyanea (5.3%) and Thalassoma bifasciatum (4.1%).
Keywords: Diet; Reef fish; Groupers; Generalist predator
Introduction
Predatory fishes are an essential component of marine food webs, being ecologically important since species diversity in the ecosystem depends mainly on their feeding habits [1], structural complexity, and food availability [2,3]. Predators control the population of their prey, thus preventing potential impacts that other trophic roles (e.g., herbivory/bioerosion) may have on coral reefs once released due to a decrease in predatory populations. Groupers (Serranidae: Epinephelinae) inhabit the littoral and sublittoral zone of tropical and subtropical seas and are considered active predators feeding upon a wide variety of fishes, crustaceans, and cephalopods, benthically or in the water column. The larger groupers (as well as others Pargos: Lutjanidae and other carnivores) vary in size and feeding behaviors to be classified as higher trophic levels and are also considered as one of the main predators of rocky and coral reef environments, placed in the higher trophic levels of the food web, therefore playing a key role in the maintenance of the communities they inhabit and in the reef ecosystems in general [3-7].
There are nearly 300 species of groupers, of which approximately 60 species can be found within the Greater Caribbean [8], many other species within the family are mesopredators (medium trophic levels), but the presence of larger groupers within coral reef ecosystems is an indicator of healthy reefs [9,10]. Groupers are also important in artisanal and commercial fisheries, making them vulnerable; a general decrease in abundance and mean catch sizes have been observed [11,12]. The species Cephalopholis cruentata is one of the most captured fish within the Caribbean and Gulf of Mexico local fisheries [13], is endemic to the Greater Caribbean and has been classified as “least concern” in the Red List of Threatened Species of the International Union for Conservation of Nature (UICN) [14].
This species reaches 42 cm in standard length and up to 2 kg in weight, inhabiting mainly reefs and the surrounding soft bottoms. It is reportedly at the top of the food web, feeding on gastropods, bivalves, crustaceans, and fishes [15]. Despite its ecological relevance to reef ecosystems and its economic importance in artisanal fisheries, information on the biology of C. cruentata is scarce, and little is known about its feeding ecology (Beukers-Stewart and Jones, 2004, Robertson et al., 2015), even the entire genus has been poorly studied [1].
Even with the existing information, the study of the feeding habits of C. cruentata in natural ecosystems is necessary to understand the ecological role that this species has in marine ecosystems, feeding regimes, diet types and the transfer of matter and energy within the food web. Therefore, they help to determine the trophic level of the species in the food web and their role in the reef ecosystem [16,17]. This information is also important to resource managers and reef conservation policies in protected marine areas such as the Puerto Morelos Reef National Park, Mexican Caribbean (PMRNP).
Accordingly, we evaluate the diet of C. cruentata by review of the stomach contents of gastrointestinal tracts (GIT) of specimens from the Puerto Morelos Reef National Park located in the northern zone of the Mexican Caribbean to ratify the species as a generalist carnivore and to advance the nutritional and ecological knowledge of the species in the region.
Materials and Methods
Study area
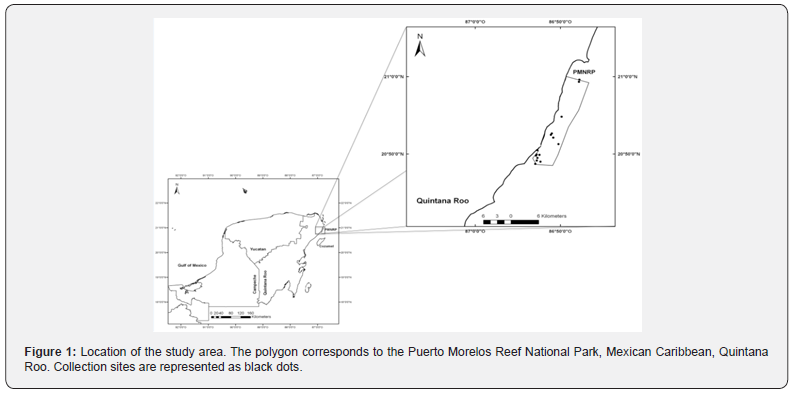
The PMRNP is located on the northeastern Yucatan Peninsula in Quintana Roo, Mexico. This marine protected area (MPA) was declared in 1998 and is among the first in Mexico to have been created through a community-based approach (Murray, 2005), with local stakeholders assuming the responsibility for elaborating the management program (published in 2000), and has an area of 9,066 ha, extending for 21 km along the NE coast of the Yucatan Peninsula and from the beach to 4.5–5 km seaward. The MPA contains a fringing reef that is close to shore (<3.5 km), which has been described in several papers [18]. Currently, major threats to the PMRNP are reef structure/composition, coral bleaching and white syndrome associated with climate change and tourism-related urban development [18,19].
Sample collection
To collect C. cruentata specimens, 37 dives were conducted at 18 reef areas, with a 3 to 18 meters depth within the PMRNP (Figure 1). Five divers participated in each diving event. Dives were carried out three times a day: morning (between 07 and 11 h), afternoon (between 12 and 17 h), and night (between 19 and 21 h), and each dive lasted 45 min, approximately. A roving diving approach was used to find fish, and they were collected using a multi-pronged pole spear. We recorded the size (standard length; cm) for each individual using a measuring board and its total weight (g) using an electronic weighing balance (ACCULAS Sartorious Group). Once the fish was on board, we dissected specimens and fixed their GIT contents in 96% v/v alcohol for subsequent analysis. Each fish and its GIT contents were labeled with an ID code.
Stomach content analysis
In the laboratory, we identified prey items found in the GIT of C. cruentata specimens to the lowest possible taxonomic level. We noted the number of empty GIT. Fishes were identified using McEachran and Fechhelm [20], Humman & Deloach [21], Fish Base (2014), and the Shorefishes of the Greater Caribbean online information system v.1.0 [15], age structure and sex determination, was obtained from Heemstra & Randall [5]. We used the keys by Abele & Kim [22] for crustaceans. We used the Food and Agriculture Organization of the United Nations [5] identification keys for both groups. Then, we counted prey items and weighed them to the nearest hundredth of a gram.
To determine if the number of stomachs examined was representative of diet determination, we calculated a species accumulation curve using the software Estimate S 9.1.0. [23,24]. We calculated curves with unconditional 95% confidence intervals using the following equation from Colwell et al. [24,25]: . We used the percentages of frequency of occurrence (%FO), percentages numerical (%N), and percentages gravimetric (%W) to determine the index of relative importance of each of the prey items found in the stomach contents of C. cruentata (Hyslop, 1980, Pinkas et al., 1971). We used the Shannon-Wiener diversity index to analyze variability in the diet (Krebs, 1999) as follows: , where Pj is the proportion of individuals in the j-th prey species.
We calculated the amplitude of the trophic niche to determine if C. cruentata shows any specialization for existing food resources using the standardized Levin index [16]: , where Pj is the proportion of individual fish that consumed a certain food resource relative to the number of resources used by the total number of fish, and n is the number of prey categories. This index ranges from 0 (minimum niche width and maximum specialization) to 1 (maximum niche width and minimum specialization).
Results
We collected 134 specimens of C. cruentata. Their total length varied from 7.5 cm to 30 cm (mean = 19.5 ± 0.4 cm). The weight ranged from 5.5 g to 525.3 g (mean = 130.3 ± 6.8 g). Forty-three (32.09 %) of the fish collected had prey items in their GIT; most were adult specimens. The prey species accumulation curve did not reach the asymptote (Figure 2).

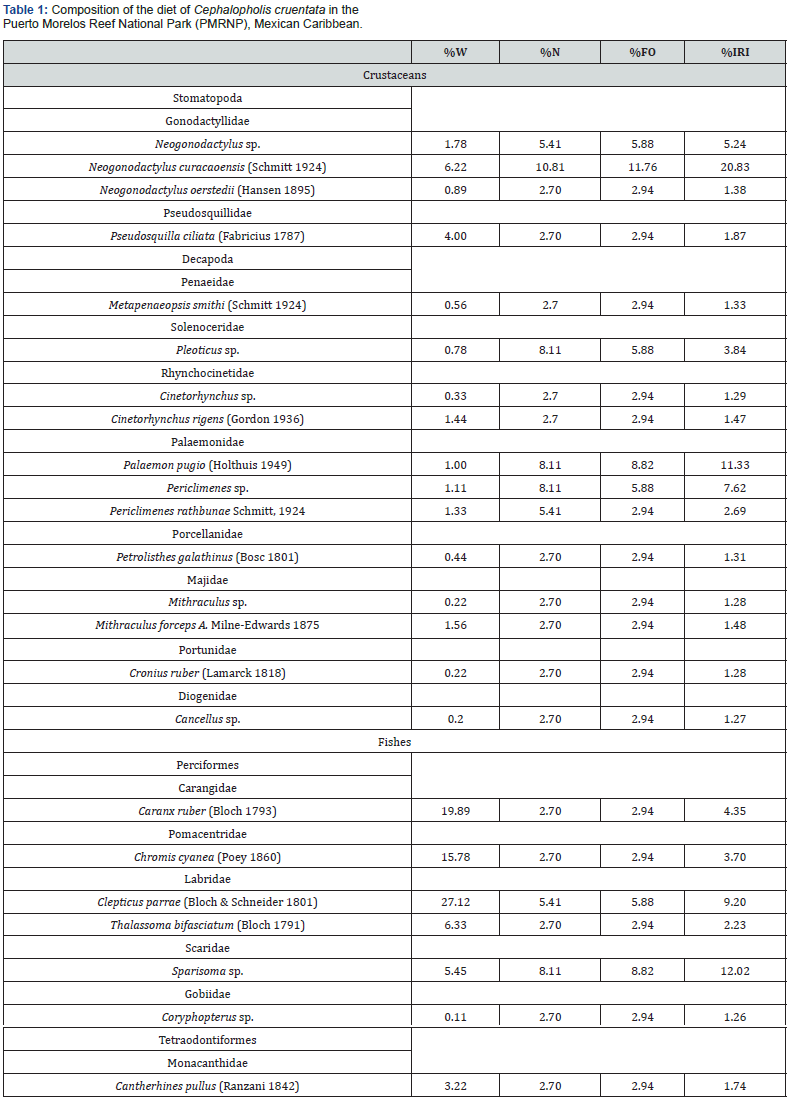
The 24 items identified as part of the C. cruentata diet are represented by 16 families, 18 genera, and 15 species (Table 1). Several food items could only be identified to the family or genus level, and, in some cases, classification was only possible as remains of fish or crustaceans. According to the %W, teleosts were the most important prey item in the diet of C. cruentata (78.25%), followed by crustaceans (21.75%). Crustaceans dominated the GIT contents numerically with 62.23 %N, while fish reported 37.77 %N. According to the %FO, the crustaceans were present in 69.7% of the stomachs, while the fish were in 30.3 %. The IRIs obtained were 35.81% for the fish group and 64.19% for crustaceans.
Families with the highest IRI values were Palaemonidae (35.37%), Gonodactylidae (27.04%), Labridae (9.49%), Scaridae (7.05%), Majidae (3.08%), Rhynchocinetidae (3.08%), Carangidae (2.46%) and Pomacentridae (2.11%) (Figure 3).

Genera with the highest IRI values were Neogonodactylus (40.89%), Periclimenes (12.49%), Sparisoma (7.9%), Palaemon (7.44%), Clepticus (6.05%), Pleoticus (4.97%), Mithraculus (3.45%) and Cinetorhynchus (3.45%) (Figure 4).
Species with the highest IRI values were Neogonodactylus curacaoensis (30.3%), Clepticus parrae (16.6%), Periclimenes rathbunae (6.7%), Caranx ruber (5.8%), Chromis cyanea (5.3%) and Thalassoma bifasciatum (4.1%) (Figure 5).


According to the %FO, 96% of the prey records were classified as accidental (Accidental f < 10); the remaining 4% correspond to secondary prey (secondary f >10 o < 50), without a preferential category. According to the Levin index, the value obtained was high (Bi = 0.6), classifying C. cruentata as a generalist carnivore. This value is congruent with the %FO, where no preference for any prey was found, and the diversity value of the consumed prey was H´ = 2.65 (bits/ind.).
Discussion
The coral grouper, Cephalopholis cruentata, is a carnivorous predator; its diet consists mainly of a variety of smaller fish, crustaceans, and to a lesser extent, cephalopods. Studies of the feeding habits of C. cruentata in the Atlantic regions of the greater Caribbean are scarce. Before this study, the only data was a general description of higher taxonomic groups on which C. cruentata feed [11,15]. The information obtained in the present study is the first to report the feeding ecology of C. cruentata for the region.
According to our results, the diet of C. cruentata in the PMRNP is composed of fish and crustaceans, which is similar to the reported for other grouper in the greater Caribbean [1,6,7,26,27] and is similar to that of the lionfish (Pterois volitans) [28], indicating a possible competition for prey items with this exotic and invasive species. The %FO determined that most of the prey are considered secondary and accidental; C. cruentata did not show a preference to any particular prey. This is supported by the results of the Levin index that classify C. cruentata as a generalist carnivore. The species accumulation curve with the data analyzed did not reach the asymptote; a larger number of organisms and a larger sample size are required to achieve it (Nakai et al., 2001, Shpigel and Fishelson, 2004, Dierking et al., 2009). This is consistent with what is known for other grouper species, for which prey species accumulation curves also do not reach the asymptote even when included in a high sample size (>200). This suggests a broad trophic niche for the group [26,29] and the need for a more extensive sampling effort.
According to the numerical index (%N) and the frequency of appearance used to estimate the importance of prey, C. cruentata had a broad trophic niche and a high percentage of crustacean prey. In contrast, fish are not encountered as frequently but have a higher contribution to dietary biomass (Table 1). According to the IRI, Palaemonidae is the most important family in the diet of C. cruentata. This family has also been reported in the diet of the congener Cephalopholis urodeta [26]. The Palaemonidae family is associated with invertebrate organisms such as corals and anemones and is reported as abundant in reef ecosystems of Mexico [30]. The group has an ecological function as a fish parasite cleaner [31]. Within this family, the genus Periclimenes is the most abundant prey. Another important family in the diet of C. cruentata is Gonodactylidae, in the order Stomatopoda, with Neogonodactylus curacaoensis being the most recorded species. This stomatopod is a predator that feeds upon different groups (fish, mollusks, annelids, crustaceans, and other invertebrates) and can capture prey of considerable size, while also serving as prey to other carnivorous organisms, such as C. cruentata [32-34].
Gravimetric percentage = %W, Numerical abundance = %N, frequency of occurrence = %FO and Index of Relative Importance = %IRI.
The fish species with the greatest importance in the diet of Cephalopholis cruentata is Clepticus parrae, from the Labridae family. Several authors have reported This family in the diet of grouper species [1,26,29]. Clepticus parrae, commonly known as the creole wrasse, feeds mainly on zooplankton and is one of the most abundant species in the Caribbean reefs [27,35]. The Scaridae family was also recorded in the diet of C. cruentata, with the prey of the genus Sparisoma being identified; however, the frequency of this family in the diet of C. cruentata was low: only three individuals were recorded. The Scaridae are herbivorous and associated with reef areas [15]. Another species recorded was Chromis cyanea from the Pomacentridae family. This species feeds on zooplankton and pelagic eggs, among other organisms. Some predatory fish species were also recorded in the diet of C. cruentata, such as C. ruber of the Carangidae family. This species feeds on fish, crustaceans, and mollusks [15].
The main prey identified in the diet of C. cruentata has also been recorded in the diet of lionfish [28], generating a possible direct competition for food between the two species. However, the lionfish maintains some advantage over the local predatory species since its prey does not identify it as a threat or potential predator [36]. The diet of Cephalopholis cruentata is very varied since it feeds on different species. Since it does not have a preference for any prey, it is considered a generalist carnivore, so more studies are required on the diet of the species to determine the complete trophic spectrum of the species; also, a comparative study between the lionfish and other top predators in Mexican Caribbean National Parks need to be conducted to understand better the invasion of the lionfish and the possible effect in the reef systems food web [37-40].
The predator-prey relationship in C. cruentata is important for maintaining the ecological balance in marine ecosystems in the Mexican Caribbean. As a top predator, C. cruentata plays a crucial role in regulating the populations of its prey. However, it may impact the composition and relative abundance of organisms and species; predation pressure maintains biodiversity and community structure. Analyzing and understanding these interactions is essential for conserving and managing reef systems and associated habitats inhabited by this predator. With the invasion of the lionfish (Pterois volitans) into the wider Caribbean, a predator with similar feeding ranges to C. cruentata, the predator-prey relationship may have cascading effects on the trophic structure's lower and upper trophic levels. Increased predation has a direct impact on the availability of food for predators. A decrease in the quantity or quality of prey can affect the fitness and reproduction of predators and their ability to survive and maintain their populations. Conversely, an uncontrolled increase in prey populations can affect the availability of food resources for other species and cause changes in community structure. In contrast, an increase in their predation of prey causes a decrease in their populations, leading to changes in their behavior and interactions with other predators and competitors. These interactions can have important implications for marine ecosystems and fisheries if the population of commercial prey of ecological interest declines due to overfishing or other factors such as water pollution or effects caused by climate change.
In summary, changes in the prey population of interest or a population of key prey species can significantly affect the people of predators, such as Cephalopholis cruentata, and vice versa. Studying these interactions is essential for knowledge, sustainability, and long-term conservation of reef ecosystems.
Acknowledgment
ATAC would like to thank the Posgrado en Ciencias del Agua, The Yucatan Scientific Research Center (Centro de Investigación Científica de Yucatán - CICY), and National Council for the Humanities, Sciences and Technologies (Consejo Nacional de Humanidades, Ciencias y Tecnologías - CONACHyT) for fellowship support. This work was supported by funding provided by The National Commission for the Knowledge and Use of Biodiversity (CONABIO)- LH003 and Michoacán University of San Nicolás de Hidalgo) CIC-UMSNH 2015.
To the National Commission of Natural Protected Areas (CONANP) members in Puerto Morelos and Captain Luis Ramos. To Juan Antonio Sánchez Jiménez, Margarita Yareli López Arroyo, Aurora Lizeth Moreno Vázquez, and Marcos Noe López Zacarías who helped in the collection of organisms, and to the members of the CPUM for their collaboration in the sampled process. To Tom Jameson from Chester Zoo for English corrections and suggestions. Organism collection was supported and allowed by fishing permit No. DGOPA.08990.031111.3073.
References
- Shpigel M, Fishelson L (2004) Food habits and prey selection of three species of groupers from the genus Cephalopholis (Serranidae: Teleostei). Environmental Biology of Fishes 24 (1): 67-73.
- Boaden AE, MJ Kingsford (2015) Predators drive community structure in coral reef fish assemblages. Ecosphere 6(4):1-33.
- Roff G, Doropoulos C, Rogers A, Bozec YM, Krueck NC, et al. (2016) The Ecological Role of Sharks on Coral Reefs. Trends Ecol Evol 31(5): 395-407.
- Parrish JD (1987) The trophic biology of snappers and groupers. In: Polovina JJ, Ralston S, Tropical snappers and groupers: biology and fisheries management (eds.), pp. 405-463.
- Heemstra PC, Randall JE (1993) FAO Species Catalogue Vol. 16, Groupers of the world (Family Serranidae, Subfamily Epinephelinae): An annotated and illustrated catalogue of the grouper, rockcod, hind, coral grouper and lyretail species known to date. FAO Fisheries Sypnosis, 125(16):
- Giménez E, Anderes B, Moreno V, Burgos R (2001) Aspectos de la conducta alimentaria del mero (Epinephelus morio) del Banco de Campeche. Ciencia Pesquera 14: 165-170.
- Puerto NE, Pérez DE, Rénan X, Brulé T (2005) Feeding habits of juvenile Cuna Bonaci Mycteroperca bonaci, (Pisces: Serranidae) along the northern coast of Yucatan, Mexico. In: Aquacom Changes, Proceedings of the Gulf and Caribbean Fisheries Institute 56: 307-320.
- Hooker HB, Santos MA, Peñaloza G, Taylor E (2010) Agregaciones Reproductivas de Grandes Serranidos en el Anp Centro de la Reserva de Biosfera Seaflower. Gulf and Caribbean Fisheries Institute 63: 166-173.
- Hodgson G, Liebeler J (2002) The global coral reef crisis - trends and solutions. Reef Check, Institute of the Environment, University of California at Los Angeles p. 77.
- Mumby PJ, Steneck RS, Edwards AJ, Ferrari R, Coleman R, et al. (2012) Fishing down a Caribbean food web relaxes trophic cascades. Marine Ecology-Progress Series 445 (20): 13-24.
- Zapelini C, Giglio VJ, Carvalho RC, Bender, MG, Gerhardinger LC (2017) Assessing Fishing Experts' Knowledge to Improve Conservation Strategies for an Endangered Grouper in the Southwestern Atlantic. Journal of Ethnobiology 37(3): 478-493.
- Silvano RA, Nora V, Andreoli, TB, Lopes PF, Begossi A (2017) The ‘ghost of past fishing’: small-scale fisheries and conservation of threatened groupers in subtropical islands. Marine Policy 75: 125-132.
- Salas MS, Velázquez IA, Torres EI, Cabrera MAV, Hernández ICH (2013) Study of fisheries in the Puerto Morelos Reef National Park.
- Rocha LA (2018) Cephalopholis cruentata. The IUCN Red List of Threatened Species: e.T132761A46916787.
- Robertson DR, Peña EA, Posada JM, Claro R (2015) Peces Costeros del Gran Caribe: sistema de Información en línea. Version 1.0 Instituto Smithsonian de Investigaciones Tropicales, Balboa, República de Panamá.
- Krebs CJ (1999) Ecological Methodology. Addison Wesley, California. pp. 620.
- Moreno SXG, Perez RP, Irigoyen AMS, Marin EE, Abitia CLA, et al. (2019) Feeding habits of the leopard grouper, Mycteroperca rosacea (Actinopterygii: Perciformes: Epinephelidae), in the central gulf of California, BCS, Mexico. Acta Ichthyologica et Piscatoria, 49(1): 9-22.
- Rodríguez MRE, Ruíz RF, Van Tussenbroek B, Barba SG, Escalante ME, et al. (2010) Environmental state and tendencies of the Puerto Morelos CARICOMP site, Mexico. Rev Biol Trop Suppl 3: 23-43.
- Hernández TL, Rebolledo VM, Merino IM, Soto M, Le Cossec A, et al. (2011) Groundwater Pollution in a Karstic Region (NE Yucatan): Baseline Nutrient Content and Flux to Coastal Ecosystems. Water Air Soil Pollut 218: 517-528.
- McEachran JE, Fechhelm JD (1998) Fishes of the Gulf of Mexico. Myxiniformes to Gasterosteiformes. University of Texas Press, Austin 1: 1112.
- Humann P, Deloach N (2014) Reef Fish Identification. Florida Caribbean Baha- mas. 4th New World Publications, Inc. Jacksonville, Florida. pp. 548.
- Abele LG, Kim W (1986) An illustrated guide to the marine decapod crustaceans of Florida. Florida Department of Environmental Regulation, Technical Series 8(1-2): 1-760.
- Moreno CE (2001) Manual de métodos para medir la biodiversidad. Textos universitarios. Universidad Veracruzana. Xalapa, Veracruz. México p. 49.
- Colwell RK, Mao CX, Chang J (2004) Interpolating, extrapolating, and comparing incidence-based species accumulation curves. Ecology 85(10): 2717-2727.
- Colwell RK, Chao A, Gotelli NJ, Lin SY, Mao CX, et al. (2012) Models and estimators linking individual-based and sample-based rarefaction, extrapolation, and comparison of assemblages. Journal of Plant Ecology 5: 3-21.
- Nakai T, Sano M, Kurokura H (2001) Feeding habits of the darkfin hind Cephalipholis urodeta (Serranidae) at Iriomote Island, southern Japan. Fisheries Science. 67: 640-643.
- Hernández I, Aguilar C, González SG (2008) Food webs of reef fish in the northwestern region of Cuba.I. stomach contents. Revista de Biología Tropical 56(2): 541-555.
- Arredondo CAT, Sanchez JJA, Avila MOG, Torres CP, Herrerias DY, et al. (2016) Spatio-temporal variation in the diet composition of red lionfish, Pterois volitans (Actinopterygii: Scorpaeniformes: Scorpaenidae), in the Mexican Caribbean: insights into the ecological effect of the alien invasion. Acta Ichthyologica et Piscatoria 46(3): 185-200.
- Dierking J, Williams ID, Walsh WJ (2009) Diet composition and prey selection of the introduced grouper species peacock hind (Cephalopholis argus) in Hawaii. Fishery Bulletin 107(4): 464-476.
- Álvarez JL, Villalobos LJ, Hendrickx ME, Escobar BE, Rodríguez AG, et al. (2014) Biodiversity of decapod crustaceans (Crustacea: Decapoda) in Mexico. Revista Mexicana de Biodiversidad 85: S208-S219.
- Fernández M, Lorio MI, Hernández D, Macchi G (2012) Studies on the reproductive dynamics of Pleoticus mulleri (Bate, 1888) (Crustacea, Decapoda, Solenoceridae) of Patagonia, Argentina. Latin American Journal of Aquatic Research 40(4): 858-871.
- Caldwell RL, Dingle H (1976) Stomatopods. Scientific American 23: 80-89.
- Reaka ML, Manning RB (1980) The distributional ecology and zoogeographical relationships of stomatopod Crustacea from Pacific Costa Rica. Smithsonian Contributions to the Marine Sciences pp. 7:1-29.
- Hendrickx ME, Salgado BJ (2002) Stomatopoda. In Biodiversity, taxonomy and biogeography of arthropods from Mexico: towards a synthesis of their knowledge. In: Morrone LJJ, Llorente JE, Bousquets HP (eds.). Universidad Nacional Autónoma de México, México, D.F, 3: 373-400.
- Grijalba BM, Castañeda ME, Acero AP (2004) Structure of an fish assemblage associated with hard bottoms in the Colombian Caribbean using the stationary visual census (CVE) technique. Actualidades Biológicas 26(81): 197-211.
- Cure K, Benkwitt C, Kindinger TL, Pickering EA, Pusack TJ, et al. (2012) Comparative behavior of red lionfish Pterois volitans on native Pacific versus invaded Atlantic coral reefs. Marine Ecology Progress Series 467: 181-192.
- Beukers SBD, Jones GP (2004) The influence of prey abundance on the feeding ecology of two piscivorous species of coral reef fish. Journal of Experimental Marine Biology and Ecology 299(2): 155-184.
- Curtis JS, Wall KR, Albins MA, Stallings CD (2017) Diet shifts in a native mesopredator across a range of invasive Lion-fish biomass. Marine Ecology Progress Series 573: 215-228.
- Hyslop EJ (1980) Stomach contents analysis, a review of methods and their application. Journal of Fish Biology 17(4): 411-429.
- Pinkas L, Oliphant MS, Iverson IL (1971) Food habits of albacore, bluefin tuna and bonito in California waters. California Department of Fish and Game, Fish Bulletin 152: 1-105.
- Murray GD (2005) Multifaceted measures of success in two Mexican marine protected areas. Society & Natural Resources 18(10): 889-905.






























