Abnormal specimens of cultured common carp, Cyprinus carpio communis L., from a fishpond in Jammu district of Union Territory of Jammu and Kashmir
Dutta S P S*
Departmental of Environmental Sciences, University of Jammu, India
Submission:November 12, 2021; Published:January 31, 2022
*Correspondence author: Dutta S P S, Departmental of Environmental Sciences, University of Jammu, India
How to cite this article: Dutta S P S. Abnormal specimens of cultured common carp, Cyprinus carpio communis L., from a fishpond in Jammu district of Union Territory of Jammu and Kashmir. Oceanogr Fish Open Access J. 2022; 14(4): 555892. DOI: 10.19080/OFOAJ.2022.14.555892
Abstract
Twelve deformed specimens of Cyprinus carpio communis (three stumpy and truncated, four highly truncated and five slightly truncated) were observed among fish collections from a fishpond in Jammu and have been reported. X-ray analysis has revealed multiple vertebral column deformities like lordosis, kyphosis, scoliosis, and coiling; vertebral compression, clustering, duplication, ankylosis and degeneration and intervertebral spaces deformities. In one fish specimen there is modification of caudal fin bones as an elongated bony structure supporting caudal fin rays. Growth of deformed fishes was lower than that of normal fish. The reason for fish aberrations could not be ascertained, but multiple possible causes are discussed.
Keywords: Cyprinus carpio communis; Deformities; Truncated and stumpy body; Jammu fishpond; Multiple factors
Introduction
Cyprinus carpio communis L., an exotic carp, is distributed in cold and warm lotic and lentic water bodies from Kashmir to Kanyakumari, in India. To meet the demand of animal food for growing population, it is cultured alone or along with other exotic and Indian major carps in reservoirs, ponds, tanks etc. The fishery department of Union territory of Jammu and Kashmir, India, has stocked this fish in ponds, almost in all the districts of Jammu region, and has even entered in natural lotic and lentic water bodies. Fish surveys in Jammu waters for last four decades have shown a sudden rise in fish deformities in both wild and cultured fish populations. However, frequencies are higher in cultured populations. Due to scanty attention by fish biologists and fishery departments on fish teratology, in India, much is not on record of Indian and exotic teleosts. Skeletal anomalies in farm teleosts are currently a major global problem in aquaculture. The incidence of skeletal anomalies is highly variable in different species and under different rearing conditions. The percentage of fish with medium to severe anomalies varies greatly not only among different farms but also among different lots within the same hatchery. Occurrence of fish deformity is an indicator of environmental quality. [1-4] Abnormalities should be given a serious attention among the cultured fish species as these cause weight loss, reproductive failure, functional disorder of vital organs, rejection by consumers and is a serious economic loss to the aquaculturists.
Materials and Methods
Deformed specimens of Cyprinus carpio communis were seen among the collection from a fishpond of Jammu district, purchased and analysed for morphological aberrations, parasitic infections and photographed. These fishes were radiographed using a medical X-ray system (AGFA)
Results
Twelve deformed specimens of Cyprinus carpio communis were seen among the fish collections from a fishpond in Ghomanhasan area, Jammu district, and are described below:
Stumpy body with highly truncated trunk and caudal peduncle and displaced fins
This dead deformed fish, collected by a fisherman from a fishpond at 8 a.m., was purchased from fish market in the month of January at 6 p.m., packed in plastic bags and brought home. At 10 p.m., fish was removed from plastic bags to fix in formaldehyde solution. Surprisingly, there was movement of fins. Immediately, it was transferred in a bucket of water and left overnight. In the morning fish was swimming like a normal fish. It was photographed, x-rayed for skeletal analysis, and released in a temple tank in the evening at 6 p.m. Revival of this dead fish after about fourteen hours is a miracle of God.
This abnormal fish, measuring 40cm and weighing 2.5kg, was recognized by highly truncated stumpy body and unequal caudal fin lobes. In a normal fish, dorsal fin origin anterior to pelvic fin origin. There is a space between the longest pectoral fin ray and pelvic fin base, pelvic fin ray and anal fin base and anal fin ray and caudal fin base. Caudal peduncle is elongated bearing at its end bilobed caudal fin (Figure 1). On the contrary, in this abnormal fish caudal peduncle is highly truncated and folded along left side of body. Caudal fin lobes are unequal with upper lobe smaller than the lower lobe. Dorsal fin origin is slightly posterior to pectoral fin origin, longest pectoral fin ray extends beyond pelvic fin base, pelvic fin ray extends anal fin base and anal fin has its origin from caudal peduncle fold and is placed above the lower caudal fin lobe (Figure 2).

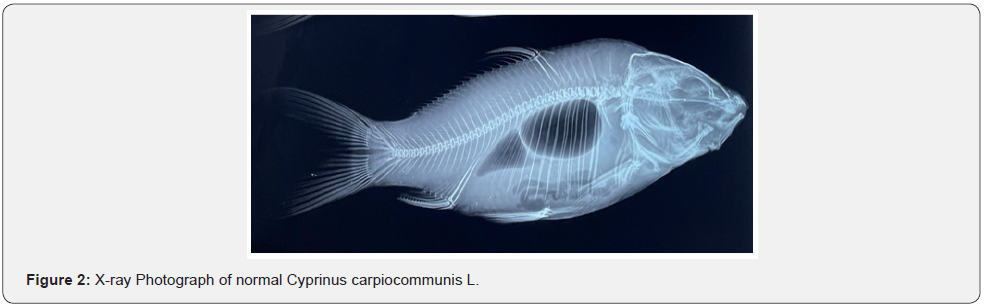
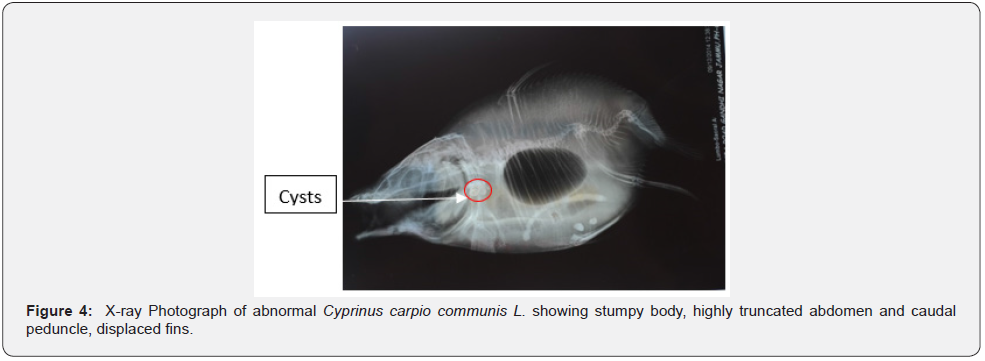
X-ray analysis has shown highly truncated caudal peduncle. After last caudal vertebra there is a curved muscular fold with a centrally placed elongated bony structure representing most probably modified caudal bones and support caudal fin rays. X-ray study has also shown presence of cysts containing larvae on opercular bone (Figure 3). X-ray analysis has revealed the presence of 32nd and 37th vertebrae (excluding urostyle) in normal and abnormal fish, respectively (Figures 2,4). Vertebral column in abnormal fish is irregular from anterior to the posterior end and is discussed as below:
i. Vertebral column between 1st -13th vertebrae, dorsally curved above air bladder lobe, is irregularly truncated with variable vertebral thickness, intervertebral spaces, and vertebral fusion.
ii. Vertebral column is semicircular between 14th to 16th vertebrae and coiled between 17th to 24th vertebrae to form a mass.
iii. Posteriorly, between 25th to 37th vertebrae, vertebral column is ventrally (lordosis) and dorsally (kyphosis) curved. 31st to 37th vertebrae are highly truncated with reduced vertebral thickness and intervertebral spaces and variable centra.
iv. Vertebrae have lost their normal biconcave structure from anterior to posterior end.
v. Urostyle and caudal bones not clear and form a fused elongated bony structure supporting caudal fin rays.
vi. Neural and haemal spines and ribs are irregular in this abnormal fish.
vii. Air bladder is bilobed in a normal fish. Whereas, in this aberrant fish only one air bladder lobe is present.
Stumpy body with highly truncated caudal peduncle, abnormal caudal fin lobes and displacement of fins
Contrary to the installation of dorsal fin anterior to pelvic fin in a normal fish, dorsal fin installation in this abnormal fish is almost opposite to the pelvic origin and longest pectoral fin ray extends pelvic fin base. Space between the longest pelvic fin ray and anal fin base, due to abnormal height, is more than the normal fish. In a normal fish there is a long caudal peduncle and longest anal fin ray falls short of bilobed caudal fin base. In this abnormal stumpy fish caudal peduncle is highly truncated and longest anal fin ray extends beyond caudal fin base. Caudal fin lobes have a deep cut and upper lobe is longer than the lower lobe (Figure 5).


X-ray analysis has revealed the presence of 32 biconcave vertebrae, after complex vertebrae, in both normal and abnormal fish. In this abnormal fish vertebra compressed and vertically elongated from anterior to the posterior side. 1st five vertebrae and their intervertebral spaces thick and centra reduced. Intervertebral spaces of 6th – 19th vertebrae slightly opaque and normal in the posterior vertebrae (Figure 6).

Stumpy body and highly truncated trunk
This aberrant fish, measuring 32cm and weighing 2 kg., was recognized by stumpy body and truncated trunk. Longest pectoral fin ray extends almost to the pelvic fin base (Figure 7). X-ray analysis has revealed the presence of 32 biconcave vertebrae in both normal and deformed fish, after complex vertebrae (Figures 2,8). In the aberrant fish first eight vertebrae highly degenerated, overlapping and fused to form three opaque structures. Neurospines not clear. Ribs irregularly fused and overlap. Posteriorly vertebrae, spines, urostyle, and caudal bones normal.

Truncated body and displacement of fins
This deformed fish specimen, measuring 16 cm and weighing 90 gms, was recognized by truncated body and fins displacement. In this aberrant fish dorsal fin installation is beyond pelvic fin origin, longest pectoral fin ray extends pelvic fin base and there is a space reduction between longest pelvic fin ray and anal aperture (Figure 9). X ray analysis has revealed a dorsally curved vertebral column with a kyphosis between 13th – 18th vertebrae. 14th – 17th vertebrae short, centra irregular and intervertebral spaces reduced (Figure 10).
Highly truncated body and fins displacement
This deformed fish, measuring 18 cm and weighing 180 gms was recognized by truncated body and extension of longest pectoral fin ray almost to pelvic fin base, pelvic fin ray beyond anal aperture and anal fin ray almost to the caudal fin base (Figure 11). X ray analysis has shown 32 biconcave vertebrae in normal and abnormal fish. There are various vertebral anomalies in this deformed fish and are:
• 1st six vertebrae short, highly compressed, and rudimentary centra. Intervertebral spaces compressed, vertically elongated, thick, and opaque.
• 7th – 9th vertebrae almost normal.
• 10th and 11th vertebrae highly compressed, elongated, and opaque.
• 12th – 14th vertebrae almost normal with reduced intervertebral spaces.
• Intervertebral spaces of 15th – 22nd vertebrae reduced and highly reduced between 23rd to 32nd overlapping vertebrae.




Truncated body and displacement of pectoral fin
Measuring 19 cm and weighing 360 gms, this truncated fish was recognized by extension of longest pectoral fin ray to the pelvic fin base. There is a reduction in space between longest pelvic fin ray and anal fin base and anal fin ray and caudal fin base. X ray analysis has shown various vertebral deformities in this deformed fish and are:
• Thick and opaque first vertebra.
• Lower half of intervertebral spaces of 2nd to 6th vertebrae thick and opaque.
• Lower half of intervertebral spaces between 7th to 11th vertebrae with thick rings.
• 12th – 26th vertebrae and their intervertebral spaces normal.
• In the remaining posterior normal vertebrae intervertebral spaces highly reduced (Figure 14).



Dorsally curved highly truncated body and displacement of fins
This abnormal fish, measuring 18.9 cm and weighing 300 gms, was recognized by dorsally curved body with extension of longest pectoral fin ray beyond pelvic base, pelvic fin ray to anal fin base and anal fin ray almost to the caudal fin base (Figure 15). X ray analysis has shown various vertebral deformities from anterior to the posterior end and are described as under:
• Vertebral column between 1st to 16th vertebrae dorsally curved.
• 1st vertebra thick with rudimentary centrum.
• 2nd vertebra highly compressed, vertically elongated, and opaque.
• Intervertebral spaces of 3rd to 7th vertebrae reduced.
• 8th – 12th vertebrae and their intervertebral spaces normal.
• Intervertebral spaces of 13th – 27th vertebrae reduced and between 28th to 32nd vertebrae highly reduced, vertebrae short and overlap (Figure 16).


Truncated trunk and displacement of fins
This deformed fish measuring 29 cm and weighing 950 gms was recognized by truncated caudal peduncle and reduction in space between longest pectoral fin ray and pelvic fin base, pelvic fin ray and anal fin base and anal fin ray and caudal fin base (Figure 17). X ray analysis has shown irregularly thick 1st to 13th vertebrae, with variable centra, and their intervertebral spaces. Posterior vertebrae normal with reduced intervertebral spaces (Figure 18).


Truncated body and displacement of pectoral fins
This aberrant specimen, measuring 22.5 cm and weighing 400 gms, was recognized by truncated body and displacement of fins. Longest pectoral fin ray extends pelvic fin base and there is a space reduction between pelvic fin ray and anal fin base and anal fin ray and caudal fin base (Figure 19). X ray analysis of this deformed fish has shown a minor kyphosis at 15th vertebra and variable vertebral deformities from anterior to posterior end
• 7th, 12th, 14th, 17th, 20th, 22nd and 24th vertebrae highly compressed, vertically elongated, and opaque.
• 1st to 6th vertebrae highly compressed, vertically elongated, and thick with thick intervertebral spaces and have variable size of centra.
• 8th – 11th, 13th, 15th – 16th, 18th, 20th – 21st vertebrae are irregularly compressed with variable size centra and intervertebral spaces.
• Intervertebral spaces of last 25th – 34th vertebrae are highly reduced (Figure 20).


Highly Truncated body and displacement of fins
Measuring 16.5 cm and weighing 130 gms, this abnormal fish was recognized by truncated body and extension of longest pectoral fin ray beyond pelvic fin base, pelvic fin to the anal fin base and anal fin ray to the caudal fin base (Figure 21). X ray analysis of this deformed fish has shown various vertebral anomalies and are described as under:
• Vertebral column dorsally curved between 1st – 15th vertebrae.
• 1st to 12th vertebrae and their intervertebral spaces irregularly thick with variable size of centra.
• Intervertebral spaces of posterior vertebrae reduced. Last two vertebrae have reduced size (Figure 22).



Truncated body and fins displacement
Measuring 22.5 cm and weighing 450 gms, this deformed fish specimen was characterised by highly truncated body and fins displacement. Longest pectoral fin ray extends about pelvic fin origin, pelvic fin ray to anal aperture and fin ray beyond caudal fin base (Figure 23). X ray of this aberrant fish has shown
• Highly thick and opaque intervertebral spaces between 1st – 12th vertebrae. Lower half of intervertebral spaces between 2nd to 6th vertebrae thick with a rudimentary ring.
• Lower half of intervertebral spaces between 7th to 17th vertebrae thick with thick rings.
• Normal intervertebral spaces of 18th – 20th vertebrae slightly reduced and highly reduced in the posterior vertebrae (Figure 24).

Highly Truncated body and fins displacement
Measuring 19.2 cm and weighing 360 gms, this highly truncated fish was characterised by extension of longest pectoral fin ray beyond pelvic fin base, pelvic fin ray beyond anal fin base and anal fin ray beyond caudal fin base (Figure 25). X ray analysis of this deformed fish has shown
Thick and opaque first vertebra.
• 2nd to 12th vertebrae and their intervertebral spaces thick.
• Lower half of intervertebral spaces of 8th – 13th vertebrae marked by thick circular rings.
• 14th to 32nd vertebrae uniformly compressed and elongated vertically with normal intervertebral spaces (Figure 26).

Discussion
Caudal peduncle in fish is supported by strong swimming muscles and are helpful in swimming. Caudal fin lobes propel the body forward and provide the lift. How these fishes and particularly fish with aberrant caudal peduncle and caudal fin lobes swim, stabilize, feed, and avoid predators is unexplainable. Vertebral aberrations are known to affect fish biology through inhibiting their free movements [5]. Such fishes with spinal deformities swim upside down or sideway and their growth is slow compared to a normal fish. Average length and weight of such abnormal fishes have been found to be lower than that of a normal fish, probably due to their inability to feed normally and to compete with the normal ones for food and other adequate resources [6].
Fish skeletal deformities have been ascribed to a wide variety of physical, chemical, and biological stressors. In fish seed producing centres, high water currents during development [7-8], cultural techniques, rearing conditions, faulty methods followed in induced breeding, mechanical shock sustained during an early phase of development, unfavorable environmental conditions during embryonic development are known to cause fish anomalies and cannot be ruled out in the present case [9-17].
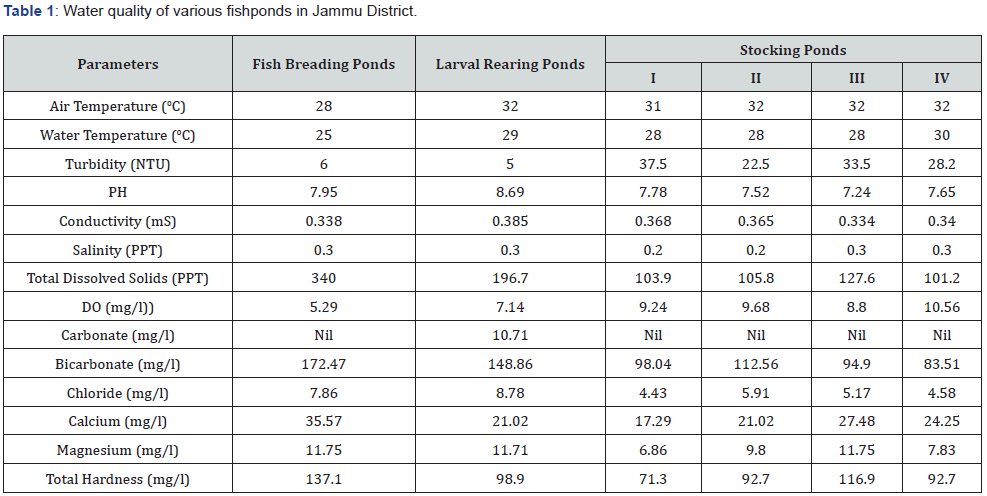
Among various abiotic factors temperature, light and temperature, low pH, salinity and low DO are known to cause fish anomalies [18-26]. Water analysis of some ponds in the area has revealed optimum levels of all the parameters as such fish anomalies, under discussion, cannot be attributed to various abiotic factors (Table 1).
Fish deformities due to water pollution [27] are not possible as the area is free from any type of large- and small-scale industry and sewage flow. Anomalies, under discussion, can also be attributed to pesticides used in nearby paddy fields and their ground water contamination and use of this contaminated water in fishponds. Fish anomalies due to pesticides contaminated water use or pesticides have also earlier been reported [7,28-34]. Anomalies in fish farms have also been ascribed to nutritional deficits [11,17,35-41]. In absence of detailed analysis of feed given to fish, it is not possible to suggest anomalies, under discussion, to inadequacy of key nutrients.
Fish anomalies have also been attributed to inbreeding and genetic defects [4,17,42-47]. As these specimens were not analysed genetically, hence it could not be ascertained whether these anomalies were hereditary or non-hereditary. Among biological factors infectious biological agents including virus, bacteria, protozoa, and other parasites are known to cause fish deformities [48-52]. Presence of cysts containing larvae in one fish specimen suggest that deformities can also be due to parasites. Presence of one air bladder in one specimen suggests that vertebral deformities can also be due to air bladder deformities [7,35,53,54]. From the above discussion, fish aberrations are caused by multiple factors [6,45-47,55,56] therefore, more detailed research is needed to exactly identify the factors causing deformities in fish farms.
References
- Kirpichnikov V S (1945) viability, rate of growth and morphology of carps of different genotypes as affected by rearing conditions. Dokl Akad Nauk Soiuza Sov Sotsialisticheskikh Resp 47(7): 503-506.
- Antunes M, Lopes Da Cunha P (2012) Skeletal anomalies in Gobius niger (Gobiidae) from Sado estuary, Portugal. Cybium 26(3): 179-184.
- Narejo N T, Mastoi A M, Laghari M Y, Lashari P K (2007) Incidence of crooked vertebral column in adult Cirrhinus mrigala (Hamilton) from Keenjhar lake (District: Thatta) Sindh, Pakistan. Pakistan J Zool 39(3): 199-201.
- Aydin I (2012) The external abnormalities of hatchery reared black sea flounder (Platichthys flesus luscus Pallas, 1814) in Turkey. Turkish Journal of Fisheries and Applied Sciences 12: 123-133.
- Jawad L A (2004) First record of an anomalus mullet fish (Mugil cephalus) from New Zealand. Tuhinga 15: 121-124.
- Al- Harbi A H (2001) Skeletal deformities in cultured common carp, Cyprinus carpio L. Asian Fisheries Science 14: 247-254.
- Chatain B (1994) Abnormal swim bladder development and lordosis in sea bass (Dicentrarchus labrax) and sea bream (Sparus auratus). Aquaculture 119(4): 371-379.
- Divanach P, Papandroulakis N, Anastasiadis P, Koumoundouros G, Kentouri M (1997) Effect of water currents on the development of skeletal deformities in sea bass (Dicentrarchus labrax L) with functional swim bladder during postlarval and nursery phase. Aquaculture 156(1-2): 145-155.
- Romanov N S (1984) Effect of cultural conditions on skull morphology in smolts of the masu salmon, Onchorhynchus masou (Brevoort). Aquaculture 41(2): 147-153.
- Leary R F, Allendorf F W, Knudsen K L (1991) Effects of rearing density on meristics and developmental stability of rainbow trout. Copeia 1991(1): 44-49.
- Gavaia P J, Dinis M T, Cancela M L (2002) Osteological development and abnormalities of the vertebral column and caudal skeleton in larval and juvenile stages of hatchery reared senegal sole (Solea senegalensis). Aquaculture 211(1-4): 305-323.
- Koumoundouros G, Divanach P, Kentori M, (2001) The effect of rearing conditions on development of saddleback syndrome and caudal fin deformities in Dentex dentex (L.). Aquaculture 200(3): 285-304.
- Bhattacharyya S (1983) Abnormal silver carps Hypopthalmichthys molitrix in fish nurseries. Environ and Ecol 1: 15-16.
- Kapoor B G, Sarkar H L (1955) Notes on four deformed specimens of the Indian carp, Labeo rohita (Ham.). Nat Inst Sci, India 21B (3): 129-136.
- Sarkar H L, Kaushik N K (1958) Notes on two deformed specimens of the Indian carp Cirrhinus mrigala (Hamilton). Proc Zool Soc 11: 39-45.
- Banerji S R, Singh M N (1978) A truncated specimen of Cirrhinus mrigala (Ham). Matsya 4: 80-82.
- Quigley D T G (1995) A lower jaw deformity in juvenile and adult Atlantic salmon (Salmo salar L.) Bulletin- European Association of Fish 15 (6): 206-209.
- Kwain W (1975) Embryonic development, early growth, and meristic variation in rainbow trout (Salmo gairdneri) exposed to combination of light intensity and temperature. Journal of Fisheries Research Board of Canada 32(3): 397-402.
- Al-Hassan L A J (1982) Vertebral deformities in fishes from Iraq and the United Arab Emirates. Arabian Gulf Iraqi Jour Mar Sci 1(1): 13-23.
- Wiegand M D, Hataley J M, Kichen C, Buchanan L (1989) Induction of developmental abnormalities in larval goldfish (Carassius auratus) under cool incubation condition. Journal of Fish Biology 35: 85-95.
- Bolla S, Holmefjord I (1988) Effect of temperature and light on development of Atlantic halibut (Hippoglossus hippoglossus) Aquaculture 74(3-4): 355-358.
- Bemaish R J (1972) Lethal pH for the white sucker (Catasomus commersonii) (Scapede). Trans Am Fish Soc 101: 355-358.
- Frognar J R (1977) Egg and larval survival of white suckers (Catastomus commersomii) (Scapede) at low pH. J Fish Res Board Can 34: 262-266.
- Lee C S, Menu B (1981) Effects of salinity on egg development and hatching in grey mullet, Mugil cephalus, L. Journal of Fish Biology 19(2): 179-188.
- Alderdice D F, Wickett W P, Brett J R (1958) Some effects of temporary exposure to low dissolved oxygen levels on Pacific salmon eggs. Journal of Fisheries Research Board of Canada 15(2): 229-249.
- Garside E T (1959) Some effects of oxygen in relation to temperature on the development of lake trout embryos. Canadian Journal of Zoology 37(5): 689-698.
- Dutta S P S (2018) Record of some deformed specimens of Cirrhinus mrigala (Ham Buch.) from river Tawi in Jammu city. The Bioscan 13(4): 831-834.
- Couch J A, Winstead J T, Goodman L R (1977) Kepone induced scoliosis and its histological consequences in fish. Science 197(4303): 585-587.
- Couch J A, Winstead J T, Hansen D J, Goodman L R (1979) Vertebral dysplasia in young fish exposed to the herbicide trifluralin. Journal of Fish Diseases 2(1): 35-42.
- Van Leeuwen C J, Helder T, Seiven W (1986) Aquatic toxicological aspects of dithiocarbamates and related compounds IV Teratogenicity and histopathology in rainbow trout (Salmo gairdneri). Aquatic Toxicology 9: 143-159.
- Middaugh D P, Fokrnie J W, Hemmer M J (1990) Vertebral abnormalities in juvenile inland silversides Menidia beryllina exposed to terbufos during embryogenesis. Diseases of Aquatic Organisms 9: 109-116.
- Dulcic J (2004) Incidence of spinal deformities in natural populations of grass goby, Zosterisessor ophiocephalus from the Karin Sea, eastern middle Adriatic. Cybium 28(1): 7-11.
- Stehr C M, Linbo J L, Incardona J P, Scholz N L (2006) The development of neurotoxicity of fibronil: notochord degeneration and locomotor defect in Zebrafish embryos and larvae. Toxicol Sci 92(1): 270-278.
- Jezierska B, Lugowska K, Witeska M (2009) The effects of heavy metals on embryonic development of fish (a review). Fish Physiol Biochem 35(4): 625-640.
- Kitajima C, Watanabe T, Tsukashima Y, Fujita S (1994) Lordotic deformation and abnormal development of swim bladders in some hatchery bred marine physoclistous fish in Japan. Journal of the World Aquaculture Society 25(1): 64-77.
- Halver J E, Ashley L M, Smith R E (1969) Ascorbic acid requirements of coho salmon and rainbow trout. Transactions of the American Fisheries Society 98(4): 762-771.
- Ashley L M (1972) Nutritional pathology. In: J E Helver (ed.), Fish nutrition, London Academic Press, Pp. 713.
- Aggarwal N K, Mahajan C L (1980) Nutritional deficiency disease in an Indian major carp, Cirrhinus mrigala (Ham) due to avitaminosis C during early growth. Journal Fish Disease 3: 231-248.
- Hodson P V, Hilton J W, Blunt B R, Slinger S J (1980) Effects of dietary ascorbic acid and chronic lead toxicity to young rainbow trout (Salmo gairdneri). Candian Journal of Fisheries and Aquatic Sciences 37(2): 1731-1741.
- Newsome C S, Piron R D (1982) Aetiology of skeletal deformities in the zebra danio fish (Brachidanio rerio, Hamilton-Buchanan). Journal of Fish Biology 21(2): 231-237.
- Lall S P, Lewis-McCrea L M (2007) Role of nutrients in skeletal metabolism
pathology in fish-An overview. Aquaculture 267(1-4): 3-19. - Aulstad D, Kittelsen A (1971) Abnormal body curvature of rainbow trout (Salmo gairdneri) inbred fry. Journal of the Fisheries Research Board of Canada 28(12): 1918-1920.
- Piron R D (1978) Spontaneous skeletal deformities in the zebra danio (Brachidanio rerio) bred for fish toxicity test. Journal of Fish Biology 13(1): 79-83.
- GJerde B, Pante Ma J R, Baevrefjord, G (2005) Genetic variation for a vertebral deformity in Atlantic salmon (Salmo salar). Aquaculture 244(1-4): 77-87.
- Andrades J A, Becerra J, Fernandez-Llebrez P (1996) Skeletal deformities in larval, juvenile and adult stages of gillhead sea bream (Sparus aurata L.). Aquaculture 141: 1-11.
- Raj A J A, Seetharaman S, Haniffa M A (2004) Skeletal deformities in few freshwater fishes from Bhavani River, Tamil Nadu. Zoos Print Jour 19(9): 1628-1629.
- Jawad L A, Öktoner A (2007) Incidence of lordosis in the freshwater mullet, Liza abu (Heckel, 1843) collected from Ataturk dam lake, Turkey. Anales de Biologia 29: 105-113.
- Lom J, Pike A, Dykova I (1991) Mxyobolus sandrae Reuss, 1906, the agent of vertebral column deformities of perch, Perca fluviatilis, in Northeast Scotland. Diseases of Aquatic Organisms 12(1): 49-53.
- Treasurer J (1992) Vertebral anomalies associated with Myxobolus sp. in perch, Perca fluviatilis in a Scottish loch. Bull Eur Assoc Fish Pathol 12(2): 63.
- Herick R P, El-Mathbouli M, Adikson M A, MacConnell E (1998) Whirling disease emergence among wild trout. Immunol Rev 166: 365-376.
- Kent M L, Watral V G, Whipps C M, Cunningham M E, Criscione C D, et al. (2004) A digenean metacercaria
(Apophallus ) and a Myxozoan (Myxobolus sp.) associated with vertebral deformities in cyprinid fishes from the Williamette river, Oregon. Journal of Aquatic Animal Health 16(3): 116-129. - Dutta S P S (2016) Some deformed specimens of Mystus bleekeri (Day) and Labeo bata (Ham. Buch) from the river Chenab in Pargwal wetland, Akhnoor, Jammu. Journal of Applied and Natural Science 8(1): 481-484.
- Trotter A J, Pankhurst P M, Buttagiene S C (2004) Morphological development of the swimbladder in hatchery reared striped trumpeter Latris lineata. Journal of Applied Ichthyology 20: 395-401.
- Grotmol S, Kryvi H, Totland G K (2005) Deformities in the notochord by the pressure from the swimbladder may cause malformation of the vertebral column in cultured Atlantic cod Gadus marhua larvae: a case study. Dis Aquat Organ 65(2): 121-128.
- Amitabh H, Firoz A M (2010) A wild specimen of Indian carp, Cirrhinus mrigala (Ham) with multiple vertebral deformities. World Journal Zoology 5(8): 167-177.
- Gemmill J F (1912) The teratology of fishes. Glassgow.
- Chatain B (1986) vessie natotoire chiz Dicentraclus labras et sparus anratus Aspects morphologiques development. Aquaculture 53: 303-311.
- Dulcic J, Soldo A (2005) Absence of caudal fin in solia (Soleidae) collected in the Northern Adriatic. Cybium 29: 308-309.
- Kitamura S, Ohara S, Suwa T, Nakagawa K (1965) Studies on vitamin requirements of Rainbow trout, Salmo gairdenri. Nippon Suisan Gakkaishi 31(10): 818-826.
- Olatunji-Akioye A O, Adeyemo O K, Akomolafe O T (2010) Photographic and radiographic study of osteological abnormalities of the head of adult African catfish (Clarias gariepinus). International Journal of Morphology 28(3): 719-722.
- Omotayo F, Abayomi O J (2011) Skeletal malformations among the Clarias species from fish mongers in Ekiti state, Continental. Continental Journal of Fisheries and Aquatic Science 5(2): 32-37.






























