Antioxidant Activity and Toxicity Test on Mangrove (Rhizophora Apiculata) from Banyuasin Waters, South Sumatra
Nadya Ayu Wirandita, Muhammad Hendri* and Riris Aryawati
Department of Mathematics and Natural Sciences, Marine Science Study Program, Sriwijaya University, Indonesia
Submission:April 13, 2023; Published:June 15, 2023
*Correspondence author: Muhammad Hendri, Department of Mathematics and Natural Sciences, Marine Science Study Program, Sriwijaya University, Indonesia Email: muhammad.hendri@unsri.ac.id
How to cite this article: Nadya Ayu Wirandita, Muhammad Hendri, Riris Aryawati. Antioxidant Activity and Toxicity Test on Mangrove (Rhizophora Apiculata) from Banyuasin Waters, South Sumatra. Oceanogr Fish Open Access J. 2023; 16(3): 555936 DOI: 10.19080/OFOAJ.2023.16.555936
Abstract
Mangrove R. apiculata can be used as a source of natural ingredients of some products because it contains secondary metabolites that are toxic and antioxidants. If Secondary metabolite compounds contained in R. apiculata mangrove is excessive, these compounds will be toxic which will later be used as an early indicator of anticancer drug ingredients and which are antioxidants, these can be used in the manufacture of nausea, anti-diarrhea and vomiting drugs. The purpose of this study was to analyze and calculate the antioxidant potential and toxicity of the extracts of the roots, stems and leaves of R. apiculata. One of the methods used to determine antioxidants is DPPH and toxicity is BSLT. The results of antioxidant and toxicity tests will be known from the IC50 and LC50 values. Based on the analysis of R. apiculata mangrove extract with methanol solvent, the IC50 values for root, stem and leaf extracts were 1,22μg/ml, 2,83μg/ml, 8,39μg/ml and the LC50 value was 976,60μg/ml, 576,75μg/ml, 1278,35μg/ml. After the analysis, it was found that the root, stem and leaf extracts of R. apiculata had very strong antioxidant potential and the stem and root extracts had toxic potential.
Keywords:Antioxidant; Toxicity; DPPH; BSLT; Secondary Metabolic Compound
Introduction
Banyuasin Regency has low land which covers parts of the Banyuasin Waters area, especially river estuaries which have potential because they have abundant mangrove ecosystems. Tanjung Api-Api is a coastal area or estuary which is categorized as a special zone where becomes the natural habitat of the mangrove community. The Tanjung Api-Api area has an abundant mangrove community. The types of mangroves found in the Tanjung Api-Api area according to Puspitasari et al. [1], are mangrove species A. marina, R. mucronata, S. alba and X. granatum. According to Rahayu [2] Tanjung Api-Api has mangrove flora including Rhizophora sp. Avicennia sp. and Sonneratia sp.
R. apiculata is a type of mangrove often found in the Tanjung Api-Api area. Another name for mangrove R. apiculata is mangrove oil. Phytochemical screening shows that mangroves contain secondary metabolites which play role as drugs or poisons. Phytochemicals are chemical materials produced by plants which are divided into two, namely primary metabolites and secondary metabolites. Primary metabolites in plants plays a role in plant growth while secondary metabolites play a role in physiological effects. Phytochemical methods are used to determine the content of secondary metabolites [3]. According to Akasia et al. [4], R. apiculata contained phenolic compounds, alkaloids, flavonoids, tannins, saponins and steroids. However, the metabolite compounds contained in R. apiculata mangrove parts such as stems, roots, and leaves have different concentrations.
Antioxidants are compounds that can inhibit free radical reactions in the body [5]. Free radicals are molecular atoms that have unpaired electrons and if the molecular atoms present in high amounts in the body, these can cause stress, cancer, diabetes and inflammation [6]. Free radicals can be formed either naturally in the body through normal metabolic processes (metabolic waste) or from external factors such as food, X-rays, air pollutants, and cigarette smoking. The body has the ability to ward off free radicals in small amounts but not in large quantities. One of the methods used to measure the level of antioxidants in samples is the DPPH method. Toxicity is a qualitative term whether there is a damage or not depending on the amount of toxic active compounds absorbed. The results obtained from the toxicity test using the BSLT method have been proven to have a positive correlation with the toxicity of anticancer compounds [3]. The BSLT method uses A. salina as the observed test object.
Mangrove R. apiculata has high bioactive compounds, one of which is in the leaves. Previous research conducted by Berawi & Marini [7], found that the roots and stems of R. apiculata contained alkaloids, flavonoids and tannins, while the leaves contained alkaloids, tannins, saponins, phenols, flavonoids and terpenoids. According to Dapas et al. [8], flavonoid and steroid compounds acted as stomach poisons and interfere with the digestive organs of the larvae which results in death. Tannin compounds have properties as antioxidants, anti-bacterial and anti-diarrhea. According to Maulana & Sasmito [9], the mangrove leaves of R. apiculata have a high concentration of bioactive compounds, namely tannins which can be toxic.
Based on the description above, it is important to test the antioxidant activity and toxicity of mangroves (R. apiculata) from Banyuasin Waters, South Sumatra using the DPPH and BSLT methods. This method was chosen because it is a method that does not take too long, is easy and costs affordable.
Material and Method
Time and place
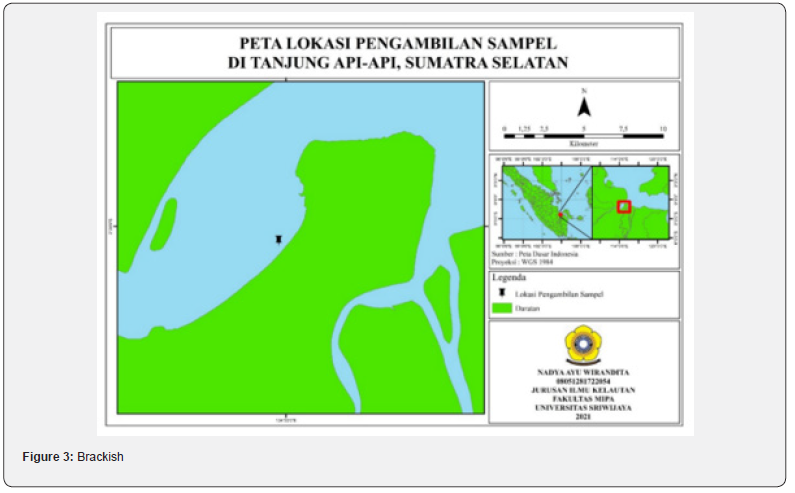
This research was conducted in December-March 2022 in Banyuasin Waters, South Sumatra and sample treatment for antioxidant tests and toxicity tests was carried out at the Marine Bioecology Laboratory and Marine Oceanography Laboratory, Faculty of Mathematics and Natural Sciences, Sriwijaya University. A map of the sampling locations is presented in figure 1.
Tools and materials
Tools used during the study: permanent marker, machete, large scissors, plastic, scales, camera, mangrove identification book [10], hand gps (global positioning system), thermometer, ph meter, hand refractometer, black cloth , tray, scissors, analytical balance, blender, filter paper 11cm in diameter, funnel, glass jar, spatula, plastic wrap, measuring cup, beaker glass, erlenmeyer, rotary evaporator, aluminum foil, dropper pipette, evaporator flask, vials, microsoft excel, Orion Aquamate 8000 spectrophotometer, stationery, aerator, plastic jar, electric light, SRCC (sedgwick rafter counting cell) and black duct tape. While materials used during the study: extract samples of mangrove leaves, stems and roots of R. apiculata, methanol solvent, A. salina, artificial sea water (ASW), water, dpph, vitamin C or ascorbic acid, aquadest and dmso (CH3)2SO.
Research procedure
Sampling, preparation and extraction of samples
The researchers obtained samples of R. apiculata mangroves from Banyuasin waters which were prepared and weighed. The parts taken were 4.9kg of leaves, 2.5kg of roots and 5.4kg of stems. The sample parts which have been taken in a fresh condition were cleaned, then sorted and cut into thin pieces, then air-dried and dried in the sun to dry. Based on research from Rumayati et al. [11], dry samples would be mashed with a blender and then filtered using a sieve to obtain powder results.
The sample extraction used in this study refers to research by Hasim et al. [12] which has been modified, sample extraction was carried out by maceration. Mangrove samples were mashed and then 100 grams were taken and macerated with methanol solvent (1:10) which had been distilled for 48 hours at room temperature. This process was carried out 3 times repetitions. The maceration solution is filtered through filter paper by using a vacuum pump, then evaporated at 40oC to obtain a crude extract.
Preparation of DPPH solution
The DPPH solution was prepared with modified reference to the research by Muthia et al. [13], 0.1 mM DPPH solution was prepared by weighing 0.002g of DPPH powder which was dissolved in 50mL with methanol solvent. The DPPH solution was prepared at room temperature in a room protected from the sun.
Preparation of Vitamin C stock solution
Vitamin C stock solution in the DPPH study was used as a positive control (comparison). A stock solution of vitamin C (ascorbic acid) with a concentration of 2000 ppm was prepared according to research by Martini et al. [14] which is modified in this present research, a 2000 ppm vitamin C stock solution was prepared by weighing 0.02g of pure vitamin C and dissolved with methanol up to 10ml into a beaker glass. The vitamin C solution or reference solution was diluted with methanol solvent to obtain a concentration of 15.625, 7.81, 3.91, 1.95, 0.98 ppm from a 2000 ppm pure vitamin C stock solution.
Preparation of test solutions
The test solution was made with reference to the modified research by Muharni et al. [15]. A 2000 ppm stock solution was arranged in the preparation of a stock extract solution by preparing 0.02g of each extract, then dissolving it using methanol with a final volume of 10ml in a beaker glass. The 2000 ppm test solution was then diluted with methanol solvent to obtain concentrations of 250, 125, 62.5, 31.25 and 15.625 ppm.
DPPH Test of R. apiculata mangrove extract
The research used for the antioxidant test in this study refers to the research by Less & Adang [16]. The test solution with 5 concentrations namely 250, 125, 62.5, 31.25 and 15.625 ppm was taken as much as 1ml at each concentration and then added 1ml of 0.1mM DPPH solution. Then the samples were incubated at room temperature and in a dark place for 30 minutes until the color changes due to DPPH activity, each sample was repeated 3 times.
The positive control (comparison) used was vitamin C (ascorbic acid) with concentrations of 15.625, 7.81, 3.91, 1.95, 0.98 ppm from dilution of 2000 ppm pure vitamin C stock solution. Vitamin C is known to have a very strong antioxidant content so that the selection of concentrations is low. According to Handayani et al. [17] absorbance measurements from antioxidant tests can use the ORION Aquamate 8000 spectrophotometer with a wavelength of 517nm.

Preparation of A. salina
The toxicity test on mangrove samples of R. apiculata began with the process of preparing a sample of nauplius A. salina by taking 1 gram of crystal A. salina, then soaking the crystal A. salina in 1 liter of artificial sea water. This process was useful in hatching with the help of lighting and aeration for 48 hours [18]. According to Sartinah et al. [19], crystal A. salina took 24 hours to hatch into nauplius. A. salina used in the BSLT test was better aged 48 hours because if it was more than 48 hours, it was afraid that A. salina would not die because of toxicity but because of the limited food in the aquarium.
Preparation of test solution
The test solution was made with reference to research by Puspitasari et al. [1]. The initial solution for the mother liquor was prepared with a concentration of 10,000 μg/mL by dissolving 1 gram of extract in 100 ml of artificial seawater. The mother liquor obtained was then diluted to obtain a concentration of 9,000, 5,000, 2,000, 1,000, 500, 250, 125, 50 μg/mL and a control solution was made without adding extract in it.
BSLT test of R. apiculata mangrove extract
The toxicity test process in this study refers to the research of Puspitasari et al. [1]. 10ml vials were prepared for the test solution and control solution. The researchers took 5ml of each test solution, then added 10 A. salina. According to Atmoko & Maruf [20], the researchers had to observe for 24 hours by looking at the number of dead and live A. salina nauplius. The control solution was treated the same as the sample without adding the extract to the artificial seawater. Each concentration was repeated 3 times compared to the control solution to see the number of A. salina nauplius mortality.
Data analysis
Antioxidant
The antioxidant test of a sample can be determined by looking at the size of the IC50. According to [21], the absorbance measured with a spectrophotometer used a wavelength of 517nm. The percentage inhibition value represented by the IC50 value was used to determine the amount of DPPH absorption resistance calculated by the formula:

From the results of the equation above, the researchers calculated the IC50 using the linear equation formula, the X axis is the concentration while the Y axis is the % inhibition value. The IC50 value according to Dewi et al. [22] is determined by the formula:

Note:
Y = 50% oxidation inhibitor
X =Inhibitor concentration (IC50 value)
a = The lines intersect on the Y axis
b = regression slope
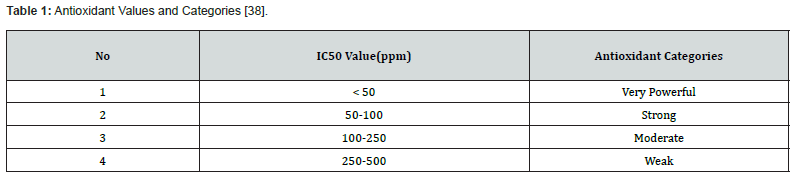
The antioxidant category in mangrove extracts was determined by the IC50 concentration value, as presented in table 1.
Toxicity
The toxicity test of a sample can be determined by looking at the size of the LC50. According to Adelia and Iskandar (2020), the LC50 value was obtained from analyzing the probit analysis method and calculating by using Microsoft Excel software. According to Nurhayati et al. [23], the effect of toxicity was analyzed by percent mortality.

The results of the equation above are then the researchers calculated LC50 with a probit value or 50% death. According to Meyer et al. [24] if there was a dead A. salina in the control, then the % of death would be determined using the Abbott formula, as follow:

Note:
T = Number of dead A. salina test
K = Number of dead A. Salina control
10 = Number of A. salina tested
After knowing the % mortality of A. salina, then the researchers tried to find the probit number through the probit table and regressing linearly.
After getting the values of a and b, the researchers did a search for the LC50 values obtained from the linear regression equation with the variables whose values are known, namely X and Y [23], so the equation of the line was obtained:

Note:
Y = Probit value
a = Regression concentration
b = Regression slope
X = Logarithm of 10 test concentrations
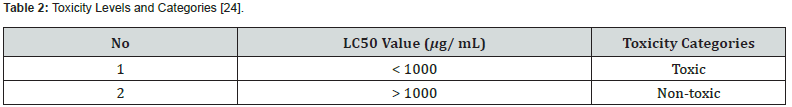
The toxicity category in mangrove extracts was determined by the LC50 concentration value, as presented in table 2.
Results and Discussion
Mangrove Rhizophora apiculata
The samples used in this study included leaves, stems, and roots of the R. apiculata mangrove species taken in the Tanjung Api-Api area, South Sumatra with coordinate points 02o22’17.70”S and 104o48’14.79”E. The Tanjung Api-api area has water parameters, namely pH 7.25, salinity 15 ppt, temperature 28oC and DO 8.04 mg/L. Referring to the quality standards according to the Decree of the Minister of State for the Environment No. 51 of 2004, namely DO >5 quality standards, pH 7-8.5, salinity up to 34 ppt and temperature 28-32oC, this can be concluded that the parameters contained in the Tanjung Api-api area are good for mangrove growth. The shape of the leaves, stems, and roots of the mangroves can be seen in figure 2.
Based on figure 2, R. apiculata stems have dark gray bark with blackish patterns, the tree height can reach up to 15m. One tree consists of several branches with an uneven bark surface. It has stilt root type with blackish brown on the outside and reddish brown on the inside. R. apiculata has green leaves which the base is red, the shape is simple elliptical with opposite branching.
The description above is in accordance with the description carried out in the research by Dekky et al. [25], stated that R. apiculata mangrove stems were dark gray in color, the leaf surface was smooth, the leaf tips were pointed with thorns, oval in shape with a length of 3-13 cm. Ulqodry TZ & Sarno [10] also mentioned that the leaves of R. apiculata were arranged in a simple, opposite direction, the strands form an elliptical shape with a length of 9-18cm and had a green leaf color.
Biomass sample extract of mangrove Rhizophora apiculata
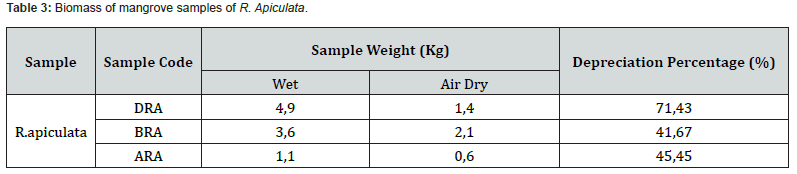
The R. apiculata mangrove sample experienced shrinkage from wet to dry weight which resulted in a shrinkage percentage presented in table 3.
Table 3 shows the sample biomass of R. apiculata mangroves has an average percentage of shrinkage of all weights of 52.85% with the highest percentage of 71.43% in the DRA sample code (R. apiculata leaves) with a wet sample weight of 4.9 kg and a dry sample weight of 1.4Kg. The lowest percentage was found in the BRA sample code (R. apiculata stem) of 41.67% with a wet sample weight of 3.6kg and a dry sample weight of 2.1kg. The highest percentage of shrinkage was found in DRA. This is suspected in the part of the mangrove leaf sample R. apiculata which has a highwater content. In accordance with research conducted by Amira (2008) in Imiliyana et al. [26], the leaf part is a photosynthetic unit that has many cell cavities filled with water and mineral nutrients so that this part has a high-water content. High water content is also related to the habitat of the R. apiculata mangrove sampling location. According to Jacob et al. [27], the relatively low water content in mangroves may be due to the mangrove habitat which has high temperature and salinity due to the influence of heat transfer from the sea.
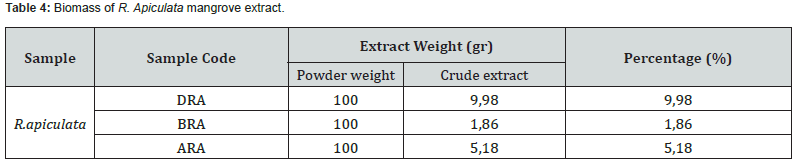
The shrinkage percentage for each sample which was air-dried and sun-dried without exposure to direct sunlight varied depending on the drying location and the drying time. It is believed that each sample has a different water content. According to research by Egra et al. [28], the water content in mangroves is air-dried in a place protected from sunlight to avoid damage to the compounds contained in it. The biomass of the R. apiculata mangrove sample extract was also calculated in addition to calculating the sample biomass from wet to dry. Based on the maceration process with methanol (polar) solvent which has been repeated three times and followed by the evaporation process, the results of the R. apiculata mangrove sample extract obtained are presented in table 4.
Table 4 shows the extraction results from the yield of 100 g of R. apiculata mangrove sample powder with methanol solvent yielding an average crude extract of 5.67%. The most extract was obtained from the DRA sample code, namely 9.98 gr (9.98%) and the least extract was found in the BRA sample code, namely 1.86 gr (1.86%). The percentage of each mangrove sample yield varies according to the solvent used in the maceration and the characteristics of the mangrove samples used. According to Purwaningsih et al. [29], temperature did not affect the amount of yield produced. Many extraction yields are influenced by the characteristics of the materials, solvents, and methods used. The maceration process carried out in this study used methanol as a solvent. Hermanda et al. [30] said that methanol solvent was a polar solvent which had advantages in extracting almost all secondary metabolite compounds in the samples tested, both polar and nonpolar. Secondary metabolites contained in the roots, stems, and leaves of R. apiculata have different concentrations. According to Acacia et al. [4], R. apiculata leaves had secondary metabolite compounds in the form of phenols, alkaloids, flavonoids, tannins, saponins and steroids. Usman [31] also said that the roots of R. apiculata contained secondary metabolites in the form of alkaloids, flavonoids and triterpenoids. Meanwhile, according to Berawi & Marini [7], the stems of R. apiculata had secondary metabolites, namely alkaloids, flavonoids and tannins.
Exact toxicity level
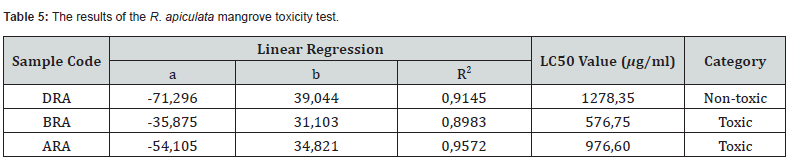
The results of the toxicity tests that have been carried out on the R. apiculata mangrove extract against nauplius A. salina are presented in table 5.
Table 5 shows the results of the toxicity test from the leaves, roots and stems extracts of the mangrove R. apiculata samples. This shows that from 3 parts of the samples, there are 2 toxic parts and 1 non-toxic part. The toxic category is seen from the LC50 value, which is found in the stems of R. apiculata (BRA) and roots of R. apiculata (ARA), while the leaves of R. apiculata (DRA) do not show toxicity. According to Sari’s research [32], R. apiculata mangrove bark extract had an LC50 value of 338.364 mg/l and according to Usman [31], R. apiculata mangrove root extract had an LC50 value of 25.7 mg/l. As well as previous research conducted by Maulana & Sasmito [9], mangrove leaf extract R. ap/l.
The results of the toxicisiiculata test showed an LC50 value was 49.45 mgtas using the BSLT method indicating that there was an extract that was not toxic to A. salina. The sample has an LC50 value of > 1000 μg/ml. In accordance with the research of Meyer et al. [24], LC50 values is > 1000 μg/ml and that was non-toxic. The high LC50 value appears due to the fact that the mortality in A. salina did not reach 50% of the amount tested at concentrations < 1000 μg/ml. Compounds can be toxic if they are able to kill 50% of A. salina within 24 hours during the test.
Stems of R. apiculata (BRA) have stronger toxic levels than leaf extracts. This can happen because the toxic compounds found in the stems, roots, and leaves are different. The higher LC50 values found in stems and roots can be influenced by several factors including age, habitat and nutrition received. According to Lambers et al. (2008) in Alfarabi & Gupita [33] variations in LC50 values found in stems, roots and leaves are caused by differences in habitat, soil nutrition, and plant age. Secondary metabolite compounds can be influenced by the nutrition and habitat of the mangroves taken.
Antioxidant activity
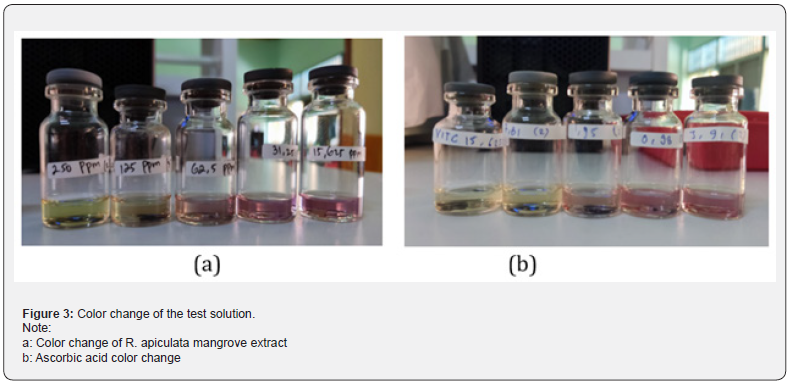
The color change of R. apiculata mangrove extract solution and ascorbic acid can be seen in figure 3.
The antioxidant activity of R. apiculata mangrove in the roots, stems and leaves can be determined by testing using the DPPH (2,2-diphenyl-1-pikri-hidrazyl) method. The way this method works is by using ascorbic acid as a comparison solution and DPPH solution which will later interact with antioxidant compounds whose initial color given is dark purple to clear yellow. Wijaya et al. [34] said that a reduction in the intensity of the color of the DPPH solution used indicated a reaction of the hydrogen atoms present in the test material with the DPPH radical molecule and the solution would turn yellow.
Absorbance measurements of roots, stems and leaves of R. apiculata with ascorbic acid as a comparison were carried out to view antioxidant activity. Absorbance measurements were carried out using a spectrophotometer at a wavelength of 517nm. According to Ardiansyah [35]’s research, ascorbic acid was used as a comparison because it was considered as a strong antioxidant, reacted quickly (less than 30 minutes), and was easy to observe and measure.
Antioxidants in the R. apiculata mangrove need to be discovered because they are very useful for research developments especially in the food and medicine fields. Research conducted by Rumagit et al. [36] found that antioxidative secondary metabolites include alkaloids, flavonoids, tannins, steroids and saponins. According to Berawi & Marini [7], the community used R. apiculata mangroves as a nausea, anti-diarrhea, vomiting, antiviral, and hypoglycemic medicine.
Antioxidant level
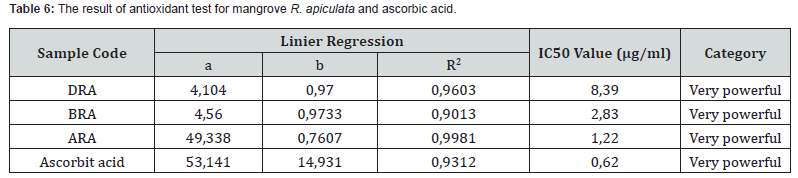
The results of antioxidant tests that have been carried out on ascorbic acid and R. apiculata mangrove extracts using the DPPH method are presented in table 6.
The level of antioxidants in the samples of roots, stems, and leaves has a low IC50 value and can be categorized as a very strong antioxidant. The IC50 value for leaf extract was 8.29μg/ ml, for stem extract was 2.83μg/ml and for root extract had the lowest value, namely 1.22μg/ml. According to Chew et al. (2011) in Jacob et al. [37], extraction results in bioactive compounds can be influenced by several factors, such as the extraction method used, the particle size of the material, storage time, conditions and the ratio of the amount of solvent to the sample.
The IC50 value used as a reference refers to the study of Hanin & Pratiwi [38]. There were four levels of IC50 value, namely an IC50 value of <50 μg/ml which is categorized as having a very strong antioxidant, an IC50 value of 50-100 μg/ml is categorized as strong, an IC50 value of 100-250 μg/ml is categorized moderate and an IC50 value of 250-500 μg/ml is categorized as weak.
Based on the research conducted, the IC50 value obtained was smaller than previous research. This could happen because several studies used high DPPH concentrations which made the antioxidant concentrations weaker. According to Balun [39] the greater decrease in DPPH absorbance will make the solution have stronger antioxidant activity.
Conclusion
The conclusions that can be obtained from the results of this study are: The sample extract of the R. apiculata mangrove has an IC50 value, namely the mangrove root extract of R. apiculata (ARA) 1.22μg/ml, the stem extract of R. apiculata (BRA) 2.83μg/ ml, R. apiculata leaves (DRA) 8.39μg/ml and R. apiculata stem extract (BRA) had an LC50 value of 576.75μg/m, R. apiculata mangrove root extract (ARA) 976.60μg/ ml and R. apiculata leaf extract (DRA) 1278.35μg/ml. The antioxidant ability of R. apiculata root, stem and leaf extracts has very strong antioxidant properties and the ability of extracts from R. apiculata stems (BRA) has stronger toxic properties than R. apiculata root extract (ARA) [40-42].
References
- Puspitasari E, Rozirwan, Hendri M (2018) Toxicity test using the brine shrimp lethality test (bslt) method on mangrove extract (Avicennia Marina, Rhizophora Mucronata, Sonneratia Alba and Xylocarpus Granatum) from banyuasin, south sumatra. Jurnal Biologi Tropis 18(1): 94.
- Rahayu S (2019) Inhibitory Power of Bioactive Compounds in Mangrove Rhizophora sp. As Antibacterial from Tanjung Api-Api Waters, South Sumatra. Indralaya : Faculty of Mathematics and Natural Sciences, Sriwijaya University 21(3): 60.
- Hasanah N, Urbach A (2018) Toxicity test of Cabbage Leaf (Brassica Oleracea var Capitata L) ethanol extract using Brine Shrimp Lethality Test (BSLT) method. Edudharma Journal 2(1): 84-88.
- Acacia AI, Son of IDNN, Son of ING (2021) Phytochemical screening of mangrove leaf extracts of Rhizophora mucronata and Rhizophora apiculata collected from the mangrove area of Tuban Village, Bali. Journal of Marine Research and Technology 4(1): 18.
- Pramesti R (2013) Antioxidant Activity of Caulerpa Serrulata Seaweed Extract Using DPPH Method (1,1 diphenyl 2 picrylhydrazyl). Buletin Oseanografi Marina 2(1): 7.
- Fitriana WD, Fatmawati S, Ersam T (2015) Antioxidant Activity Test on DPPH and ABTS from Fractions Moringa Leaves (Moringa oleifera). Bandung, 20-21 October 2016. Bandung: Snips. p. 657.
- Berawi KN, Marini D (2018) Literature review of the effectiveness of oil palm stem bark (Rhizopora apiculata) as an antioxidant. Journal of Agromedicine 5(1): 416.
- Dapas CC, Koleangan HSJ, Sangi M (2014) Analysis of Secondary Metabolites and Toxicity Test of Sea Onion (Proiphys amboinensis (L.) ) Stem Extract. Journal of Mipa Unsrat Online 3(2): 148.
- Maulana DM, Sasmito BB (2021) The dose effect of mangrove leaf extract (Rhizophora apiculata) on anticancer activity in hela cells. Journal of SCRTE 5(1): 8.
- Ulqodry TZ, Sarno (2017) Mangrove Conservation Textbook. Palembang: UPT Publishers and Printing, University of Sriwijaya.
- Rumayati, Idiawati N, Destiarti L (2014) Test of antioxidant activity, total phenol and toxicity of lakum leaf and stem extracts (Cayratia trifolia (L) Domin). Journal of Equatorial Chemistry 3(3): 31.
- Hasim, Andrianto D, Lestari ED, Faridah DN (2017) Antioxidant activity of white dragon fruit (Hylocereus undatus) extract using DPPH and Rancimat methods. J Gizi Pangan 12(3): 204.
- Muthia R, Saputri R, Verawati SA (2019) Antioxidant activity test of mundar (garcinia forbesii king.) peel ethanol extract using the DPPH (2,2-diphenyl-1-picrylhydrazil) method. Jurnal Pharmascience 6(1): 77.
- Martini I, Azzahra IF, Perdana F (2017) Antioxidant activities of n-hexan, ethyl acetate, and methanol extracts of dewandaru leaves (Eugenia uniflora l.). Jurnal Ilmiah Farmako Bahari 8(2): 34.
- Muharni M, Elfita E, Masyita M (2015) Isolation of secondary metabolite compounds from n-hexane extract of brotowali plant (Tinosporacrispa L.). Molekul 10(1): 40.
- Kurang RY, Adang B (2018) Phytochemical screening and antioxidant activity tests of soursop (Annona Muricata L.) leaves with the 1,1-diphenyl-2-pikrylhydrazyl (DPPH) method. Partner 23(1): 569.
- Handayani V, Ahmad AR, Sudir M (2014) Antioxidant Activity Test of Patikala Flower and Leaf Methanol Extract (Etlingera elatior (Jack) RMSm) Using the DPPH Method. Journal Pharm Sci Res 1(2): 89.
- Baud GS, Sangi MS, Koleangan HSJ (2014) Analysis of secondary metabolites and toxicity test of the ethanol extract of broken bones (Euphorbia tirucalli l.) stems using the brine shrimp lethality test (BSLT) method. Scientific Journal of Science 14(2): 108.
- Sartinah A, Yamin Y, Nurhasanah, Arba M, Akib NI, et al. (2020) Acute Toxicity Test of Ketapang Laut (Terminalia Catappa L.) Bark Extract and Fraction Using the BSLT Method. Jurnal Farmasi, Sains, dan Kesehatan 6(1): 4.
- Atmoko T, Maaruf A (2009) Toxicity test and phytochemical screening of plant extract orangutan feed source on larvae. Research Journal of Forests and Nature Conservation 6(1): 39.
- Paputungan Z, Wonggo D, Kaseger BE (2017) Phytochemical test and antioxidant activity of Sonneratia alba mangrove fruit in Nunuk Village, Pinolosian District, South Bolaang Mongondow Regency. Fisheries product technology media 5(3): 98.
- Dewi SR, Ulya N, Argo BD (2018) Flavonoid content and antioxidant activity of Pleurotus ostreatus extract. Journal of Agricultural Engineering Rona 11(1): 4.
- Nurhayati APD, Abdulgani N, Febrianto R (2006) Toxicity test of Eucheuma alvarezii extract against Artemia salina as a preliminary study of anti-cancer potential. Jurnal Akta Kimindo 2(1): 42.
- Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, et al. (1982) Brine Shrimp: A Convenient General Bioassay for Active Plant Constituent. Planta Medica 45(5): 31-34.
- Dekky, Linda R, Wardoyo ERP (2016) Inventory of Mangrove Types Found in the Tanjung Bila Area, Pemangkat District, Sambas Regency. Protobiont Journal 5(3): 57.
- Imiliyana A, Muryono M, Purnobasuki H (2011) Estimation of carbon stock in Rhizophora Stylosa tree stands at camplong beach, sampang-madura. Researchgate, p. 1-15.
- Jacoeb AM, Purwaningsih S, Rinto (2011) Anatomy, bioactive components and antioxidant activities of Api-Api mangrove leaves (avicennia marina). Journal of Processing of Indonesian Fishery Products 14(2): 147.
- Egra S, Rofin M, Adiwena M, Jannah N, Kuspradini H, et al. (2019) Antimicrobial Activity of Mangrove (Rhizophora mucronata) Extract in Inhibiting the Growth of Ralstonia solanacearum Causes Wilt Disease. Agrovigor 12(1): 27.
- Purwaningsih S, Salamah E, Sukarno YP, Deskawati E (2013) Antioxidant activity of mangrove fruit (Rhizophora mucronata Lamk) at different temperature. JPHPI 16(3): 202.
- Hermanda R, Widayat W, Rijai L (2016) Antibacterial Activity of Methanol Extract of Merung Plant Root (Coptosapelta tomentosa) Against Escherichia coli and Staphylococcus aureus Inside: Proceedings of the National Pharmacy Seminar; Samarina, Samarinda: Tropical Pharmaceutical Research and Development Laboratory, pp. 326.
- Usman U (2017) Phytochemical Test and Antibacterial Test of Rhizopora Apiculata Mangrove Root against Escherichia coli and Staphylococcus aureus Jurnal Kimia dan Pendidikan Kimia 2(3): 170.
- Sari N (2017) Larvicidal Activity Test of n-Hexane, Ethyl Acetate and 96% Ethanol Extract of Oil Mangrove Root (Rhizophora apiculata Blume). Against Aedes aegypti L Mosquito Larvae. Makassar: Alauddin State Islamic University.
- Alfarabi M, Widyadhari G (2018) Toxicity test and phytochemical identification of Rimbang (Solanum torvum Swartz) Journal of Biology 11(2) :113.
- Wijaya DP, Paendong JE, Abidjulu J (2014) Phytochemical Screening and Antioxidant Activity Test of Rice Leaves (Phrynium capitatum) using the DPPH (1,1-diphenyl-2-picrylhidrazyl) method. Jurnal Mipa Unsrat 3(1): 13.
- Ardiansyah A (2016) Extraction and Formulation of Holothuria scabra Oral Suspension as a Source of Antioxidants. Oceanology and Limnology in Indonesia 1(1): 35.
- Rumagit HM, Runtuwene MRJ, Sudewi S (2015) Phytochemical assessment and antioxidant activity test of ethanol extract of sponge Lamellodysidea herbacea. Pharmacon Jurnal Ilmiah Farmasi 4(3): 184.
- Jacoeb AM, Suptijah P, Zahidah (2013) Chemical composition, bioactive components and antioxidant activity of lindur fruit (Bruguiera gymnorrhiza). JPHPI 16(1): 90.
- Hanin NNF, Pratiwi R (2017) Phenolic Content, Flavonoids and Antioxidant Activity of Fertile and Sterile Sea Fern ( Acrostichum aureum L.) Leaf Extract in the Mangrove Area of Kulon Progo, Yogyakarta. Journal of Tropical Biodiversity and Biotechnology 2(1): 54.
- Balun SVK (2018) Effectiveness Test of Rhizophora Apiculata Mangrove Leaf Extract as Antibacterial Against Staphilococcus Aureus. Malang: Fisheries Product Technology Study Program, Department of Aquatic Resources Management, Faculty of Fisheries and Marine Sciences, Universitas Brawijaya, p. 48.
- Adelia Y, Iskandar D (2020) Effectiveness Test of Lamtoro Seed Extract (Leucaena leucocephala) as Insecticide against American Cockroach (Periplaneta americana) Journal of Chemical Research 11(2): 74.
- Bahriul P, Rahman N, Diah AWM (2014) Antioxidant Activity Test of Bay Leaf Extract (Syzygium Polyanthum) Using 1,1-Diphenyl-2-Pikrylhidrazil. Kim Academic Journal 3(3): 371.
- Noor YR, Khazali M, Suryadiputra INN (2006) Guide to Introduction to Mangrove in Indonesia. Bogor : PHKA/WI-IP.






























