The Megafauna Invertebrates on the Continental Shelf of the Yucatan Peninsula: Ecological Characterization
Torruco Daniel* and González Solis M Alicia
Center for Research and Advanced Studies of the IPN. Merida Unit. Coral Reefs Functional Groups Laboratory, Mexico
Submission:October 31, 2023;Published:November 15, 2023
*Correspondence author: Torruco Daniel, Center for Research and Advanced Studies of the IPN. Merida Unit. Coral Reefs Functional Groups Laboratory, Mexico
How to cite this article:Torruco D, González Solis M A. The Megafauna Invertebrates on the Continental Shelf of the Yucatan Peninsula: Ecological Characterization. Oceanogr Fish Open Access J. 2023; 16(5): 555949. DOI: 10.19080/OFOAJ.2023.16.555949
Abstract
During 2016 and 2018, three oceanographic field trips were carried out on the Yucatan Shelf in the Gulf of Mexico, to obtain a baseline that characterizes the region in several components that allow the establishment of the biological-ecological conditions. From this general objective, this work is based on the study of the invertebrates of the megafauna, captured with commercial trawl nets. In a comprehensive approach of all the cruises that have been obtained under the general project, 47 benthic trawls were carried out covering an area of 90 260.8 m2, the depth range was from 40 to 650 m. The Phyla number was 9, with 302 species in 26 147 organisms weighing 359.13 kg·ha-1. The total ecological diversity of megafauna measured under the Shannon-Wiener was 5.86 bits·ind-1. Five species are the most dominant, however, only one sponge reaches 10% dominance of the entire community. Megafauna is diverse, with the highest abundance of groups and species at sites close to coral reefs. About the interactions that occur with the variables of the environment, the community descriptors of phyla No., richness, abundance, and biomass were related to factors such as oxygen and heavy metals, but more frequently to the abundance and biomass of fish, presenting evidence that biological interactions are fundamental in the structuring of megafauna.
Keywords: Megafauna; Invertebrates; Biomass; Abundance; Gulf of Mexico
Introduction
The increasing exploitation of natural resources poses a growing threat to many ecosystem functions and associated services [1]. In marine systems, the increase in water temperatures, overexploitation by fishing, and construction of facilities in offshore factors are just some of the stressors that progressively change the benthic structure of the communities and influence ecosystem functioning (e.g., cycle biogeochemistry, sediment mixing, interspecific relationships, etc.) [2-5]. Consequently, descriptors easily key ecosystem functions are becoming more important for both politics as well as science. Over time, coastal ecosystems have allowed man to have a large number and variety of resources to meet his needs, not only of the riverside inhabitants but also of those further away.
However, despite this situation on the coasts, there is a notable lag in Mexico's knowledge, use, and proper management of these resources. The scientific society and the leader underscore the urgent need for a broader set of diversity indicators aligned with valued functions. The taxonomic identity, abundance, or biomass of species alone have little power to explain ecosystem processes, as these are determined by the traits of the ecological effect of the organisms involved [6,7] and their habitats. Trait-based indices can be a promising tool for meeting social, political, and environmental demands.
Key services such as biogeochemical cycles, bottom mineralization, oxygen regulation, etc. are strongly influenced by invertebrate activities in reprocessing (ventilation, etc.) in and over sediments [8-11]. Especially in shallow marine areas, bio irrigation benthic fauna activity is important for biogeochemical cycles [12-14]. In addition to this ecological importance, they are the base of the food chain of vertebrates and other invertebrates, they have the ability to modify the characteristics of the sediment in which they are found, affecting other smaller groups such as the macro and meiofauna [15], they have close relationships with aspects such as sedimentation [16], quantity and quality of organic matter [17], distance from the coast [18], depth [19], latitude [20] and present various ecological relationships (predation, mutualism, parasitism, symbiosis, commensalism) [21].
As a group, megafaunal invertebrates are heterogeneous in several facets, ranging from the phases of their life cycles, their motility, their size, their permanence in their habitats, and their behavior [16] (Williams et al. 2016). Above all, if some groups have colonial representatives such as the poriferan, cnidarians, phoronids, and hemichordates, they have a particular morphology (depending on the phylum), which makes it difficult to treat them statistically-mathematically and consequently to interpret the role they play in the total ecosystem. On the other hand, benthic communities are the most affected by anthropogenic activities (fisheries, oil and gas extraction, mining, waste dumping, etc.). Therefore, it is essential to have a record that serves as a baseline to differentiate these effects from those caused by nature. The growing coastal development, and the rapid and extensive extraction of natural resources in general, should alert leaders and those responsible for authorizing these activities. That is why, to correct the lack of information to form this baseline, we are analyzing the invertebrates that occur in the Yucatan continental shelf.
Of the works that have been carried out with the megafauna are those of Torruco et al. [22], Murawski et al. [23], and those of Ramírez et al. [24] with the ichthyofauna fraction, other studies include invertebrates but it is a single phylum, or a single group of a certain phylum as is the case of crustaceans [25,26] with invertebrates as a group, we have the works of Rubio-Polania et al. [27] and Torruco et al. [28].
Materials and Methods
Area of Study
The continental shelf of the northern Yucatan Peninsula is characterized by having a very low slope (~1:1000), and a very characteristic circulation with consistent and periodic behavior, where the current pattern is dominated by the action of the trade winds that give it momentum predominantly to the west. [29] Tidal variability can modulate the intensity of the currents but does not affect their direction. Meza-Padilla et al. [30] suggest that the Yucatan Current comes from the Caribbean and generates the loop current, it also has an important influence on the currents in the Yucatecan shelf, it is possible to observe within the shelf weak eddies and trends of currents towards the coast and away from the continental shelf. On the other hand, the bodies of water that influence the northern Yucatan shelf have a marked seasonal behavior. The sea surface is dominated by Caribbean surface water and both with high temperatures (25 to 30oC), which are strongly influenced by thermohaline processes that occur on the Peninsula's north coast, which generate waters with a high salt content (hyperhalines), due to the intense evaporation that occurs in lagoons and shallow regions. These waters are exported from the Yucatan shelf by the ocean currents and reach the Gulf of Mexico carrying properties and materials on their way [32].

Sixteen stations were defined in each of the three cruises, covering the Yucatan shelf (Figure 1). A total of 47 trawls were performed with commercial nets (18 m opening, 20 m length, 1 ½ inch mesh span, and a 1 ¼ inch collector flake). The trawls were carried out by commercial shrimp boats and each of them lasted approximately 30 minutes at a speed of 2-3 knots.
Methodology
From the captured fauna, the invertebrates were separated from the megafauna, weighed, and separated by taxonomic groups. Subsequently, they were frozen for transfer to the laboratory where they were identified, counted, measured, and weighed. The data were standardized to org/ha and were used to develop species-density matrices (abundance and biomass) for each station to later perform statistical analyses to obtain images of the community descriptors of this fauna.
Statistical analysis
The ecological descriptors that were quantified in the different communities are indicators of ecological processes that regulate both the community itself and the different types of habitats that have a certain abundance and specific richness. In this case, it is presented the hypothesis that species richness and density of the different groups do not present variations in the different areas sampled. If this hypothesis is not accepted, then the variations in density may be related to the abundance and/or biomass of some groups that channel resources more efficiently, giving a relationship of dependency inter and intraspecific, as well as differences in habitat complexity. Under the premise that a total characterization of the area is convenient in this integrative report to make a baseline [32], the megafauna was analyzed jointly with all the groups, referring to each cruise.
For ecological diversity (H'), the Shannon-Wiener index [33] was used, and Pielou's [34] proposal J, was used to approach equitability. An important parameter in this characterization is given by the Importance Value Index (IVI), which defines the dominance of each species in the community [35], where they are conjugated with various parameters such as relative biomass, relative abundance, and relative frequency. Finally, to define the degree of relationship between the variables recorded for the area with the community descriptors of the invertebrates of the megafauna (Richness, Biomass, Abundance, and Phyla Number), a multiple correlation was first carried out to take as a selection criterion the highest weights β, and then a canonical multivariate analysis was carried out to obtain the environmental variables that have the greatest influence with the community descriptors of the invertebrates [36], the selected variables were: Lead, Cadmium, Nickel, Aluminium, Vanadium, REDOX, Organic Matter, Fine Sand, Very Fine Sand, Gravel, Salinity, Oxygen, Temperature, Total Aliphatic, Total Hydrocarbons, Total Carbon, Total PaH's, Fish Biomass, Fish Abundance and Depth.
Results
Given the lack of information on the continental shelf of the State of Yucatan, the characteristics of each of the cruises carried out are shown in table 1. The trawls carried out in this area in the three cruises were 47, in all the cruises a similar number of trawls were carried out (15-16); the longest drag time was performed on the second cruise and a larger area was also swept (Table 1). The depth range was from 40 m to 650 m, in general, 30 trawls were made less than 100 m and 17 more than 100 m (Table 1).
The number of groups was 9 represented by the following Phyla: Arthropoda, Mollusca, Echinodermata, Cnidaria, Spongia, Sipunculida, Phoronida, Annelida, and Cordata. The last four were sporadic and not very diverse, while the first four were diverse and plentiful, though not on all cruises. Species richness was generally high, with a total of 302 species, the highest value reported on the second cruise (228). The greatest abundance occurred on the same cruise ship (16,082 org·ha-1) and consequently, the largest biomass (216.7 kg·ha-1). The highest diversity value was recorded on the second cruise, reaching 5,855 bits·ind-1; however, all cruises had values greater than 4 bits·ind-1 and in general, all megafauna reached a value of 5.86 bits·ind-1 (Table 1). Equitability showed the highest value on the second cruise (greater than 0.8), although the total value was 0.747.
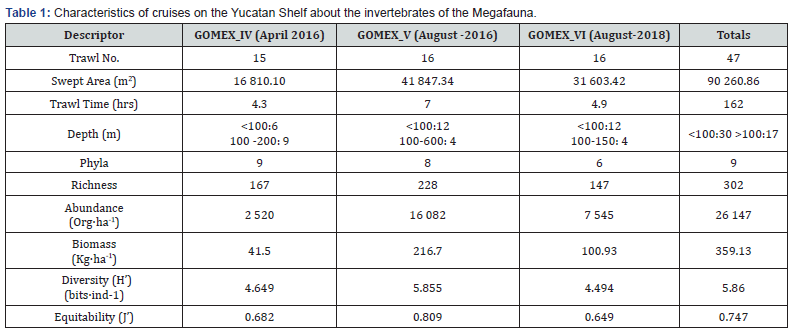
Analyzing the descriptors of the community with depth, for each of the cruisers, a series of graphs are presented that show the position of the different sampling sites and the values obtained, to have a reliable idea of how many Phyla the visualized descriptors represent.
Phyla number
On the first cruise, the greatest number of Phyla were recorded at shallow depths (≤50 m) and the lowest number at depths around 100-200m (Figure 2A). For the second cruise, the number of Phyla was highest in the shallow localities around 100-200m (6 Phyla), but also at these depths, the lowest number of phyla (4) occurred. At depths greater than 500 m the number of Phyla was high (6 Phyla) (Figure 2B). In the third crossing, the highest number of Phyla obtained was recorded in the area between 40 and 60 m (6 Phyla), the number of Phyla decreases towards 100 m, although it is variable (2-5 Phyla) (Figure 2C).
Species richness
For the first cruise, species richness presented the highest values (55-52 species) in shallow depths (<50 m), at 100 m the values are lower (19-4 species), and around 200 m the range is 19-5 species, only the number of phyla is reduced (Figure 2D). For the second cruise, the highest species richness (85 species) was at shallow depths (<200 m) but the lowest value was also recorded (8 species); from the 600m area, 48 species with several phyla were present (Figure 2E). In the last cruise, species richness was more evenly distributed in each cruise from 44 to 140 m; however, the greatest number occurred at depths of about 30 m (Figure 2F).
Abundance
The abundance recorded on the first cruise is very high at depths of around 200m (1075 org·ha-1 the highest value), and decreases in shallower areas, presenting its minimum value (18 org·ha-1) at depths of around 100m (Figure 2G). In the second, the highest abundance was obtained in about 200m (3476 org·ha-1) and the lowest abundance occurred in localities near 100m (111 org·ha-1) (Figure 2H). In the last cruise, the highest abundance was around 60 m (2029 org·ha-1), and the lowest was around 140 m (90 org·ha-1), the other sites fluctuated between 1118-147 org·ha-1 (Figure 2I).
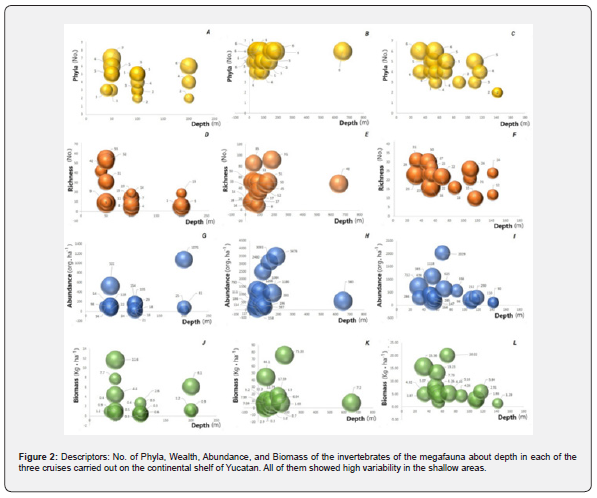
Biomass
The biomass of the first cruise follows a pattern like its abundance, as it is high in depths around 50m (11.6 kg·ha-1 as the highest value), decreases by about 100 m (0.3 kg·ha-1), in all sites near this depth, the biomasses are low, increasing slightly at 200 m (0.9-6.1 kg·ha-1) (Figure 3J). On the second cruise, the highest biomass reached was 75.33 kg·ha-1 in areas close to 200 m. In stations around 100 m, the biomasses are variable from 0.3 to 44.1 kg·ha-1, at depths greater than 500 m, there is a biomass of 7.2 kg·ha-1 (Figure 3K). The highest biomass of the last cruise was around 70 m (20.02 kg·ha-1), and the lowest was between 100 and 120 m (1.86 kg·ha-1); However, between 30 and 40 m there was also a site with low biomass (1.87 kg·ha-1) (Figure 3L).
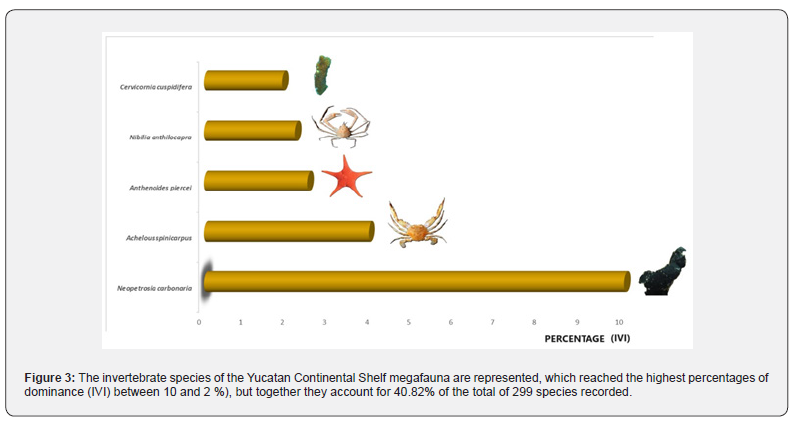
Dominance
Regarding the dominance of the species, in the invertebrates of the megafauna, there is no (awesome) overwhelming dominance, since the most oppressive species barely reaches 10% of the IVI (index of the importance value). Five species are the most important: two sponges, two decapod crustaceans, and a starfish (Figure 3), representing the groups of Sponges (Neopetrosia carbonaria and Cervicornia cuspidora), Echinoderms (Anthenoides piercei) and Crustaceans (Achelous spinicarpus and Nibilida anthilocapra). The group of molluscs and cnidarians, being abundant and of many species, but present in a single cruise, did not reach higher percentages in the index.
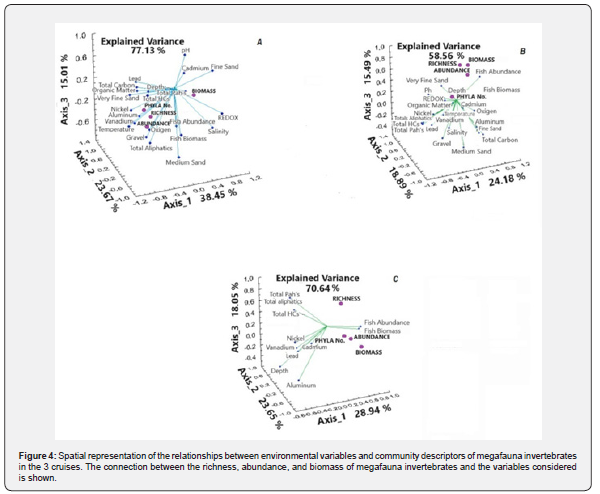
Canonical analysis
Concerning the canonical analysis, in the first cruise the abundance of the invertebrates of the megafauna shows a relationship with dissolved oxygen, while the phyla number and richness are close to the metals nickel and vanadium, the biomass, on the contrary, moves away from them and approaches the Total PaHs; The 3 canonical axes explain 77.12% of the variance (Figure 4A). In the second cruise, the biomass, richness, and abundance of the invertebrates of the megafauna are related to the abundance of fish; while the phyla number is related to the depth, both variables are very close to the origin, in this case, the variance explained by the 3 axes is 58.56%, 18.5% lower than the first cruise (Figure 4B). For the last cruise, the arrangement of phyla numbers, abundance, and biomass of megafauna invertebrates are located close to fish abundance and biomass, while species richness is located solitary; the value of the variance explained by the three axes is 70.64%, 6.48% lower than the first cruise and 12.08% higher than the second (Figure 4C).
Discussion
The use of organisms as bioindicators in the quality assessment and monitoring of diverse ecosystems has been widely documented [37,38]. Among them, benthic invertebrates have been recognized as organisms that meet the requirements and in turn offer numerous advantages to be considered as indicators of quality in aquatic ecosystems, such as
a) being found in all aquatic systems, thus favouring comparative studies
b) their sedentary nature, which allows effective spatial analysis of the effects of disturbances [39].
There are now numerous biotic indicators based on information from various benthic groups to assess the environmental status and quality of the benthic habitat of estuarine and marine ecosystems at local and regional scales [40]. These indicators range from the most simplistic based on information on the richness and diversity (e.g. H', richness indices) of the benthic community, to the most complex employing multidimensional models to integrate biological information and environmental factors to build descriptive and predictive models of the state of the ecosystem [41]. In this context, the following can be inferred from the analysis of the data: The area with the greatest variability was the shallow zone (<100-200 m) since the highest values were obtained in all descriptors, but also the lowest. Beyond 500 m, the values obtained were average. The abundance-biomass relationship indicates that organisms of variable size were found in the studied areas and consequently abundances and biomasses were also variable.
Some authors [23,42] mention that shallow and coastal environments are highly variable because the characteristics of the communities are influenced by the environment, which are the essential factors that define their structure and consequently the functioning of benthic communities. However, in these cruises there was an ambiguity, 107 species were represented by only 1 individual, including these, 193 more species were only present in one cruise reaching a total of 300 species with this characteristic, while 128 species were more frequent in 2 or all 3 cruises as predicted by Preston [43]. This scheme has great significance in the ecological diversity of megafauna invertebrates, achieving high values. The diversities for each group could be lower than those of the total invertebrates, with similar values reported for specific groups by other authors [44-46]. The phyla Arthropoda, Mollusca, Echinodermata, and Cnidaria were the most abundant and diverse, which is consistent with what was reported for the Gulf of Mexico [47-49].
It is predicted that marine communities, in general, should show significant variations in local and more specific species richness within sites because of ocean transport [44]. But in our case, with megafauna invertebrates, these positional effects on local species richness are also modified by a variety of other variables (i.e. reproductive aggregations, growth habitats, patches with different sedimentological characteristics and/or size, temperatures, oxygenation, etc.), so that in all three cruises this descriptor is spatially solitary. However, an important aspect in the explanation of the lack of patterns could be the patchy habitat that occurs on the bottom and that would maintain the causal mechanisms of the absence of patterns, in addition to the proximity to coral reefs, which would act as a provider and reservoir of species. Although the approach detects the responses of individual taxa to environmental stress, it can be difficult to confirm which ecological functions are driving these responses, organisms that appear to function similarly in ecological roles do not always respond to stressors in the same way [50,51]. Because, although they share some important attributes, they are likely to differ in other, more subtle ways.
It has been postulated that, in the tropics at small sites, the ratio of species richness should be controlled by the local environment [52,53], as is possibly the case on the Yucatan continental shelf, where physical/biological factors seem to be the most important factors in the region address the abundances and biomasses of invertebrate fauna on all cruises. With abundance, it would probably be physical and chemical factors, where there is evidence of certain dynamics of the bottom that change over time and that originate complex relationships between these descriptors and oceanic variables, while with biomass it is plausible that there is a relationship with the abundance and biomass of fish (predators), which would give evidence that there is a certain relationship of intraspecific interdependence. However, we should not rule out that both scenarios may also occur at the same time, giving rise to a complex result between these relationships.
Concerning dominance, the results indicate that there is no one species that achieves preponderant dominance over the others in the megafauna, which makes a flexible coexistence and implies that the interactions between the species are lax, where each species and/or groups of species obtain the necessary energy from different sources to prevail and consequently, they have multiple responses to changes in their environment. About the groups, the dominance of crustaceans and echinoderms is like that reported by other authors [54-57], who have also reported these groups as numerically dominant. Two species of sponges are among the most dominant and the limit imposed on the 5 most important species, is a consequence of the habitat where they occur (close to coral reefs). The identified species are organisms with great adaptive capacities and their dominance may be regulated by the trophic spectrum or by their permanence in a given habitat [7,58-60].
Conclusion
a) To understand the community structures in the three cruises and the processes that influence the determination of biodiversity, we have the following:
b) 9 phyla were recorded in the area, with 302 species, reaching a total diversity of 5.86 bits/ind.
c) Five important taxonomic groups were detected: crustaceans, echinoderms, sponges, cnidarians and molluscs.
d) The dominance of megafauna species was given by sponges, crustaceans, and echinoderms in general. However, with this dominance in cruises of adjacent areas [61] there is an alternation of species in these groups, where molluscs and cnidarians enter this alternation.
e) The abundance and biomasses showed differences and are not due to the number of trawls made in each one, since they were similar, highlighting differences between the three cruises, nor was it a reflection of the time of year since the last two cruises were in August and were different. Consequently, there is still no determining pattern of richness, abundance, and biomass in this area. However, it can be shown that the variability in the shallow zone is very evident since both species’ richness, abundance, and biomass can have maximum and minimum values in that area and only depend on the position of the season.
f) The greatest variability in community descriptors (richness, abundance, and biomass) is presented at stations near coral reefs. - No annual, interannual, or seasonal pulses were detected.
g) There is no strong evidence of descriptors' dependence on depth.
h) The abundance/biomass ratio indicates that organisms of varying size and abundance occur in all areas.
i ) There will likely be a differentiation in the platform as described by Gonzalez (1989) with the group of molluscs, but to test this hypothesis it is convenient to carry out larger cruise ships with a greater number of stations.
j) With this fraction of the megafauna, no significant signs of disturbance were detected on these cruises.
Acknowledgement
This research has been financed by SENER_CONACYT Hydrocarbons Project 201441. This research is a contribution from the Gulf of Mexico Research Consortium (CIGoM).
References
- Naeem S, Duffy JE, Zavaleta E (2012) The functions of biological diversity in an age of extinction. Science 336(6087): 1401-1406.
- Tillin H, Hiddink J, Jennings S, Kaiser M (2006) Chronic bottom trawling alters the functional composition of benthic invertebrate communities on a sea-basin scale. Sea Ecol Prog Be 318: 31-45.
- Coates DA, Deschutter Y, Vincx M, Vanaverbeke J (2014) Enrichment and shifts in macrobenthic assemblages in an offshore wind farm area in the Belgian part of the North Sea. Sea Environ Res 95: 1-12.
- Solan M, Hauton C, Godbold JA, Wood CL, Leighton TG, et al. (2016) Anthropogenic sources of underwater sound can modify how sediment-dwelling invertebrates mediate ecosystem properties. Sci Rep 6: 205-240.
- Torruco D, González SMA, Torruco GAD (2018a) Comparative analysis of mollusks in the Los Petenes Biosphere Reserve and coastal lagoons in southeastern Mexico. Bull Mar Coast Res 47(1): 25-44.
- Díaz S, Cabido M (2001) Vive la difference: plant functional diversity matters to ecosystem processes. Trends Ecol Evol 16(11): 646-655.
- Dewenter I, Emmerson M, Potts SG, Gagic V, Bartomeus I, et al. (2015) Functional identity and diversity of animals predict ecosystem functioning better than species-based indices. Proc R Soc London B: Biol Sci 282: 20-26.
- Wilkinson MT, Richards PJ, Humphreys GS (2009) Breaking ground: pedological, geological, and ecological implications of soil bioturbation. Earth Sci Rev 97(1-4): 257-272.
- De Bello F, Lavorel S, Díaz S, Harrington R, Cornelissen JH, et al. (2010) Towards an assessment of multiple ecosystem processes and services via functional traits. Biodivers Conserv 19: 2873-2893.
- Kristensen E, Penha LG, Delefosse M, Valdemarsen T, Quintana CO, et al. (2012) What is bioturbation? The need for a precise definition for fauna in aquatic sciences. Sea Ecol Prog Be 446: 285-302.
- Valença AW, Vanek SJ, Meza K, Canto R, Olivera E, et al. (2017) Land use as a driver of soil fertility and biodiversity across an agricultural landscape in the Central Peruvian Andes. Ecol Appl 27(4): 1138-1154.
- Collie J, Escanero G, Valentine P (1997) Effects of bottom fishing on the benthic megafauna of Georges Bank Sea. Ecol Prog Ser 155: 159-172.
- Mermillod BF, François CF, Rosenberg R (2005) Biodiversity of benthic invertebrates and organic matter processing in shallow marine sediments: an experimental study. J Exp Mar Biol Ecol 315(2): 187-209.
- Braeckman U, Foshtomi MY, Van Gansbeke D, Meysman F, Soetaert K, et al. (2014) Variable importance of macrofaunal functional biodiversity for biogeochemical cycling in temperate coastal sediments. Ecosys 17: 720-737.
- Iken K, Brey T, Wand U, Voigt J, Junghans P (2001) Food web structure of the benthic community at the Porcupine Abyssal Plain (NE Atlantic): A stable isotope analysis. Prog Oceanogr 50(1-4): 383-405.
- Birchenough SNR, Reiss H, Degraer S, Mieszkowska N, Borja A, et al. (2015) Climate change and marine benthos: a review of existing research and future directions in the North Atlantic. WIREs Clim. Chang 6(2): 203-223.
- Tecchio S, Ramírez L, Sardà EVAF, Company JB (2011) Biodiversity of deep-sea demersal megafauna in western and central Mediterranean basins. Scient Mar 75(2): 341-350.
- Durden JM, Bett BJ, Jones DOB, Huvenne VAI, Ruhl HA (2015) Abyssal hills - hidden source of increased habitat heterogeneity, benthic megafaunal biomass, and diversity in the deep sea. Prog Oceanogr 137: 209-218.
- Bluhm BA, Iken K, Hardy SM, Sirenko BI, Holladay BA (2009) Community structure of epibenthic megafauna in the Chukchi Sea. Aquat Biol 7: 269-293.
- Linse K, Barnes DKA, Enderlein P (2006) Body size and growth of benthic invertebrates along an Antarctic latitudinal gradient. Deep Sea Res Part II Top Stud Oceanogr 53(8-10): 921-931.
- Buhl ML, Ellingsen KE, Buhl MP, Skaar KL, Gonzalez MG (2016) Trawling disturbance on megabenthos and sediment in the Barents Sea: Chronic effects on density, diversity, and composition. ICES J Mar Sci 73: 98-114.
- Williams A, Schlacher T, Rowden A, Althaus F, Clarck M, Bowden D (2016) Recent advances in seamount ecology a contribution to the census of marine life. Mar Ecol 31: 183-199.
- Torruco D, González SMA, Torruco GAD (2018b) Fish diversity, distribution and their relationship with environmental variables in the southern Gulf of Mexico. Jour Trop Biol 66(1): 438-456.
- Murawski SA, Peebles EB, Gracia A, Tunnell JW, Armenteros M (2018) Comparative Abundance, Species Composition, and Demographics of Continental Shelf Fish Assemblages throughout the Gulf of Mexico. Marine and Coastal Fisheries: Dynamics, Management, and Ecosystem Science 10(3): 325-346.
- Ramírez JM, Vázquez BAR, Gracia A (2019) Ichthyofaunal list of the continental slope of the southern Gulf of Mexico. ZooKeys.
- Santana MLD, De Grave S, Simões N (2013) New records of caridean shrimps (Decapoda: Caridea) from shallow water along the northern Yucatan peninsula coasts of Mexico. Nauplius 21(2): 225-238.
- Rios LGV, Espinosa MJC, Zetina MC, Aguilar CC, Ramírez EA (2013) The lobster fishery Panulirus argus in the Gulf of Mexico and Mexican Caribbean Sea. INE.
- Rubio PJC, Torruco D, González SMA, Ordaz BJF, Caamal JY (2018) Benthic megafauna of outer margins of the continental shelf of Yucatan Peninsula. Regio Stud Mar Scien 24: 184-195.
- Torruco D, Gonzalez SMA, Torruco GAD (2018c) Invertebrate Megafauna in the Perdido Fold Belt Polygon, Gulf of Mexico. Mexico Ocean Fish 8(4).
- Zavala HJ, Morey SL, O Brien JJ (2003) Seasonal circulation on the western shelf of the Gulf of Mexico using a high-resolution numerical model. Jour Geophy Res.
- Meza PR, Enriquez C, Liu Y, Appendini CM (2019) Ocean circulation in the western Gulf of Mexico using self‐organizing maps. Jour Geophy Res Oceans 124(6): 4152-4167.
- Enriquez C, Mariño TI, Jerónimo MG, Capurro FL (2013) Thermohaline processes in a tropical coastal zone. Continental Shelf Research 69: 101109.
- Edgar GJJ, Bustamante RHH, Fariña JM, Calvopina M, Martínez C, et al. (2004) Bias in evaluating the effects of marine protected areas: the importance of baseline data for the Galapagos Marine Reserve. Environ Conserv 31(3): 212-218.
- Magurran AE (1988) Ecological diversity and its measurement. Princeton University Press. Princeton, New Jersey pp. 179.
- Pielou EC (1975) Ecological diversity. John Wiley and Sons. New York pp. 210.
- Brower JE, Zar JH (1977) Field and laboratory methods for general ecology. Wm. C. Brown Co. Publishers. Dubuque, Iowa. pp. 194.
- Sokal RR, Rohlf FJ (2012) Biometry 4a (Ed.) Freeman WH and Co New York.
- Borja A, Franco J, Muxika I (2004) The biotic indices and the Water Framework Directive: the required consensus in the new benthic monitoring tools. Mar Pollut Bull 48(3-4): 405-408.
- Chen Y (2013) Impacts of Multi Resolution Species Distributional Information Analyzing Macroecological Patterns. J Ecosys Ecograph 3: 137.
- Hyland J (2004) Developing Indicators of Stress in the Marine Benthos: The UNESCO/IOC Ad Hoc Benthic Indicator Group. En: Indicators of stress in the marine benthos. Intergovernmental Oceanographic comisió Workshop Report No. 195. UNESCO 8.
- Díaz DLA, Ignacio FJI, Álvarez TP, Ramírez FO, Lopez LLG (2004) The sustainability of fisheries in the Gulf of Mexico. In: Caso M, Pisanty I, Ezcurra E (eds), Environmental diagnosis of the Gulf of Mexico. Secretariat of Environment and Natural Resources, National Institute of Ecology, A. C. Harte Research Institute for the Gulf of Mexico Studies, CDMX, México pp. 727-755.
- Brown A, Thatje S (2014) Explaining bathymetric diversity patterns in marine benthic invertebrates and demersal fishes: physiological contributions to adaptation of life at depth. Biol Rev 89(2): 406-426.
- Olenin S, Ducrotoy JP (2006) The concept of biotope in marine ecology and coastal management. Mar Poll Bull 53(1-4): 20-29.
- Preston FW (1962) The canonical distribution of commonness and rarity. Ecology 43: 185-215.
- Vides CMP (2011) Distribution of the benthic megafauna of the Colombian Caribbean associated with environmental variables of the seabed. Bol Inv Mar Cost 40(2): 249-270.
- Hernández ÁI, Guerra CE, Bracho C, Rada M, Ocaña FA, et al. (2018) Variation in species diversity of deep-water megafauna assemblages in the Caribbean across depth and ecoregions. PLoS ONE 13(8): e0201269.
- Alvarado JJ, Chacón MJ, Aspfeifa SL, Cortés J (2022) Diversity of Deep-sea echinoderms from Costa Rica. Front Mar Sci 9: 918878.
- Pequegnat WE, Gallaway BJ, Pequegnat LH (1990) Aspects of the ecology of the deep-water fauna of the Gulf of Mexico. Am Zool 30(1): 45-64.
- Wei CL, Rowe GT, Fain HG, Scheltema AH, Wilson GDF, et al. (2010) Bathymetric zonation of deep-sea macrofauna in relation to export of surface phytoplankton production. Sea Ecol Prog Ser 399: 1-14.
- Solís MFA, Laguarda FA, Durán GA, Vázquez BAR, Gracia A (2014) Biodiversity of echinoderms (Echinodermata) from the Mexican deep sea. The Final Frontier the Deep Ocean 215-253.
- Ramsay K, Kaiser MJ, Hughes RN (1998) Responses of benthic scavengers to fishing disturbance by towed gears in different habitats. J Exp Mar Biol Ecol 224(1): 73-89.
- Ruíz T, Vázquez BAR, Gracia A (2013) Associations of epibenthic megacrustaceans in the Campeche Sound, Gulf of Mexico. Rev Mex Biodiver 84: 280-290.
- Terborgh JW, Faaborg J (1980) Saturation of bird communities in the West Indies. Am Nat 116(2): 178-195.
- Gotelli NJ, Anderson MJ, Arita HT, Chao A, Colwell RK, et al. (2009) Patterns and causes of species richness: a general simulation model for macroecology. Ecolo Letters 12(9): 873-886.
- Torruco D (1988) Structure of the necrobenthic fauna of the sublittoral in front of Laguna Verde, Veracruz. Master's Thesis. CINVESTAV U. Mé Mexico.
- Escobar BE (2000) The biodiversity of the deep sea in Mexico. Biodiversitas 29: 2-6.
- Nilsen V, Zamora P (2006) Commercial species II: Crustaceans and mollusks. In: Nilsen V, Quesada AMA (ed). Coastal Marine Environments of Costa Rica. Interdisciplinary Coastal Marine Commission of the Exclusive Zone of Costa Rica pp. 105-118.
- Huidobro CL, JVallarta ZRF, Izábal MJD, Martínez MVH, Del Campo HD, et al. (2018) Benthic Ecosystem in the Campeche Sound: Bathymetry, Oceanography and Biology. Gulf of Mexico and Caribbean Sea Campaign. Informe Té INAPESCA p. 54.
- Flint RW, Rabalais N (1981) Environmental studies of a marine system: South Texas outer continental shelf. Texas: Texas University Press.
- Torruco D, Chávez EA, González MA (2006) Spatio-temporal variation of the structural organization of demersal communities in the southwestern Gulf of Mexico. Rev Biol Trop 55(2): 509-536.
- Calderón PJM, Moreno CE (2019) Beta diversity based on dissimilarity indices: Its partition into turnover components and differences in richness. In: Moreno CE (Ed) Biodiversity in a changing world: Theoretical and methodological foundations for its study. Autonomous University of the State of Hidalgo/Libermex, CDMX. pp. 203-222.
- Rubio PJC, González SMA, Enriquez C, Árcega CF, Ceja MV, et al. (2022) Community structure of megabenthos of Perdido Fold Belt (Tamaulipas, Mexico) and its relationship with the oceanographic and sediment parameters including potential pollutants. Mar Biol Res. 18(7-8): 477-494.






























